Visual Abstract
Keywords: CKD, diabetes, diabetes mellitus, GFR, kidney
Abstract
Key Points
Shorter measured GFR protocols are accurate and precise compared with the reference standard measured GFR protocol in patients with preserved GFR.
These shorter protocols can potentially improve the adoption of GFR measurement more widely by reducing procedural time and cost.
Background
Measured GFR (mGFR) using exogenous tracers is recommended in a number of settings. Plasma one-compartment multisample protocols (MSPs) are the most commonly used, with iohexol being the dominant tracer. The accuracy of MSPs has mostly been evaluated in the setting of reduced GFR where delayed initial and final samples are recommended. Much less is known about MSPs when GFR is not decreased, and the default protocol tends to include initial sampling at 120 minutes and final sampling at 240 minutes after iohexol injection. The recent Kidney Disease Improving Global Outcomes 2024 Clinical Practice Guideline for the Evaluation and Management of CKD includes research recommendations for the development of shorter more efficient mGFR protocols. The objective of this study was to assess the performance of shorter MSPs with earlier initial (60 and 90 minutes) and final (150, 180, and 210 minutes) sampling times in individuals with preserved GFR. Reference mGFR (R-mGFR) was calculated using five samples collected between 120 and 240 minutes.
Methods
Four different combinations of shorter sampling strategies were investigated. Performance was evaluated using measurements of bias, precision, and accuracy (P2, P5, and mean absolute error).
Results
The mean R-mGFR of the 43 participants was 102.3±13.7 ml/min per 1.73 m2. All shorter mGFRs had biases <1 ml/min per 1.73 m2 and mean absolute error <1.6 ml/min per 1.73 m2. All shorter mGFRs were within 5% of the R-mGFR, and the majority were within 2%.
Conclusions
These results demonstrate that shortening the mGFR procedure in individuals with preserved GFR provides similar results to the current standard while significantly decreasing procedure time.
Introduction
The GFR is routinely used as a measure of kidney function.1 GFR can be eGFR or measured GFR (mGFR).1 eGFRs are calculated using equations that incorporate endogenous marker concentration(s) along with various combinations of patient-specific characteristics.2,3 Although convenient, eGFR measurements are known to be inaccurate at the individual patient level.4,5 This inaccuracy is particularly pronounced in those with better preserved GFR.6–9 This should be taken into consideration in research and clinical care settings such as medication dosing, live kidney donor eligibility assessment, hyperfiltration identification, CKD diagnosis, and kidney function outcome selection in certain clinical trials.10,11
mGFR is the gold standard for assessing GFR because it directly measures the clearance of an infused exogenous tracer.12 Iohexol has emerged in recent years as the dominant tracer owing to its widespread availability, lack of radiation exposure, and low cost.13 Several mGFR protocols are used, including urinary clearance and plasma clearance methodologies. One-compartment plasma clearance multisample protocols (MSPs) are used most frequently and have been validated against urinary inulin clearance.14 In one-compartment MSPs, initial and final sampling times depend on the expected GFR because the filtration rate determines (1) when the elimination phase of the tracer is reached and hence when sampling can start and (2) when tracer concentrations become sufficiently low that analytical imprecision affects GFR results. In those with significantly reduced GFR (<30 ml/min per 1.73 m2), later initial (4 hours postinjection) and final (12 hours postinjection) MSP have been shown to be the most accurate, whereas shorter initial (2 hours postinjection) and final (4 hours postinjection) MSPs are more accurate in patients with preserved GFR.14
Few one-compartment MSP studies have evaluated sampling strategies in patients with GFRs >60 ml/min per 1.73 m2, and none have initiated sampling before 2 hours.14–17 However, the limited available evidence suggests that individuals with preserved GFRs reach the terminal elimination phase sooner than 2 hours. Hence, earlier and shorter sampling strategies may be possible.18–20 Shortening the protocol would improve feasibility and reduce procedural costs, which are barriers to more widespread implementation of mGFR testing,2,3,21 but the impact on accuracy is unknown. The recently published Kidney Disease Improving Global Outcomes 2024 Clinical Practice Guideline for the Evaluation and Management of CKD research recommendations include the development of more efficient and shorter GFR measurement protocols.1
Accordingly, this study aimed to assess the performance of shorter one-compartment MSP mGFR protocols using earlier initial and final sampling times than those currently used in individuals with preserved GFR.
