Abstract
The one- and two-electron enzymic reduction of the bioreductive alkylating agents 2-methylmethoxynaphthoquinone (quinone I) and 2-chloromethylnaphthoquinone (quinone II) was studied with purified NADPH-cytochrome P-450 reductase and DT-diaphorase respectively, and characterized in terms of kinetic constants, oxyradical production, thiol oxidation and DNA-strand-break formation. The catalytic-centre activity values indicated that DT-diaphorase catalysed the reduction of quinone I far more efficiently than NADPH-cytochrome P-450 reductase, although the Km values of the two enzymes for this quinone were similar (1.2-3.0 microM). The one-electron-transfer flavoenzyme also catalysed the reduction of quinone II, but the behaviour of DT-diaphorase towards this quinone did not permit calculation of kinetic constants. A salient feature of the redox transitions caused by the one- and two-electron catalysis of these quinones was the different contributions of disproportionation and autoxidation reactions respectively. In the former case, about 26% of NADPH consumed was accounted for in terms of autoxidation (as H2O2 formation), whereas in the latter, the autoxidation component accounted for most (98%) of the NADPH consumed. This difference was abrogated by superoxide dismutase, which enhanced autoxidation during NADPH-cytochrome P-450 catalysis to a maximal value. E.s.r. analysis indicated the formation of superoxide radicals, the signal of which was suppressed by superoxide dismutase and unaffected by catalase. The one- and two-electron reduction of these quinones in the presence of GSH was accompanied by formation of thiyl radicals. Although superoxide dismutase suppressed the thiol radical e.s.r. signal in both instances, the enzyme enhanced GSSG accumulation during NADPH-cytochrome P-450 catalysis of quinone I, whereas it inhibited GSSG formation during reduction of the quinone by DT-diaphorase. One- and two-electron reduction of quinone I led to calf thymus DNA-strand-break formation, a process that (a) was substantially decreased in experiments performed with dialysed DNA and in the presence of desferal and (b) was partially sensitive to superoxide dismutase and/or catalase. These findings are rationalized in terms of the occurrence of metal ions ligated to DNA, protecting against the toxic effects of superoxide radicals generated during enzymic reduction of quinones.
Full text
PDF
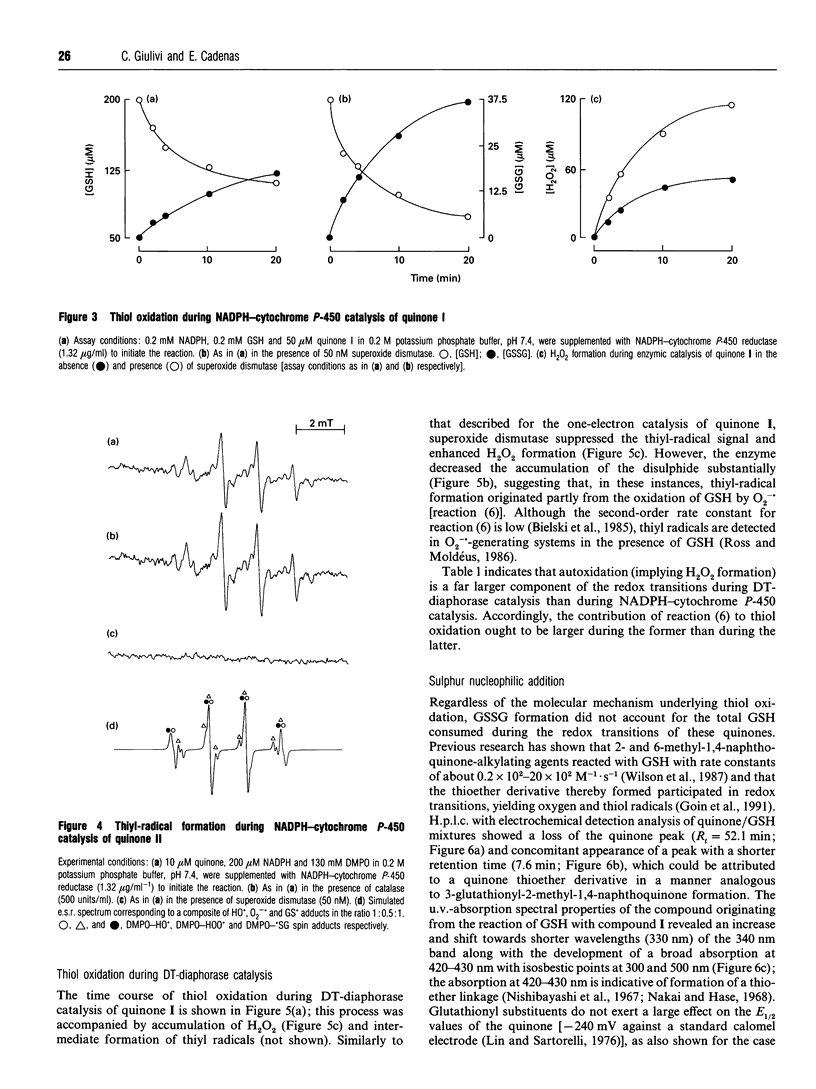
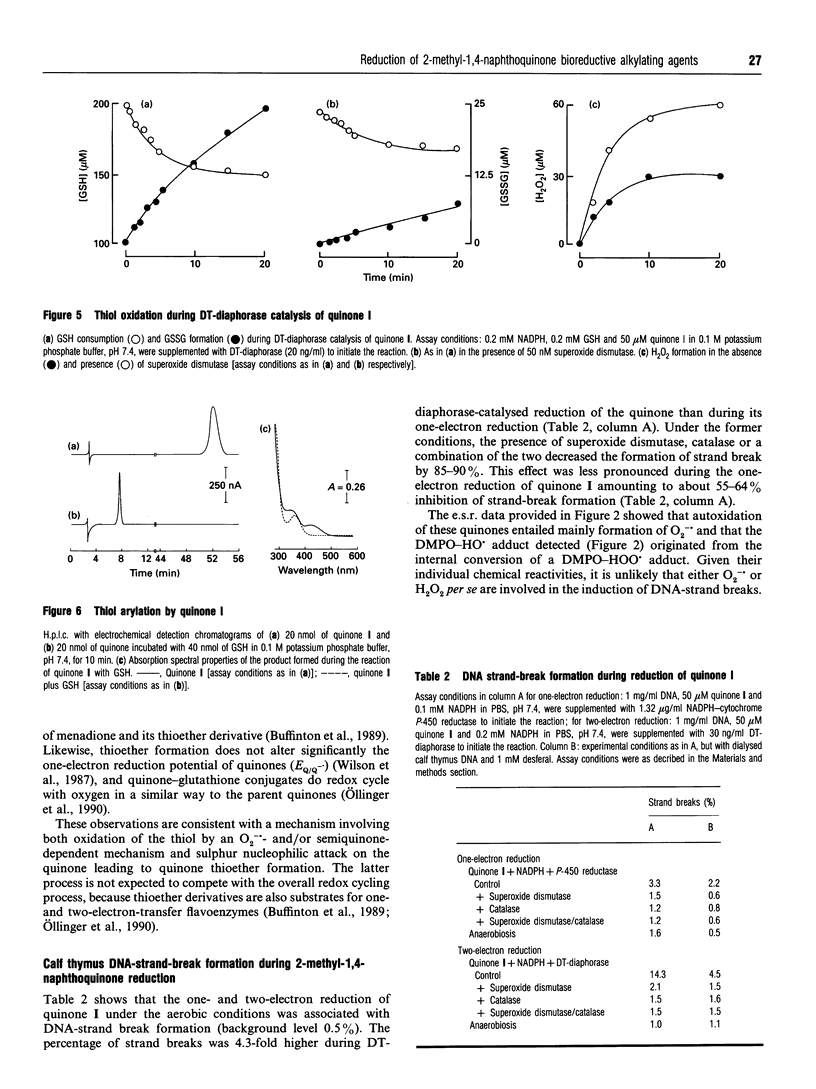



