Abstract
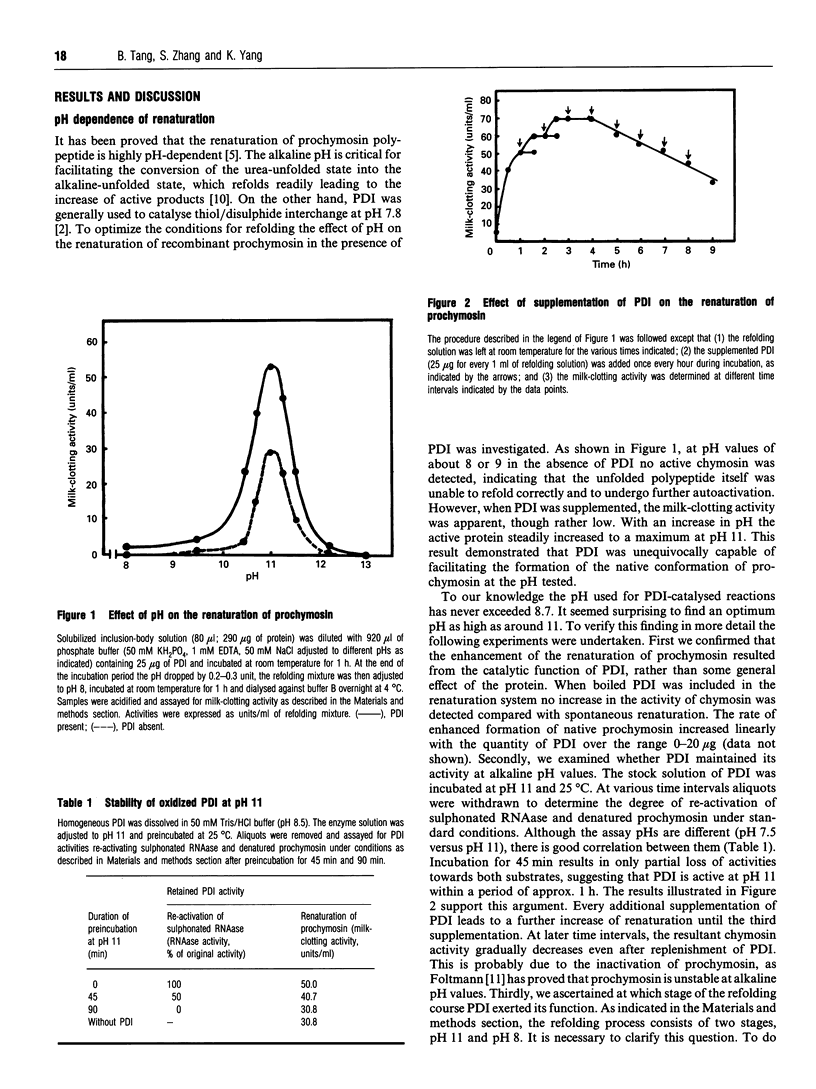
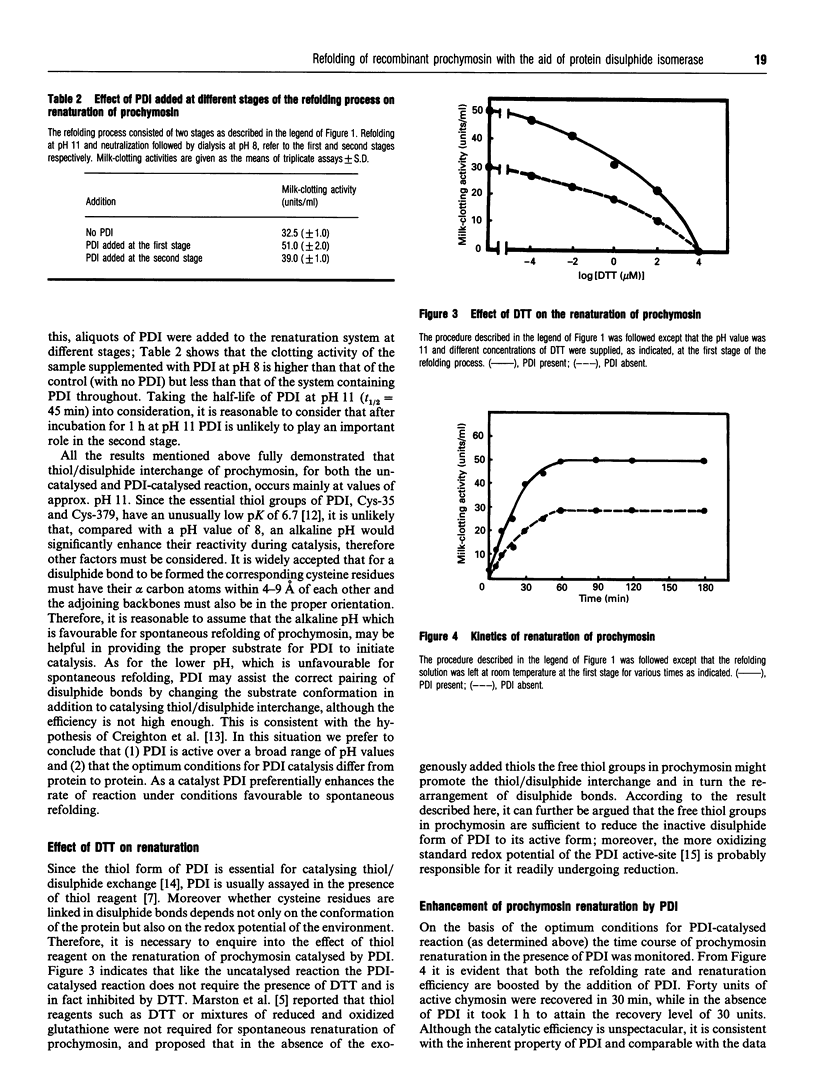
Protein disulphide isomerase (PDI) was shown to be able to accelerate the refolding of unfolded recombinant prochymosin and to enhance the overall yield of active protein. Unlike previous reports in this study PDI was found to be active at pH values as high as 11. The coincidence of the similar apparent optimum pH values of uncatalysed and PDI-catalysed reactions suggests that conditions favourable to spontaneous refolding of proteins may help PDI to catalyse thiol/disulphide interchange. Under the conditions described here no exogenously added dithiothreitol was required for PDI-catalysed renaturation, implying that the disulphide form of PDI was reduced to its active form by the free thiol groups in prochymosin molecules.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Creighton T. E., Hillson D. A., Freedman R. B. Catalysis by protein-disulphide isomerase of the unfolding and refolding of proteins with disulphide bonds. J Mol Biol. 1980 Sep 5;142(1):43–62. doi: 10.1016/0022-2836(80)90205-3. [DOI] [PubMed] [Google Scholar]
- Emtage J. S., Angal S., Doel M. T., Harris T. J., Jenkins B., Lilley G., Lowe P. A. Synthesis of calf prochymosin (prorennin) in Escherichia coli. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3671–3675. doi: 10.1073/pnas.80.12.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltmann B. A review on prorennin and rennin. C R Trav Lab Carlsberg. 1966;35(8):143–231. [PubMed] [Google Scholar]
- Freedman R. B., Brockway B. E., Lambert N. Protein disulphide-isomerase and the formation of native disulphide bonds. Biochem Soc Trans. 1984 Dec;12(6):929–932. doi: 10.1042/bst0120929. [DOI] [PubMed] [Google Scholar]