Methods
Study Participants and Design
Participants were recruited from a 4-year prospective, observational study evaluating the cardiovascular, renal, and bone health profile of young adults with type 1 diabetes.22,23 The mGFR procedure was undertaken at three time points (baseline, midpoint, and end of study) in a subset of participants. The study was approved by the SickKids Research Ethics Board (File Number 100055749), and participants provided informed consent in accordance with the Declaration of Helsinki. The end-of-study mGFR visit (year 2022–2023) was used for this analysis.
mGFR Testing
Preprocedural Requirements
Participants were instructed to adhere to sodium replete (2–4 mmol/kg per day) and protein diet (1.0–1.5 g/kg real body weight/day) 7 days before mGFR and abstain from alcohol and tobacco consumption 72 hours before the test. Caffeine and nonsteroidal anti-inflammatory medications were abstained for 48 hours, and exercise was avoided for 24 hours before the test. Participants were fasted on arrival, consuming only water starting at 10 pm the night before the procedure. The nighttime insulin was adjusted to target a blood sugar of around 10 mmol/L at 8:00 am.
Test Procedure
A detailed description of the procedure has been previously published.6 In brief, after 2 hours of glucose stabilization (range 7–13 mmol/L), an iohexol reference (blank) blood sample was drawn, and a 5 ml bolus of iohexol (OMNIPAQUE, GE Health Care) was subsequently administered over a 2-minute period. Blood samples were drawn from the contralateral arm to iohexol injection at 60, 90, 120, 150, 180, 210, and 240 minutes after iohexol injection.
Iohexol Clearance Analysis
After each iohexol blood collection, the sample was spun by centrifuge (Allegra X-30R Centrifuge) at 2500 rpm for 10 minutes, and the serum was extracted and frozen at −80°C. Iohexol was measured using liquid chromatography-tandem mass spectrometry at Queen's University (Kingston, Canada) using a modification of the method of Annesley and Clayton, reported in previous clinical and preclinical studies.14,24,25 Briefly, 1 μg of iohexol-d5 internal standard (Toronto Research Chemicals) was added to 20 μl of serum sample (or 11-point calibrator, 0.9–860 μg/ml), and the solution was diluted with 430 μl water. Protein precipitation was facilitated by addition of 100 μl of 0.2 M zinc sulfate and 450 μl methanol with vortex mixing after each addition. After centrifugation, 30 μl of supernatant was combined with 970 μl of 10% (v/v) methanol. Ten-microliter aliquots were analyzed by liquid chromatography-tandem mass spectrometry using an ACQUITY/Xevo TQ-S system (Waters) equipped with a HSS-T3 column (1.7 μm; 2.1×100 μm; Waters) held at 40°C. Chromatography was facilitated by a linear gradient elution of 5% to 80% mobile phase B (methanol containing 0.1%[v/v] glacial acetic acid) in mobile phase A (water, containing 0.1%[v/v] glacial acetic acid) over 3 minutes at a flow rate of 400 μl/min. Mass transitions used for quantification included m/z 822.1>804.1 and 827.1>809.1 for iohexol and iohexol-d5, respectively. Reproducibility and accuracy of iohexol measurements was based on analysis of serum samples distributed by an iohexol external quality assurance program (EQUALIS, Uppsala, Sweden), which served as quality control samples analyzed during each assay. Average iohexol concentrations for EQUALIS samples were within 3.2% of the all-laboratory mean (range: −1 to +7.8%). Interassay coefficients of variation were <10%.
