Abstract
Objectives
To evaluate 1-year bimekizumab efficacy in PsA from the patient perspective using the 12-item PsA Impact of Disease (PsAID-12) questionnaire.
Methods
BE OPTIMAL (NCT03895203; biologic DMARD [bDMARD]-naïve), BE COMPLETE (NCT03896581; inadequate response/intolerance to TNF inhibitors [TNFi-IR]) and BE VITAL (NCT04009499; open-label extension) assessed bimekizumab 160 mg every 4 weeks in patients with PsA. Post hoc analyses of patient-reported disease impact, assessed by the PsAID-12 questionnaire, are reported to 1 year (collected to Week 40 in BE COMPLETE).
Results
Overall, 1,112 total patients were included (698 bimekizumab, 414 placebo). Rapid improvements observed with bimekizumab treatment at Week 4 continued to Week 16 and were sustained to 1 year. At 1 year, mean (SE) change from baseline in PsAID-12 total score was comparable between bimekizumab-randomized patients and patients who switched to bimekizumab at Week 16 (bDMARD-naïve bimekizumab –2.3 [0.1], placebo/bimekizumab –2.2 [0.1]; TNFi-IR bimekizumab –2.5 [0.1], placebo/bimekizumab –2.2 [0.2]). Proportions of bimekizumab-randomized patients achieving clinically meaningful within-patient improvement (≥3-point decrease from baseline) at Week 16 were sustained to 1 year (bDMARD-naïve 49.0%; TNFi-IR 48.5%) and were similar for placebo/bimekizumab patients (bDMARD-naïve 44.4%; TNFi-IR 40.6%). Across studies and arms, 35.3% to 47.8% of patients had minimal or no symptom impact at 1 year. Improvements were observed to 1 year across all single-item domains, including pain, fatigue and skin problems.
Conclusion
Bimekizumab treatment resulted in rapid and sustained clinically meaningful improvements in disease impact up to 1 year in bDMARD-naïve and TNFi-IR patients with PsA.
Trial registration
BE OPTIMAL: NCT03895203; BE COMPLETE: NCT03896581; BE VITAL: NCT04009499 (ClinicalTrials.gov)
Keywords: PsA, bimekizumab, PROM, health-related QoL, rheumatology, Severity of Illness Index
Graphical abstract
Rheumatology key messages.
Bimekizumab treatment resulted in rapid and sustained reductions in disease impact, assessed using the PsAID-12.
High percentages of patients achieved clinically meaningful levels of improvement with bimekizumab treatment.
One-year bimekizumab treatment improved PsA symptoms, resulting in sustained improvements in health-related quality of life.
Introduction
PsA is a chronic, inflammatory disease that manifests in a myriad of ways, including peripheral and axial joint disease, enthesitis, dactylitis and psoriatic skin disease [1–4]. The multi-faceted nature of PsA results in long-term health-related quality of life (HRQoL) detriment encompassing physical, social and emotional aspects of patients’ lives, especially when compared with both the general population and patients with other chronic diseases, such as rheumatoid arthritis [5–7]. Pain, fatigue and skin problems—including itchiness, redness and swelling—are particularly impactful symptoms. These symptoms reduce patients’ engagement in daily activities and, more generally, negatively impact social and emotional wellbeing [8–10].
The profound impact of PsA’s symptomatology on patients’ HRQoL, extending beyond physical symptoms, reinforces the importance of the formal evaluation of disease impact from the patient perspective in addition to assessment of clinical disease activity. This approach is recognized by the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA)-Outcome Measures in Rheumatology (OMERACT) working group through the inclusion of HRQoL in the PsA Core Domain Set [11]. To this end, GRAPPA-OMERACT has provisionally endorsed, for inclusion in the PsA core outcome measurements, the following: the HAQ-Disability Index (HAQ-DI) and the Physical Functioning subscale of the 36-item Short-Form Health Survey (SF-36 PF) to specifically assess the physical function domain, and the 12-item PsA Impact of Disease (PsAID-12) questionnaire to assess the multi-faceted impact of PsA on HRQoL [12, 13]. The PsAID-12 questionnaire was the first patient-reported outcome measure developed specifically for PsA with input from patients, and covers a broad spectrum of symptoms which are patient priorities [14]. Psychometric analyses have demonstrated that PsAID-12 scores are valid, reliable and responsive in patients with PsA [15].
