Abstract
Vancomycin resistance in enterococci is an increasing clinical problem, and several phenotypes have been identified. We demonstrate here that the resistance mechanism in the constitutively vancomycin-resistant Enterococcus gallinarum BM4174 involves an altered pathway of peptidoglycan synthesis and hydrolysis of the normal precursors in the vancomycin-sensitive pathway. A ligase encoded by the vanC gene catalyses synthesis of D-Ala-D-Ser and substitutes this dipeptide for D-Ala-D-Ala in peptidoglycan precursors. It is presumed that this substitution lowers the affinity of vancomycin for its target site. Destruction of D-Ala-D-Ala (D,D-peptidase activity) and of UDP-MurNAc-L-Ala-D-isoGlu-L-Lys-D-Ala-D-Ala by removal of the terminal D-Ala residue (D,D-carboxypeptidase activity) ensures that the normal vancomycin-sensitive pathway of peptidoglycan synthesis cannot function in the resistant strain.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen N. E., Hobbs J. N., Jr, Richardson J. M., Riggin R. M. Biosynthesis of modified peptidoglycan precursors by vancomycin-resistant Enterococcus faecium. FEMS Microbiol Lett. 1992 Nov 1;77(1-3):109–115. doi: 10.1016/0378-1097(92)90140-j. [DOI] [PubMed] [Google Scholar]
- Arthur M., Courvalin P. Genetics and mechanisms of glycopeptide resistance in enterococci. Antimicrob Agents Chemother. 1993 Aug;37(8):1563–1571. doi: 10.1128/aac.37.8.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur M., Molinas C., Depardieu F., Courvalin P. Characterization of Tn1546, a Tn3-related transposon conferring glycopeptide resistance by synthesis of depsipeptide peptidoglycan precursors in Enterococcus faecium BM4147. J Bacteriol. 1993 Jan;175(1):117–127. doi: 10.1128/jb.175.1.117-127.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barna J. C., Williams D. H. The structure and mode of action of glycopeptide antibiotics of the vancomycin group. Annu Rev Microbiol. 1984;38:339–357. doi: 10.1146/annurev.mi.38.100184.002011. [DOI] [PubMed] [Google Scholar]