GFR Calculations
MSP GFRs were calculated using a one-compartment slope intercept method.13 The GFR was corrected for the missing fast phase using the Brochner-Mortensen equation,26 followed by body surface area indexing using the Dubois and Dubois equation.27 The reference mGFR (R-mGFR) was calculated using iohexol measurements from blood samples drawn at 120, 150, 180, 210, and 240 minutes (120–240 minutes). The 129- to 240-minute time frame has been previously validated in adults (≥18 years) with preserved eGFR (≥60 ml/min per 1.73 m2).14 Four different combinations of sampling points were used for calculating shorter mGFRs (S-mGFR): the first four samples (60–150 minutes, F4-mGFR); middle four samples (90–180 minutes, M4-mGFR); first five samples (60–180 minutes, F5-mGFR); and middle five samples (90–210 minutes, M5-mGFR). The coefficient of determination (R2) of each mGFR regression was reviewed, and those with R2<0.975 had their regression curves reviewed to identify obvious outliers, which were eliminated as recommended.20 Because the study aim was to assess the accuracy of the earlier and shorter protocols, no data points with higher-than-expected iohexol concentrations based on the regression curves reviewed were eliminated from either the 60-minute or 90-minute time points in the main analysis. High 60-minute time points were excluded in a secondary analysis to assess their impact on the S-mGFR calculations. MSP protocols with three or fewer time points were not evaluated because they have been shown to be more biased and less precise and do not allow for R2 determination if an outlier is excluded.14 GFR was estimated using the CKD in children-1 equation,28 which we have previously shown to be the most accurate eGFR equation in this cohort.6
Statistical Analysis
The baseline characteristics of the patients are presented as mean±SD, counts, and percentages. Bias was calculated by subtracting R-mGFR from the S-mGFRs, with a negative bias indicating R-mGFR underestimation. Precision, a measure of the dispersion of the individual biases around the median bias, was defined as the interquartile range. Accuracy, which reflects both bias and precision, was assessed as the percentage of study participants with S-mGFR values within 5% (P5) and 2% (P2) of the R-mGFR and as the mean absolute error (MAE). Statistical comparisons between S-mGFR MAE values were made via the Wilcoxon rank-sum test. MAE was selected as the primary outcome measure because it incorporates both bias and precision, is more sensitive than the P5 or P2, and is less affected by outliers than root mean square error. Confidence intervals for MAE, median bias, P2, and precision were obtained by identifying the 2.5th and 97.5th percentiles based on 100,000 bootstrap samples. Bland-Altman plots were created to visualize the mean difference between R-mGFR and S-mGFRs values and the limits of agreement (bias±1.96 SD).29 Regression plots were created to visualize the association between body mass index (BMI) and bias for the early sampling protocols, and Pearson correlation coefficients were calculated.
Results
The demographic and clinical characteristics of the 43 participants are shown in Table 1. The mean age was 23.9±1.9 years, the mean BMI was 27.7±6.8 kg/m2, and 51% were female sex.
Table 1.
Patient characteristics
| Characteristics | Value |
|---|---|
| Age, yr | 23.9±1.9 |
| Female sex, n (%) | 22 (51) |
| BMI, kg/m2 | 27.7±6.8 |
| BSA, m2 | 2.00±0.27 |
| Diabetes duration, yr | 16.0±3.0 |
| HbA1c, % | 7.7±1.2 |
| Creatinine, mg/dl | 0.74±0.14 |
| Urine ACR (median [Q1–Q3]) (mg/g) | 8.0 (4.5–12.4) |
| eGFRCKiD-1, ml/min per 1.73 m2 | 95.5±15.9 |
Values are shown as mean±SD unless otherwise indicated. ACR, albumin to creatinine ratio; BMI, body mass index; BSA, body surface area; eGFRCKiD-1, eGFR CKD in children; HbA1c, hemoglobin A1c.
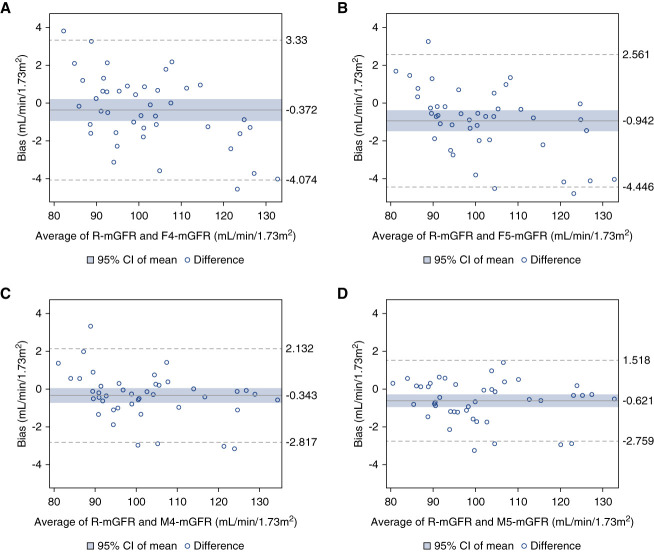
Nine single iohexol concentrations beyond 90 minutes were eliminated from the entire dataset as obvious outliers. The mean R-mGFR in this cohort was 102.3±13.7 (range: 80.4–134.7) ml/min per 1.73 m2. The performance of the four S-mGFRs is presented in Table 2. The median bias was <1 ml/min per 1.73 m2 for all S-mGFRs. Precision was similar and high among all S-mGFRs, with the lowest interquartile range in the M4-mGFR. The M4-mGFR MAE (0.9 ml/min per 1.73 m2) was significantly lower than the F4-mGFR (P < 0.001) and F5S-mGFR (P < 0.001) but was not significantly different when compared with M5-mGFR MAE (P = 0.20). More than 85% of the M4-mGFR and M5-mGFR protocols had less than a 2% difference from the R-mGFR (Table 2). Bland-Altman plots assessing S-mGFRs compared with R-mGFR are shown in Figure 1. There was no apparent trend in increasing bias by mGFR (Figures 1 and 2). There was no association between early sampling protocol biases and BMI (P values for all correlation coefficients >0.4) (Figure 3).