The increasing availability of newer treatments for PsA provides an opportunity to improve our understanding of their long-term effects from the patient perspective, including impact on symptom reduction, physical function and overall disease impact. Doing so will be important for shared decision-making, allowing clinicians and patients to select the most appropriate treatment.
Bimekizumab is a monoclonal IgG1 antibody that selectively inhibits IL-17F in addition to IL-17A. The 52-week efficacy and tolerability of bimekizumab has been demonstrated in patients with active PsA who were biologic DMARD (bDMARD)-naïve, or who have had prior inadequate response or intolerance to TNF inhibitors (TNFi-IR) [16, 17]. Bimekizumab efficacy has also been demonstrated for these patient populations up to 1 year using HRQoL measures, including HAQ-DI and SF-36 [16, 17].
Here, we report on the 1-year bimekizumab efficacy from the patient perspective using the PsAID-12 questionnaire in bDMARD-naïve and TNFi-IR patients with active PsA.
Methods
Study design and participants
Full methodological details of the BE OPTIMAL (NCT03895203) and BE COMPLETE (NCT03896581) studies, and their open-label extension BE VITAL (NCT04009499), have been reported previously [18–20]. In brief, BE OPTIMAL and BE COMPLETE were two randomized, phase 3 multicentre studies, placebo-controlled to Week 16, that assessed bimekizumab in bDMARD-naïve and TNFi-IR patients with active PsA, respectively. Patients in BE OPTIMAL were randomized 3:2:1 to receive either subcutaneous bimekizumab 160 mg every 4 weeks (Q4W), placebo or reference (subcutaneous adalimumab 40 mg every 2 weeks [Q2W]). At Week 16, patients receiving placebo switched to receive bimekizumab (placebo/bimekizumab). Those initially randomized to receive bimekizumab or adalimumab continued their dosing until Week 52. The BE OPTIMAL study was not powered for statistical comparisons of adalimumab to bimekizumab or placebo; therefore, results should not be compared between the adalimumab treatment arm and the bimekizumab or placebo treatment arms. Patients in BE COMPLETE were randomized 2:1 to subcutaneous bimekizumab 160 mg Q4W, or placebo. Patients completing Week 52 of BE OPTIMAL or Week 16 of BE COMPLETE were eligible to enter BE VITAL, in which all patients received bimekizumab 160 mg Q4W, including those randomized to placebo in BE COMPLETE (placebo/bimekizumab). BE COMPLETE plus BE VITAL is referred to as ‘BE COMPLETE’ hereafter.
The study designs for the two phase 3 studies up to Week 52 are shown in Supplementary Fig. 1, available at Rheumatology online. Key inclusion and exclusion criteria have been reported previously. Briefly, patients had a documented diagnosis of adult-onset, active PsA for at least six months prior to study screening [18–20].
PsAID-12
The PsAID-12 questionnaire is a self-administered, PsA-specific, patient-reported outcome measure that assesses PsA impact during the week preceding completion. The PsAID-12 questionnaire was developed in 12 languages (English, Estonian, Flemish, French, German, Hungarian, Italian, Norwegian, Romanian, Russian, Spanish and Turkish) [21]. Additional translations needed for BE OPTIMAL and BE COMPLETE were generated using a similar translation process that followed the Translation and Cultural adaptation–Principles of Good Practice, as recommended by the International Society for Pharmacoeconomics and Outcomes Research [22].