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonini I., Lin T. S., Cosby L. A., Dai Y. R., Sartorelli A. C. 2- and 6-methyl-1,4-naphthoquinone derivatives and potential bioreductive alkylating agents. J Med Chem. 1982 Jun;25(6):730–735. doi: 10.1021/jm00348a023. [DOI] [PubMed] [Google Scholar]
- Brunmark A., Cadenas E. Redox and addition chemistry of quinoid compounds and its biological implications. Free Radic Biol Med. 1989;7(4):435–477. doi: 10.1016/0891-5849(89)90126-3. [DOI] [PubMed] [Google Scholar]
- Brunmark A., Cadenas E. Reductive addition of glutathione to p-benzoquinone, 2-hydroxy-p-benzoquinone, and p-benzoquinone epoxides. Effect of the hydroxy- and glutathionyl substituents on p-benzohydroquinone autoxidation. Chem Biol Interact. 1988;68(3-4):273–298. doi: 10.1016/0009-2797(88)90021-x. [DOI] [PubMed] [Google Scholar]
- Buffinton G. D., Ollinger K., Brunmark A., Cadenas E. DT-diaphorase-catalysed reduction of 1,4-naphthoquinone derivatives and glutathionyl-quinone conjugates. Effect of substituents on autoxidation rates. Biochem J. 1989 Jan 15;257(2):561–571. doi: 10.1042/bj2570561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadenas E., Ernster L. Quinoid compounds: high-performance liquid chromatography with electrochemical detection. Methods Enzymol. 1990;186:180–196. doi: 10.1016/0076-6879(90)86108-8. [DOI] [PubMed] [Google Scholar]
- Chen H. H., Ma J. X., Forrest G. L., Deng P. S., Martino P. A., Lee T. D., Chen S. Expression of rat liver NAD(P)H:quinone-acceptor oxidoreductase in Escherichia coli and mutagenesis in vitro at Arg-177. Biochem J. 1992 Jun 15;284(Pt 3):855–860. doi: 10.1042/bj2840855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman J. B., Gilfor D., Farber J. L. Dissociation of the accumulation of single-strand breaks in DNA from the killing of cultured hepatocytes by an oxidative stress. Mol Pharmacol. 1989 Jul;36(1):193–200. [PubMed] [Google Scholar]
- Cresteil T., Jaiswal A. K. High levels of expression of the NAD(P)H:quinone oxidoreductase (NQO1) gene in tumor cells compared to normal cells of the same origin. Biochem Pharmacol. 1991 Aug 8;42(5):1021–1027. doi: 10.1016/0006-2952(91)90284-c. [DOI] [PubMed] [Google Scholar]
- Fariss M. W., Reed D. J. High-performance liquid chromatography of thiols and disulfides: dinitrophenol derivatives. Methods Enzymol. 1987;143:101–109. doi: 10.1016/0076-6879(87)43018-8. [DOI] [PubMed] [Google Scholar]