Table 2.
Performance of different measured GFR sampling combinations compared with reference mGFR calculation
| Performance Measure | R-mGFR (120–240 min) | F4-mGFR (60–150 min) | F5-mGFR (60–180 min) | M4-mGFR (90–180 min) | M5-mGFR (90–210 min) |
|---|---|---|---|---|---|
| Mean mGFR±SD, ml/min per 1.73 m2 | 102.3±13.7 | 101.9±12.7 | 101.3±12.7 | 101.9±13.2 | 101.6±13.5 |
| Median bias (95% CI), ml/min per 1.73 m2 | — | −0.4 (−1.1 to 0.6) | −0.7 (−1.2 to −0.3) | −0.4 (−0.5 to −0.1) | −0.5 (−0.8 to −0.1) |
| Precision: IQR of bias (95% CI), ml/min per 1.73 m2 | — | 2.4 (1.6 to 3.1) | 1.9 (0.9 to 3.1) | 1.2 (0.5 to 1.6) | 1.4 (0.9 to 1.9) |
| MAE (95% CI), ml/min per 1.73 m2 | — | 1.5a (1.2 to 1.9) | 1.5a (1.2 to 1.9) | 0.9 (0.8 to 1.2) | 0.9b (0.7 to 1.2) |
| P5 (%) | — | 100 | 100 | 100 | 100 |
| P2 (%) (95% CI) | — | 74 (61 to 86) | 74 (61 to 86) | 86 (74 to 95) | 88 (79 to 98) |
Bias is defined as median (95% confidence interval) difference between the reference measured GFR and sampling combination. Precision is defined as the interquartile range (95% confidence interval) of the bias. Mean absolute error is defined as the absolute mean (95% confidence interval) difference between the reference mGFR and sampling combination. P5/P2 are the percentage of shorter measured GFRs within 5% and 2% of the reference measured GFR. CI, confidence interval; IQR, interquartile range; MAE, mean absolute error; mGFR, measured GFR; P5, percentage within 5%; R-mGFR, reference mGFR.
P < 0.001 compared with M4-mGFR.
P = 0.203 compared with M4-mGFR.
Figure 1.
Bland Altman plots of agreement between S-mGFRs and R-mGFR. (A) R-mGFR and F4-mGFR, (B) R-mGFR and F5-mGFR, (C) R-mGFR and M4-mGFR, and (D) R-mGFR and M5-mGFR. The solid blue line represents the mean difference between S-mGFRs and R-mGFR, the blue shaded area is the 95% CI of the mean bias, and the hatched lines are two SDs of the data away from the mean. CI, confidence interval; mGFR, measured GFR; R-mGFR, reference mGFR; S-mGFR, shorter mGFR.
Figure 2.
Bivariate linear regression plots between R-mGFR and S-mGFR. (A) R-mGFR versus F4-mGFR, (B) R-mGFR versus F5-mGFR, (C) R-mGFR versus M4-mGFR, and (D) R-mGFR versus M5-mGFR. The solid blue line is the line of identity.
Figure 3.
Bivariate linear regression plots between S-mGFR bias and BMI with Pearson correlation coefficients (ρ). (A) R-mGFR versus F4-mGFR, (B) R-mGFR versus F5-mGFR, (C) R-mGFR versus M4-mGFR, and (D) R-mGFR versus M5-mGFR. The solid blue line is the regression, and the shaded blue area is the 95% CI. BMI, body mass index.