The questionnaire includes 12 physical and psychological single-item domains that cover a broad spectrum of symptoms impacting HRQoL [21]. The 12 single-item domains are pain, fatigue, skin problems, work and/or leisure activities, functional capacity, discomfort, sleep disturbance, coping, anxiety, embarrassment and/or shame, social participation and depression. Each single-item domain is rated with a 0–10 numerical rating scale, and the total score, also on a 0–10 scale, is calculated by multiplying the single-item domain scores by a weighting factor and subsequently summing them; higher scores indicate worse status [21].
Clinically meaningful within-patient improvement (a decrease of ≥3 points from baseline in patients with baseline scores ≥3) and disease severity thresholds have been identified for total and most single-item domain scores [15].
In BE OPTIMAL, the PsAID-12 questionnaire was completed electronically at baseline and Weeks 4, 16, 24, 36 and 52. In BE COMPLETE, the PsAID-12 questionnaire was completed electronically at baseline and Weeks 4, 12, 16 and 40.
Statistical analysis
All outcomes reported in the current manuscript are presented side-by-side for BE OPTIMAL and BE COMPLETE; results are analysed descriptively for similarities and differences between the bimekizumab and placebo/bimekizumab groups, as well as between studies. Results for the adalimumab arm are presented in the Supplementary Appendix, available at Rheumatology online, along with results for the pooled population of bDMARD-naïve and TNFi-IR patients. Results for the pooled population are provided up to Week 16, after which assessment time points and study designs differed, preventing pooling of data.
Results from planned analyses of mean change from baseline (CfB) in PsAID-12 total and single-item domain scores are provided up to Week 52 for BE OPTIMAL and up to Week 40 for BE COMPLETE. Furthermore, post hoc analyses were performed to analyse PsAID-12 levels and changes as categories. To explore the effect of treatment on disease impact, change in PsAID-12 total score was analysed according to thresholds for PsAID-12 clinically meaningful within-patient improvement. To assess impact as a state, PsAID-12 levels were analysed according to categories of disease impact/severity thresholds. Favourable impact status (defined here as minimal or no symptom impact) was identified as PsAID-12 total scores of ≤1.15 [15].
Multiple imputation (MI) was used for continuous variables; in this case, absolute scores and CfB. Non-responder imputation (NRI) was used for clinically meaningful within-patient improvement, a binary outcome. Observed case (OC) data are reported at baseline and for disease severity states.
Ethics approval
The BE OPTIMAL, BE COMPLETE and BE VITAL studies were done in accordance with the Declaration of Helsinki and the International Conference on Harmonisation Guidance for Good Clinical Practice. Ethics approval was obtained from the relevant institutional review boards at participating sites. All patients provided written informed consent in accordance with local requirements. Separate ethics approval for the current study was not obtained, as it was a post hoc analysis.
Results
Patient disposition and baseline characteristics
In total, 1,112 patients were randomized to placebo or bimekizumab across BE OPTIMAL and BE COMPLETE; 414 were randomized to placebo and 698 to bimekizumab. Of the 712 patients randomized to placebo or bimekizumab in BE OPTIMAL, 645 (90.6%) completed Week 52. Of the 400 patients in BE COMPLETE, 360 (90.0%) completed Week 40.
TNFi-IR patients were, on average, older with longer time since first PsA diagnosis; however, baseline mean PsAID-12 scores, along with mean Psoriasis Area and Severity Index (PASI) score, tender joint count (TJC) and swollen joint count (SJC) were generally comparable between treatment groups and studies (Table 1).
Table 1.