- Finkelstein E., Rosen G. M., Rauckman E. J. Spin trapping of superoxide and hydroxyl radical: practical aspects. Arch Biochem Biophys. 1980 Mar;200(1):1–16. doi: 10.1016/0003-9861(80)90323-9. [DOI] [PubMed] [Google Scholar]
- Floyd R. A. DNA-ferrous iron catalyzed hydroxyl free radical formation from hydrogen peroxide. Biochem Biophys Res Commun. 1981 Apr 30;99(4):1209–1215. doi: 10.1016/0006-291x(81)90748-8. [DOI] [PubMed] [Google Scholar]
- Gant T. W., Rao D. N., Mason R. P., Cohen G. M. Redox cycling and sulphydryl arylation; their relative importance in the mechanism of quinone cytotoxicity to isolated hepatocytes. Chem Biol Interact. 1988;65(2):157–173. doi: 10.1016/0009-2797(88)90052-x. [DOI] [PubMed] [Google Scholar]
- Goin J., Gibson D. D., McCay P. B., Cadenas E. Glutathionyl- and hydroxyl radical formation coupled to the redox transitions of 1,4-naphthoquinone bioreductive alkylating agents during glutathione two-electron reductive addition. Arch Biochem Biophys. 1991 Aug 1;288(2):386–396. doi: 10.1016/0003-9861(91)90211-z. [DOI] [PubMed] [Google Scholar]
- Goldberg I. H. Free radical mechanisms in neocarzinostatin-induced DNA damage. Free Radic Biol Med. 1987;3(1):41–54. doi: 10.1016/0891-5849(87)90038-4. [DOI] [PubMed] [Google Scholar]
- Goldstein S., Czapski G. The role and mechanism of metal ions and their complexes in enhancing damage in biological systems or in protecting these systems from the toxicity of O2-. J Free Radic Biol Med. 1986;2(1):3–11. doi: 10.1016/0748-5514(86)90117-0. [DOI] [PubMed] [Google Scholar]
- Graham D. G. Catecholamine toxicity: a proposal for the molecular pathogenesis of manganese neurotoxicity and Parkinson's disease. Neurotoxicology. 1984 Spring;5(1):83–95. [PubMed] [Google Scholar]
- Halliwell B. Oxidants and human disease: some new concepts. FASEB J. 1987 Nov;1(5):358–364. [PubMed] [Google Scholar]
- Halliwell B. Superoxide-dependent formation of hydroxyl radicals in the presence of iron salts is a feasible source of hydroxy radicals in vivo. Biochem J. 1982 Aug 1;205(2):461–463. doi: 10.1042/bj2050461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander P. M., Bartfai T., Gatt S. Studies on the reaction mechanism of DT diaphorase. Intermediary plateau and trough regions in the initial velocity vs substrate concentration curves. Arch Biochem Biophys. 1975 Aug;169(2):568–576. doi: 10.1016/0003-9861(75)90201-5. [DOI] [PubMed] [Google Scholar]
- Hollander P. M., Ernster L. Studies on the reaction mechanism of DT diaphorase. Action of dead-end inhibitors and effects of phospholipids. Arch Biochem Biophys. 1975 Aug;169(2):560–567. doi: 10.1016/0003-9861(75)90200-3. [DOI] [PubMed] [Google Scholar]