For the four individuals in whom the 60-minute time point subjectively appeared high, biases were recalculated with the 60-minute time point removed (Table 3). There was some small improvement in bias in some but not all measures. These four individuals had BMIs in the healthy weight (two) and overweight (two) categories.
Table 3.
Performance measures pre-elimination and postelimination of the 60-minute sample in four patients
| Patient | R-mGFR (120–240 min) ml/min per 1.73 m2 | BMI (kg/m2) | 60-min Sample Elimination | Bias F4-mGFR (60–150 min) ml/min per 1.73 m2 | Bias F5-mGFR (60–180 min) ml/min per 1.73 m2 |
|---|---|---|---|---|---|
| A | 83.7 | 22.01 | Pre | 2.1 | 1.5 |
| Post | −0.7 | 0.6 | |||
| B | 86.0 | 28.16 | Pre | −0.2 | 0.8 |
| Post | −3.3 | 0.5 | |||
| C | 134.7 | 27.13 | Pre | −4.0 | −4.0 |
| Post | −0.6 | −0.6 | |||
| D | 86.2 | 21.48 | Pre | 1.2 | 0.3 |
| Post | 0.5 | 2.0 |
Bias is the difference between the reference measured GFR and the shorter measured GFRs (F4 and F5-mGFR). A negative bias indicates if the shorter protocols underestimate the reference measured GFR. BMI, body mass index; mGFR, measured GFR; R-mGFR, reference mGFR.
Discussion
The goal of this study was to evaluate the performance of MSP mGFR protocols using earlier initial and final blood samples (S-mGFR) than the conventionally used 120- to 240-minute R-mGFR in individuals with preserved GFR. All S-mGFRs performed well, with mean biases <1 ml/min per 1.73 m2 and MAEs < 1.6 ml/min per 1.73 m2. All S-mGFRs were within 5% of the R-mGFR, and the majority were within 2%.
The M4-mGFR and M5-mGFR calculations had the lowest bias, greatest precision, and highest accuracy among the S-mGFRs. However, it is unclear whether the very slight improved performance of the M4-mGFR and M5-mGFR over the even shorter F4-mGFR protocol is clinically meaningful. The benefits from a cost and convenience perspective of the shorter early initial/final sample F4-mGFR protocol may reasonably be considered to outweigh the slightly improved performance of the longer protocols. Indeed, the F4-mGFR had a procedure time of 150 minutes, a 90-minute reduction compared with the R-mGFR, while still maintaining a very high degree of accuracy (MAE 1.5 ml/min per 1.73 m2, P2 74%). Furthermore, the calculation of the R2 with visual inspection of the regression curves allows for the identification of seemingly high early values, which can be eliminated from the GFR calculation if appropriate.
An important yet theoretical concern of initiating sampling before 120 minutes is that patients may not have reached the elimination phase when full equilibration of the tracer is complete and decreasing plasma levels do not solely reflect renal elimination, which is a prerequisite for using one-compartment mGFR protocols.13 While this is the case in patients with low GFR,14,30 it has not been established in those with higher GFRs. The limited available evidence suggests instead that the elimination phase may be attained earlier than 120 minutes.18–20 The M4-mGFR and M5-mGFR did do slightly better than the F4/F5-mGFRs which may be due to the latters' sampling during the transition between distribution and equilibration phases at 60 minutes. Visual inspection of the regression curves identified higher than expected 60-minute data points in only four patients. This may result from some persistence of the distribution phase but could also be due in part to preanalytic variation or analytic imprecision. When these data points were removed in the secondary analysis, patient bias improved in most (but not all) S-mGFR calculations (Table 3). This improvement, however, was minute (at most 3 ml/min per 1.73 m2 or 3%), indicating that the impact of potentially capturing the later end of the distribution phase on GFR calculation is infrequent and negligible. Although the volume of iohexol distribution may be higher in the setting of obesity, resulting in delayed attainment of the terminal elimination phase, the bias of the early protocols was not significantly associated with BMI. Furthermore, none of the four individuals whose visual inspection of the regression curves revealed a higher than anticipated 60-minute iohexol concentration were either obese or very obese.