Baseline patient characteristics, disease activity and PsAID-12 total and single-item domain scores
| BE OPTIMAL (bDMARD-naïve) |
BE COMPLETE (TNFi-IR) |
|||
|---|---|---|---|---|
| Placebo (n = 281) | Bimekizumab 160 mg Q4W (n = 431) | Placebo (n = 133) | Bimekizumab 160 mg Q4W (n = 267) | |
| Patient characteristics | ||||
| Age, years, mean (SD) | 48.7 (11.7) | 48.5 (12.6) | 51.3 (12.9) | 50.1 (12.4) |
| Male, n (%) | 127 (45.2) | 201 (46.6) | 60 (45.1) | 130 (48.7) |
| Time since first PsA diagnosis, years, mean (SD) | 5.6 (6.5)a | 6.0 (7.3)b | 9.2 (8.1)c | 9.6 (9.9)d |
| Disease activity | ||||
| TJC (of 68 joints), mean (SD) | 17.1 (12.5) | 16.8 (11.8) | 19.3 (14.2) | 18.4 (13.5) |
| SJC (of 66 joints), mean (SD) | 9.5 (7.3) | 9.0 (6.2) | 10.3 (8.2) | 9.7 (7.5) |
| PASI,e mean (SD) | 7.9 (5.6) | 8.2 (6.8) | 8.5 (6.6) | 10.1 (9.1) |
| PsAID-12 scores, mean (SE) | ||||
| Total score | 4.1 (0.1) | 4.0 (0.1) | 4.4 (0.2) | 4.5 (0.1) |
| Pain | 5.5 (0.1) | 5.3 (0.1) | 5.9 (0.2) | 5.8 (0.1) |
| Fatigue | 4.8 (0.2) | 4.6 (0.1) | 4.9 (0.2) | 5.2 (0.2) |
| Skin problems | 4.4 (0.2) | 4.4 (0.1) | 5.4 (0.2) | 5.1 (0.2) |
| Work and/or leisure activities | 4.5 (0.2) | 4.4 (0.1) | 4.8 (0.2) | 4.8 (0.2) |
| Functional capacity | 4.6 (0.2) | 4.6 (0.1) | 5.1 (0.2) | 5.0 (0.2) |
| Discomfort | 4.3 (0.2) | 4.3 (0.1) | 4.8 (0.2) | 4.9 (0.2) |
| Sleep disturbance | 3.8 (0.2) | 3.5 (0.1) | 3.7 (0.3) | 4.0 (0.2) |
| Coping | 3.9 (0.2) | 3.6 (0.1) | 4.2 (0.2) | 4.1 (0.2) |
| Anxiety, fear and uncertainty | 2.5 (0.2) | 2.1 (0.1) | 2.0 (0.2) | 2.3 (0.2) |
| Embarrassment and/or shame | 2.5 (0.2) | 2.3 (0.1) | 2.7 (0.3) | 2.8 (0.2) |
| Social participation | 2.7 (0.2) | 2.5 (0.1) | 3.2 (0.2) | 3.1 (0.2) |
| Depression | 1.4 (0.1) | 1.1 (0.1) | 1.4 (0.2) | 1.8 (0.2) |
Randomized set. PsAID-12 scores range from 0 to 10; higher scores indicate a worse status.
n = 279.
n = 423.
n = 132.
n = 266.
For patients with psoriasis involving ≥3% of BSA at baseline (BE OPTIMAL bimekizumab n = 217, placebo/bimekizumab n = 140 and BE COMPLETE bimekizumab n = 176, placebo/bimekizumab n = 88).
bDMARD: biologic DMARD; BSA: body surface area; PASI: Psoriasis Area and Severity Index; PsAID-12: 12-item PsA Impact of Disease; Q4W: every 4 weeks; SJC: swollen joint count; TJC: tender joint count; TNFi-IR: inadequate response or intolerance to TNF inhibitors.
Disease impact at baseline
At baseline, mean (SE) PsAID-12 total score for bDMARD-naïve and TNFi-IR patients was in the range 4.0 (0.1)–4.5 (0.1) across treatment arms and study populations. Most PsAID-12 single-item domain scores were >4; mean (SE) baseline single-item domain scores ranged from 1.1 (0.1) (depression) to 5.9 (0.2) (pain) across treatment arms and study populations. At baseline, both bDMARD-naïve and TNFi-IR patients reported being most impacted by pain, fatigue and skin problems, and least impacted by depression (Table 1). Additional baseline patient demographics and disease activity measures are shown in Supplementary Table 1, available at Rheumatology online.