- Imlay J. A., Linn S. DNA damage and oxygen radical toxicity. Science. 1988 Jun 3;240(4857):1302–1309. doi: 10.1126/science.3287616. [DOI] [PubMed] [Google Scholar]
- Koppenol W. H. A thermodynamic appraisal of the radical sink hypothesis. Free Radic Biol Med. 1993 Jan;14(1):91–94. doi: 10.1016/0891-5849(93)90513-t. [DOI] [PubMed] [Google Scholar]
- Kyle M. E., Nakae D., Sakaida I., Miccadei S., Farber J. L. Endocytosis of superoxide dismutase is required in order for the enzyme to protect hepatocytes from the cytotoxicity of hydrogen peroxide. J Biol Chem. 1988 Mar 15;263(8):3784–3789. [PubMed] [Google Scholar]
- Lin A. J., Sartorelli A. C. Potential bioreductive alkylating agents-VI. Determination of the relationship between oxidation-reduction potential and antineoplastic activity. Biochem Pharmacol. 1976 Jan 15;25(2):206–207. doi: 10.1016/0006-2952(76)90293-8. [DOI] [PubMed] [Google Scholar]
- Lin A. J., Shansky C. W., Sartorelli A. C. Potential bioreductive alkylating agents. 3. Synthesis and antineoplastic activity of acetoxymethyl and corresponding ethyl carbamate derivatives of benzoquinones. J Med Chem. 1974 May;17(5):558–561. doi: 10.1021/jm00251a026. [DOI] [PubMed] [Google Scholar]
- Lind C., Cadenas E., Hochstein P., Ernster L. DT-diaphorase: purification, properties, and function. Methods Enzymol. 1990;186:287–301. doi: 10.1016/0076-6879(90)86122-c. [DOI] [PubMed] [Google Scholar]
- Lown J. W., Sim S. K., Majumdar K. C., Chang R. Y. Strand scission of DNA by bound adriamycin and daunorubicin in the presence of reducing agents. Biochem Biophys Res Commun. 1977 Jun 6;76(3):705–710. doi: 10.1016/0006-291x(77)91557-1. [DOI] [PubMed] [Google Scholar]
- Mason R. P., Rao D. N. Thiyl free radical metabolites of thiol drugs, glutathione, and proteins. Methods Enzymol. 1990;186:318–329. doi: 10.1016/0076-6879(90)86125-f. [DOI] [PubMed] [Google Scholar]
- Monks T. J., Hanzlik R. P., Cohen G. M., Ross D., Graham D. G. Quinone chemistry and toxicity. Toxicol Appl Pharmacol. 1992 Jan;112(1):2–16. doi: 10.1016/0041-008x(92)90273-u. [DOI] [PubMed] [Google Scholar]
- Monks T. J., Highet R. J., Lau S. S. 2-Bromo-(diglutathion-S-yl)hydroquinone nephrotoxicity: physiological, biochemical, and electrochemical determinants. Mol Pharmacol. 1988 Oct;34(4):492–500. [PubMed] [Google Scholar]