- Billot-Klein D., Gutmann L., Sablé S., Guittet E., van Heijenoort J. Modification of peptidoglycan precursors is a common feature of the low-level vancomycin-resistant VANB-type Enterococcus D366 and of the naturally glycopeptide-resistant species Lactobacillus casei, Pediococcus pentosaceus, Leuconostoc mesenteroides, and Enterococcus gallinarum. J Bacteriol. 1994 Apr;176(8):2398–2405. doi: 10.1128/jb.176.8.2398-2405.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugg T. D., Wright G. D., Dutka-Malen S., Arthur M., Courvalin P., Walsh C. T. Molecular basis for vancomycin resistance in Enterococcus faecium BM4147: biosynthesis of a depsipeptide peptidoglycan precursor by vancomycin resistance proteins VanH and VanA. Biochemistry. 1991 Oct 29;30(43):10408–10415. doi: 10.1021/bi00107a007. [DOI] [PubMed] [Google Scholar]
- Dutka-Malen S., Leclercq R., Coutant V., Duval J., Courvalin P. Phenotypic and genotypic heterogeneity of glycopeptide resistance determinants in gram-positive bacteria. Antimicrob Agents Chemother. 1990 Oct;34(10):1875–1879. doi: 10.1128/aac.34.10.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutka-Malen S., Molinas C., Arthur M., Courvalin P. Sequence of the vanC gene of Enterococcus gallinarum BM4174 encoding a D-alanine:D-alanine ligase-related protein necessary for vancomycin resistance. Gene. 1992 Mar 1;112(1):53–58. doi: 10.1016/0378-1119(92)90302-6. [DOI] [PubMed] [Google Scholar]
- Evers S., Reynolds P. E., Courvalin P. Sequence of the vanB and ddl genes encoding D-alanine:D-lactate and D-alanine:D-alanine ligases in vancomycin-resistant Enterococcus faecalis V583. Gene. 1994 Mar 11;140(1):97–102. doi: 10.1016/0378-1119(94)90737-4. [DOI] [PubMed] [Google Scholar]