This study had several limitations. First, there is no urinary clearance reference standard; therefore, we cannot comment on the accuracy of the protocols relative to a gold standard urinary clearance mGFR. Such urinary clearance comparator studies are lacking in patients with preserved GFR and are required to further refine and validate plasma clearance protocols. Second, the patient population is small and conducted in young people with type 1 diabetes, and confirmatory studies in larger populations in other clinical settings (nondiabetics, varied BMIs, etc.) are required. Third, the study included patients with both well-preserved mGFRs and eGFRs. It is well recognized that the eGFR is imprecise at high levels, which could cause concerns using the eGFR for protocol selection. Performance studies of abridged protocols are warranted in patients with eGFRs between 60 and 90 ml/min per 1.73 m2 where there is a paucity of data assessing sampling protocol accuracy. Third, we did not provide a formal economic analysis because this is beyond the scope of the current analysis and would likely vary from site to site depending on infrastructure and personnel costs. It is, however, reasonable to assume the shortened protocols would incur significant cost savings and improve efficiency, allowing for greater throughput.
MGFR has been recommended over eGFR in a variety of settings, such as kidney donor assessment, drug dosing, instances where creatinine is inaccurate, and in certain clinical trials where a single time point GFR is the end point or where eGFR may be biased by the intervention.1,9,11 Despite this, mGFR remains infrequently performed in most jurisdictions. The recent Kidney Disease Improving Global Outcomes CKD guidelines specifically highlight the need to develop and validate more efficient mGFR protocols.1 The results of this study indicate that shortening the mGFR procedure in patients with preserved GFRs by collecting earlier initial and final blood samples provides very similar results to the current standard while decreasing procedural length. The cost savings and improved feasibility of these shortened protocols should reduce some of the barriers to more widespread adoption of GFR measurement.
Acknowledgments
The authors want to acknowledge Dr. James Scholey, Department of Physiology, Faculty of Medicine, University of Toronto and Dr. Farid Mahmud, Department of Pediatrics, Division of Endocrinology and Research Institute, Sick Kids for securing funding for this project. We would also like to acknowledge the participants with type 1 diabetes for their support and participation in this study, as well as Martin Kauffman from the Department of Biomedical and Molecular Sciences at Queen's University in Kingston, Ontario, Canada for his contributions toward the measurement and reporting of biochemical investigations for this study.
Footnotes
C.A.W. and K.G.-S. contributed equally to this work and are considered co-first authors.
Disclosures
Disclosure forms, as provided by each author, are available with the online version of the article at http://links.lww.com/KN9/A574.
Funding
This work was supported by Juvenile Diabetes Research Foundation Canada (Canadians Seeking Solutions and Innovations to Overcome CKD [Can-SOLVE CKD] Network) and Canadian Institute of Health Research-Strategies for Patient Oriented Research (Canadians Seeking Solutions and Innovations to Overcome CKD [Can-SOLVE CKD] Network).
Author Contributions
Conceptualization: Kaveh Gaynor-Sodeifi, Etienne Sochett, Christine A. White.
Data curation: Kaveh Gaynor-Sodeifi, Patrick A. Norman, Etienne Sochett, Christine A. White.
Formal analysis: Kaveh Gaynor-Sodeifi, Patrick A. Norman, Christine A. White.
Funding acquisition: Etienne Sochett, Christine A. White.
Investigation: Michelle Furman, Kaveh Gaynor-Sodeifi, Etienne Sochett, Christine A. White.
Methodology: Kaveh Gaynor-Sodeifi, Patrick A. Norman, Etienne Sochett, Christine A. White.
Project administration: Michelle Furman, Christine A. White.
Resources: Michelle Furman, Kaveh Gaynor-Sodeifi, Christine A. White.
Software: Kaveh Gaynor-Sodeifi, Christine A. White.
Supervision: Etienne Sochett, Christine A. White.
Validation: Patrick A. Norman, Etienne Sochett, Christine A. White.
Visualization: Patrick A. Norman, Christine A. White.
Writing – original draft: Kaveh Gaynor-Sodeifi, Etienne Sochett, Christine A. White.
Writing – review & editing: Michelle Furman, Kaveh Gaynor-Sodeifi, Patrick A. Norman, Etienne Sochett, Christine A. White.