- Freedman R. B., Bulleid N. J., Hawkins H. C., Paver J. L. Role of protein disulphide-isomerase in the expression of native proteins. Biochem Soc Symp. 1989;55:167–192. [PubMed] [Google Scholar]
- Hawkins H. C., Blackburn E. C., Freedman R. B. Comparison of the activities of protein disulphide-isomerase and thioredoxin in catalysing disulphide isomerization in a protein substrate. Biochem J. 1991 Apr 15;275(Pt 2):349–353. doi: 10.1042/bj2750349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins H. C., Freedman R. B. The reactivities and ionization properties of the active-site dithiol groups of mammalian protein disulphide-isomerase. Biochem J. 1991 Apr 15;275(Pt 2):335–339. doi: 10.1042/bj2750335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins H. C., de Nardi M., Freedman R. B. Redox properties and cross-linking of the dithiol/disulphide active sites of mammalian protein disulphide-isomerase. Biochem J. 1991 Apr 15;275(Pt 2):341–348. doi: 10.1042/bj2750341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillson D. A., Lambert N., Freedman R. B. Formation and isomerization of disulfide bonds in proteins: protein disulfide-isomerase. Methods Enzymol. 1984;107:281–294. doi: 10.1016/0076-6879(84)07018-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marston F. A. The purification of eukaryotic polypeptides synthesized in Escherichia coli. Biochem J. 1986 Nov 15;240(1):1–12. doi: 10.1042/bj2400001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugrue R., Marston F. A., Lowe P. A., Freedman R. B. Denaturation studies on natural and recombinant bovine prochymosin (prorennin). Biochem J. 1990 Oct 15;271(2):541–547. doi: 10.1042/bj2710541. [DOI] [PMC free article] [PubMed] [Google Scholar]