- Monks T. J., Highet R. J., Lau S. S. Oxidative cyclization, 1,4-benzothiazine formation and dimerization of 2-bromo-3-(glutathion-S-yl)hydroquinone. Mol Pharmacol. 1990 Jul;38(1):121–127. [PubMed] [Google Scholar]
- Morrison H., Di Monte D., Nordenskjöld M., Jernström B. Induction of cell damage by menadione and benzo(a)pyrene-3,6-quinone in cultures of adult rat hepatocytes and human fibroblasts. Toxicol Lett. 1985 Oct;28(1):37–47. doi: 10.1016/0378-4274(85)90007-4. [DOI] [PubMed] [Google Scholar]
- Nishibayashi H., Nakai N., Sato R. Specificity of naphthoquinones as cofactor for NADPH oxidation by liver microsomes. J Biochem. 1967 Aug;62(2):215–222. doi: 10.1093/oxfordjournals.jbchem.a128651. [DOI] [PubMed] [Google Scholar]
- O'Brien P. J. Molecular mechanisms of quinone cytotoxicity. Chem Biol Interact. 1991;80(1):1–41. doi: 10.1016/0009-2797(91)90029-7. [DOI] [PubMed] [Google Scholar]
- Ollinger K., Buffinton G. D., Ernster L., Cadenas E. Effect of superoxide dismutase on the autoxidation of substituted hydro- and semi-naphthoquinones. Chem Biol Interact. 1990;73(1):53–76. doi: 10.1016/0009-2797(90)90108-y. [DOI] [PubMed] [Google Scholar]
- Ollinger K., Llopis J., Cadenas E. Study of the redox properties of naphthazarin (5,8-dihydroxy-1,4-naphthoquinone) and its glutathionyl conjugate in biological reactions: one- and two-electron enzymatic reduction. Arch Biochem Biophys. 1989 Dec;275(2):514–530. doi: 10.1016/0003-9861(89)90398-6. [DOI] [PubMed] [Google Scholar]
- Ordoñez I. D., Cadenas E. Thiol oxidation coupled to DT-diaphorase-catalysed reduction of diaziquone. Reductive and oxidative pathways of diaziquone semiquinone modulated by glutathione and superoxide dismutase. Biochem J. 1992 Sep 1;286(Pt 2):481–490. doi: 10.1042/bj2860481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powis G. Free radical formation by antitumor quinones. Free Radic Biol Med. 1989;6(1):63–101. doi: 10.1016/0891-5849(89)90162-7. [DOI] [PubMed] [Google Scholar]
- Riley R. J., Workman P. DT-diaphorase and cancer chemotherapy. Biochem Pharmacol. 1992 Apr 15;43(8):1657–1669. doi: 10.1016/0006-2952(92)90694-e. [DOI] [PubMed] [Google Scholar]
- Ross D., Moldeus P. Thiyl radicals--their generation and further reactions. Adv Exp Med Biol. 1986;197:329–335. doi: 10.1007/978-1-4684-5134-4_30. [DOI] [PubMed] [Google Scholar]
- Siegel D., Beall H., Senekowitsch C., Kasai M., Arai H., Gibson N. W., Ross D. Bioreductive activation of mitomycin C by DT-diaphorase. Biochemistry. 1992 Sep 1;31(34):7879–7885. doi: 10.1021/bi00149a019. [DOI] [PubMed] [Google Scholar]