Data Sharing Statement
Data cannot be shared. Raw data are not publicly available because of restrictions by the University of Toronto's Research Ethics Board. Data that support this study's findings are available on request from the corresponding author (cw38@queensu.ca).
References
- 1.Kidney Disease Improving Global Outcomes (KDIGO) CKD Group. KDIGO 2024 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2024;105(4S):S117–S314. doi: 10.1016/j.kint.2023.10.018 [DOI] [PubMed] [Google Scholar]
- 2.Porrini E Ruggenenti P Luis-Lima S, et al. Estimated GFR: time for a critical appraisal. Nat Rev Nephrol. 2019;15(3):177–190. doi: 10.1038/s41581-018-0080-9 [DOI] [PubMed] [Google Scholar]
- 3.Levey AS, Coresh J, Tighiouart H, Greene T, Inker LA. Measured and estimated glomerular filtration rate: current status and future directions. Nat Rev Nephrol. 2020;16(1):51–64. doi: 10.1038/s41581-019-0191-y [DOI] [PubMed] [Google Scholar]
- 4.Scarr D Lovblom LE Bjornstad P, et al. Estimated glomerular filtration rate calculated by serum creatinine lacks precision and accuracy in adults with type 2 diabetes with preserved renal function. J Diabetes Complications. 2023;37(9):108562. doi: 10.1016/j.jdiacomp.2023.108562 [DOI] [PubMed] [Google Scholar]
- 5.Shafi T Zhu X Lirette ST, et al. Quantifying individual-level inaccuracy in glomerular filtration rate estimation: a cross-sectional study. Ann Intern Med. 2022;175(8):1073–1082. doi: 10.7326/M22-0610 [DOI] [PubMed] [Google Scholar]
- 6.Gaebe K White CA Mahmud FH, et al. Evaluation of novel glomerular filtration rate estimation equations in adolescents and young adults with type 1 diabetes. J Diabetes Complications. 2022;36(1):108081. doi: 10.1016/j.jdiacomp.2021.108081 [DOI] [PubMed] [Google Scholar]
- 7.Inker LA Eneanya ND Coresh J, et al. New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med. 2021;385(19):1737–1749. doi: 10.1056/NEJMoa2102953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaillard F Courbebaisse M Kamar N, et al. Impact of estimation versus direct measurement of predonation glomerular filtration rate on the eligibility of potential living kidney donors. Kidney Int. 2019;95(4):896–904. doi: 10.1016/j.kint.2018.11.029 [DOI] [PubMed] [Google Scholar]
- 9.Delanaye P Melsom T Ebert N, et al. Iohexol plasma clearance for measuring glomerular filtration rate in clinical practice and research: a review. Part 2: why to measure glomerular filtration rate with iohexol?. Clin Kidney J. 2016;9(5):700–704. doi: 10.1093/ckj/sfw071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ebert N Bevc S Bokenkamp A, et al. Assessment of kidney function: clinical indications for measured GFR. Clin Kidney J. 2021;14(8):1861–1870. doi: 10.1093/ckj/sfab042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White C, Siegal D, Akbari A, Knoll GA. Use of kidney function end points in kidney transplant trials: a systematic review. Am J Kidney Dis. 2010;56(6):1140–1157. doi: 10.1053/j.ajkd.2010.08.015 [DOI] [PubMed] [Google Scholar]
- 12.Schwartz GJ, Furth S, Cole SR, Warady B, Munoz A. Glomerular filtration rate via plasma iohexol disappearance: pilot study for chronic kidney disease in children. Kidney Int. 2006;69(11):2070–2077. doi: 10.1038/sj.ki.5000385 [DOI] [PubMed] [Google Scholar]
- 13.Delanaye P Ebert N Melsom T, et al. Iohexol plasma clearance for measuring glomerular filtration rate in clinical practice and research: a review. Part 1: how to measure glomerular filtration rate with iohexol? Clin Kidney J. 2016;9(5):682–699. doi: 10.1093/ckj/sfw070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White CA Akbari A Allen C, et al. Simultaneous glomerular filtration rate determination using inulin, iohexol, and (99m)Tc-DTPA demonstrates the need for customized measurement protocols. Kidney Int. 2021;99(4):957–966. doi: 10.1016/j.kint.2020.06.044 [DOI] [PubMed] [Google Scholar]