- Gutmann L., Billot-Klein D., al-Obeid S., Klare I., Francoual S., Collatz E., van Heijenoort J. Inducible carboxypeptidase activity in vancomycin-resistant enterococci. Antimicrob Agents Chemother. 1992 Jan;36(1):77–80. doi: 10.1128/aac.36.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handwerger S., Pucci M. J., Volk K. J., Liu J., Lee M. S. The cytoplasmic peptidoglycan precursor of vancomycin-resistant Enterococcus faecalis terminates in lactate. J Bacteriol. 1992 Sep;174(18):5982–5984. doi: 10.1128/jb.174.18.5982-5984.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq R., Dutka-Malen S., Duval J., Courvalin P. Vancomycin resistance gene vanC is specific to Enterococcus gallinarum. Antimicrob Agents Chemother. 1992 Sep;36(9):2005–2008. doi: 10.1128/aac.36.9.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messer J., Reynolds P. E. Modified peptidoglycan precursors produced by glycopeptide-resistant enterococci. FEMS Microbiol Lett. 1992 Jul 1;73(1-2):195–200. doi: 10.1016/0378-1097(92)90608-q. [DOI] [PubMed] [Google Scholar]
- Nieto M., Perkins H. R. Modifications of the acyl-D-alanyl-D-alanine terminus affecting complex-formation with vancomycin. Biochem J. 1971 Aug;123(5):789–803. doi: 10.1042/bj1230789. [DOI] [PMC free article] [PubMed] [Google Scholar]
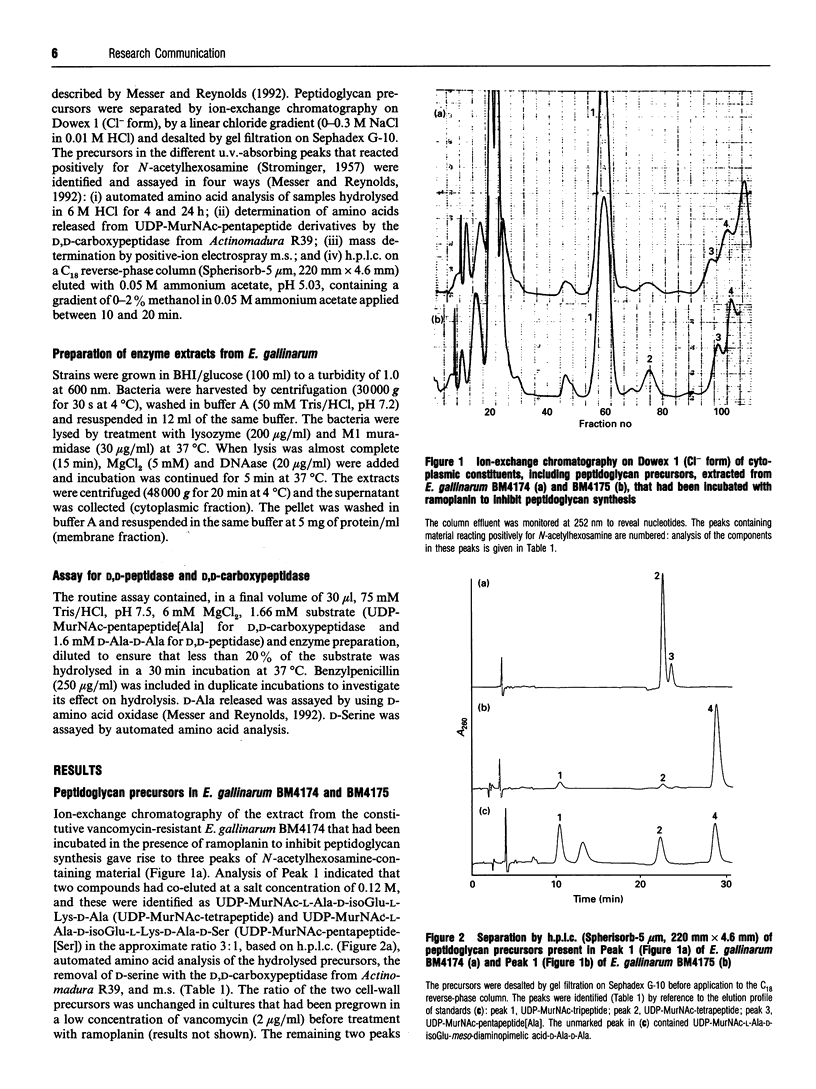
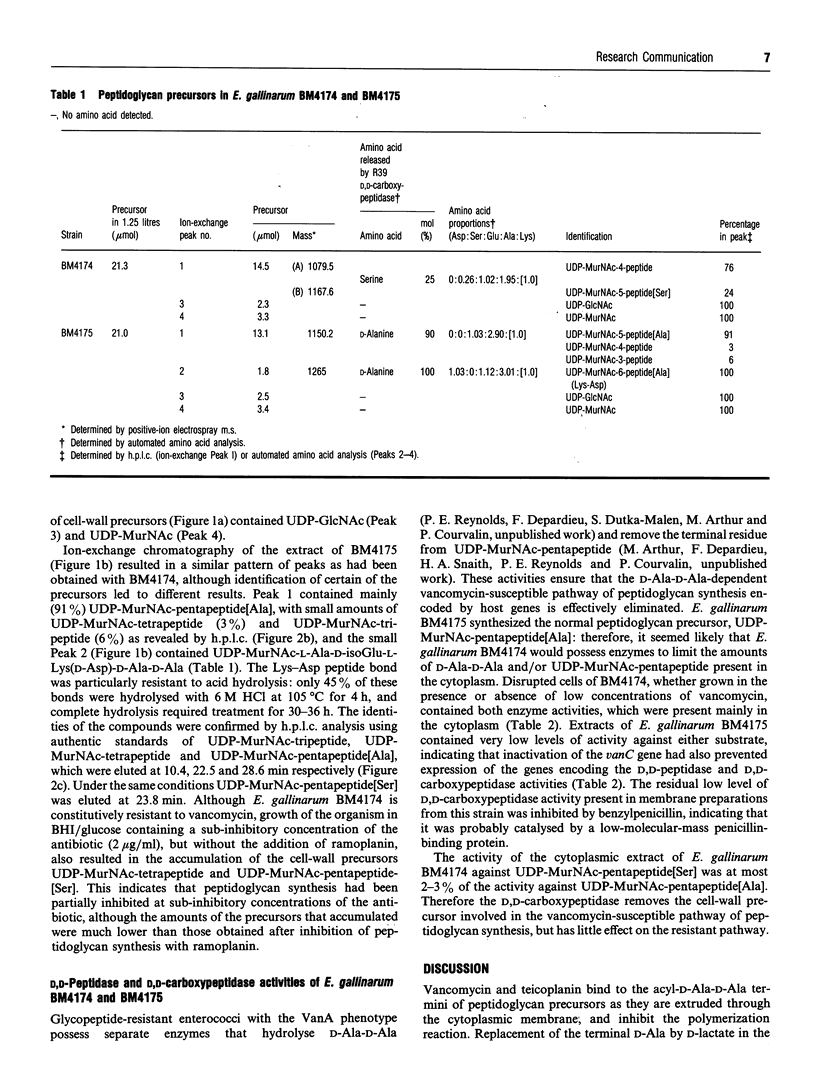
- STROMINGER J. L. Microbial uridine-5'-pyrophosphate N-acetylamino sugar compounds. I. Biology of the penicillin-induced accumulation. J Biol Chem. 1957 Jan;224(1):509–523. [PubMed] [Google Scholar]
- Vincent S., Knight R. G., Green M., Sahm D. F., Shlaes D. M. Vancomycin susceptibility and identification of motile enterococci. J Clin Microbiol. 1991 Oct;29(10):2335–2337. doi: 10.1128/jcm.29.10.2335-2337.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent S., Minkler P., Bincziewski B., Etter L., Shlaes D. M. Vancomycin resistance in Enterococcus gallinarum. Antimicrob Agents Chemother. 1992 Jul;36(7):1392–1399. doi: 10.1128/aac.36.7.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]