- Singer B., Kuśmierek J. T. Chemical mutagenesis. Annu Rev Biochem. 1982;51:655–693. doi: 10.1146/annurev.bi.51.070182.003255. [DOI] [PubMed] [Google Scholar]
- Thor H., Smith M. T., Hartzell P., Bellomo G., Jewell S. A., Orrenius S. The metabolism of menadione (2-methyl-1,4-naphthoquinone) by isolated hepatocytes. A study of the implications of oxidative stress in intact cells. J Biol Chem. 1982 Oct 25;257(20):12419–12425. [PubMed] [Google Scholar]
- Wardman P., Wilson I. Control of the generation and reactions of free radicals in biological systems by kinetic and thermodynamic factors. Free Radic Res Commun. 1987;2(4-6):225–232. doi: 10.3109/10715768709065287. [DOI] [PubMed] [Google Scholar]
- Wilson I., Wardman P., Lin T. S., Sartorelli A. C. One-electron reduction of 2- and 6-methyl-1,4-naphthoquinone bioreductive alkylating agents. J Med Chem. 1986 Aug;29(8):1381–1384. doi: 10.1021/jm00158a010. [DOI] [PubMed] [Google Scholar]
- Wilson I., Wardman P., Lin T. S., Sartorelli A. C. Reactivity of thiols towards derivatives of 2- and 6-methyl-1,4-naphthoquinone bioreductive alkylating agents. Chem Biol Interact. 1987 Mar;61(3):229–240. doi: 10.1016/0009-2797(87)90003-2. [DOI] [PubMed] [Google Scholar]
- Winterbourn C. C., Cowden W. B., Sutton H. C. Auto-oxidation of dialuric acid, divicine and isouramil. Superoxide dependent and independent mechanisms. Biochem Pharmacol. 1989 Feb 15;38(4):611–618. doi: 10.1016/0006-2952(89)90206-2. [DOI] [PubMed] [Google Scholar]
- Winterbourn C. C. Cytochrome c reduction by semiquinone radicals can be indirectly inhibited by superoxide dismutase. Arch Biochem Biophys. 1981 Jun;209(1):159–167. doi: 10.1016/0003-9861(81)90268-x. [DOI] [PubMed] [Google Scholar]
- Winterbourn C. C., Gutteridge J. M., Halliwell B. Doxorubicin-dependent lipid peroxidation at low partial pressures of O2. J Free Radic Biol Med. 1985;1(1):43–49. doi: 10.1016/0748-5514(85)90028-5. [DOI] [PubMed] [Google Scholar]
- Winterbourn C. C. Superoxide as an intracellular radical sink. Free Radic Biol Med. 1993 Jan;14(1):85–90. doi: 10.1016/0891-5849(93)90512-s. [DOI] [PubMed] [Google Scholar]
- van Ommen B., Ploemen J. H., Bogaards J. J., Monks T. J., Gau S. S., van Bladeren P. J. Irreversible inhibition of rat glutathione S-transferase 1-1 by quinones and their glutathione conjugates. Structure-activity relationship and mechanism. Biochem J. 1991 Jun 15;276(Pt 3):661–666. doi: 10.1042/bj2760661. [DOI] [PMC free article] [PubMed] [Google Scholar]