- 15.Sterner G, Frennby B, Mansson S, Nyman U, Van Westen D, Almen T. Determining 'true' glomerular filtration rate in healthy adults using infusion of inulin and comparing it with values obtained using other clearance techniques or prediction equations. Scand J Urol Nephrol. 2008;42(3):278–285. doi: 10.1080/00365590701701806 [DOI] [PubMed] [Google Scholar]
- 16.Delanaye P Vidal-Petiot E Stehle T, et al. Comparison of plasma clearance with early-compartment correction equations and urinary clearance in high GFR ranges. Kidney Int Rep. 2021;6(6):1622–1628. doi: 10.1016/j.ekir.2021.03.886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dubourg L Lemoine S Joannard B, et al. Comparison of iohexol plasma clearance formulas vs. inulin urinary clearance for measuring glomerular filtration rate. Clin Chem Lab Med. 2021;59(3):571–579. doi: 10.1515/cclm-2020-0770 [DOI] [PubMed] [Google Scholar]
- 18.Chantler C, Garnett ES, Parsons V, Veall N. Glomerular filtration rate measurement in man by the single injection methods using 51Cr-EDTA. Clin Sci. 1969;37(1):169–180. PMID: 4980763 [PubMed] [Google Scholar]
- 19.Jacobsson L. A method for the calculation of renal clearance based on a single plasma sample. Clin Physiol. 1983;3(4):297–305. doi: 10.1111/j.1475-097x.1983.tb00712.x [DOI] [PubMed] [Google Scholar]
- 20.Delanaye P Flamant M Dubourg L, et al. Single- versus multiple-sample method to measure glomerular filtration rate. Nephrol Dial Transplant. 2018;33(10):1778–1785. doi: 10.1093/ndt/gfx345 [DOI] [PubMed] [Google Scholar]
- 21.Tondel C Bolann B Salvador CL, et al. Iohexol plasma clearance in children: validation of multiple formulas and two-point sampling times. Pediatr Nephrol. 2017;32(2):311–320. doi: 10.1007/s00467-016-3436-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sochett EB Dominicis M Vali R, et al. Relationship between risk factors for impaired bone health and HR-pQCT in young adults with type 1 diabetes. Front Endocrinol (Lausanne). 2023;14:1144137. doi: 10.3389/fendo.2023.1144137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levin A Adams E Barrett BJ, et al. Canadians seeking solutions and Innovations to Overcome chronic kidney disease (Can-SOLVE CKD): form and function. Can J Kidney Health Dis. 2018;5:2054358117749530. doi: 10.1177/2054358117749530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Annesley TM, Clayton LT. Ultraperformance liquid chromatography-tandem mass spectrometry assay for iohexol in human serum. Clin Chem. 2009;55(6):1196–1202. doi: 10.1373/clinchem.2008.121533 [DOI] [PubMed] [Google Scholar]
- 25.Turner ME Laverty KJ Jeronimo PS, et al. Validation of a routine two-sample iohexol plasma clearance assessment of GFR and an evaluation of common endogenous markers in a rat model of CKD. Physiol Rep. 2017;5(9):e13205. doi: 10.14814/phy2.13205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bröchner-Mortensen J. A simple method for the determination of glomerular filtration rate. Scand J Clin Lab Invest. 1972;30(3):271–274. doi: 10.3109/00365517209084290 [DOI] [PubMed] [Google Scholar]
- 27.Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition. 1989;5(5):303–313. PMID: 2520314 [PubMed] [Google Scholar]
- 28.Pierce CB, Munoz A, Ng DK, Warady BA, Furth SL, Schwartz GJ. Age- and sex-dependent clinical equations to estimate glomerular filtration rates in children and young adults with chronic kidney disease. Kidney Int. 2021;99(4):948–956. doi: 10.1016/j.kint.2020.10.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Int J Nurs Stud. 2010;47(8):931–936. doi: 10.1016/j.ijnurstu.2009.10.001 [DOI] [PubMed] [Google Scholar]
- 30.Gaspari F Perico N Ruggenenti P, et al. Plasma clearance of nonradioactive iohexol as a measure of glomerular filtration rate. J Am Soc Nephrol. 1995;6(2):257–263. doi: 10.1681/ASN.V62257 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data cannot be shared. Raw data are not publicly available because of restrictions by the University of Toronto's Research Ethics Board. Data that support this study's findings are available on request from the corresponding author (cw38@queensu.ca).