Change in PsAID-12 total and single-item domain scores
Total score
Bimekizumab-randomized patients in both study populations showed rapid improvement in mean (SE) PsAID-12 total score as early as Week 4; CfB for bDMARD-naïve bimekizumab –1.2 (0.1), placebo –0.2 (0.1) and TNFi-IR bimekizumab –1.4 (0.1), placebo –0.1 (0.1) (Fig. 1, Table 2). These improvements continued to Week 16, with bimekizumab-randomized patients demonstrating greater mean (SE) CfB in PsAID-12 total scores compared with placebo-randomized patients in both bDMARD-naïve patients (bimekizumab –1.8 [0.1], placebo –0.5 [0.1]) and TNFi-IR patients (bimekizumab –2.2 [0.1], placebo –0.3 [0.2]). Improvements at Week 16 were sustained to Week 52/40 for those on bimekizumab, while patients who switched to bimekizumab at Week 16 achieved similar improvements to bimekizumab-randomized patients in both study populations (bDMARD-naïve bimekizumab –2.3 [0.1], placebo/bimekizumab –2.2 [0.1]; TNFi-IR bimekizumab –2.5 [0.1], placebo/bimekizumab –2.2 [0.2]).
Figure 1.
PsAID-12 total mean score distribution at baseline and Weeks 4, 16 and 52/40 [MI]. Randomized set. Markers for mean and median may cross in cases where mean and median overlap. bDMARD: biologic DMARD; MI: multiple imputation; PsAID-12: 12-item PsA Impact of Disease; Q1: lower quartile; Q3: upper quartile; Q4W: every 4 weeks; TNFi-IR: inadequate response or intolerance to TNF inhibitors
Table 2.
Change from baseline in PsAID-12 total and single-item domain scores [MI]
| BE OPTIMAL (bDMARD-naïve) |
BE COMPLETE (TNFi-IR) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo/bimekizumab 160 mg Q4W (n = 281) |
Bimekizumab 160 mg Q4W (n = 431) |
Placebo/bimekizumab 160 mg Q4W (n = 133) |
Bimekizumab 160 mg Q4W (n = 267) |
|||||||||
| Week 4 | Week 16 | Week 52 | Week 4 | Week 16 | Week 52 | Week 4 | Week 16 | Week 40 | Week 4 | Week 16 | Week 40 | |
| CfB in PsAID-12 scores, mean (SE) | ||||||||||||
| Total score | −0.2 (0.1) | −0.5 (0.1) | −2.2 (0.1) | −1.2 (0.1) | −1.8 (0.1) | −2.3 (0.1) | −0.1 (0.1) | −0.3 (0.2) | −2.2 (0.2) | −1.4 (0.1) | −2.2 (0.1) | −2.5 (0.1) |
| Pain | 0.0 (0.1) | −0.6 (0.1) | −2.9 (0.2) | −1.3 (0.1) | −2.2 (0.1) | −2.9 (0.1) | −0.1 (0.2) | −0.4 (0.2) | −2.6 (0.3) | −1.5 (0.1) | −2.6 (0.2) | −2.9 (0.2) |
| Fatigue | −0.2 (0.1) | −0.7 (0.1) | −2.2 (0.2) | −1.0 (0.1) | −1.6 (0.1) | −2.3 (0.1) | 0.1 (0.2) | −0.3 (0.2) | −1.9 (0.2) | −1.3 (0.1) | −2.2 (0.2) | −2.4 (0.2) |
| Skin problems | −0.2 (0.1) | −0.4 (0.2) | −2.9 (0.2) | −2.0 (0.1) | −2.7 (0.2) | −3.1 (0.2) | 0.1 (0.2) | −0.5 (0.2) | −3.6 (0.3) | −2.2 (0.2) | −3.2 (0.2) | −3.7 (0.2) |
| Work and/or leisure activities | −0.1 (0.1) | −0.6 (0.2) | −2.4 (0.2) | −1.4 (0.1) | −2.1 (0.1) | −2.7 (0.1) | −0.3 (0.2) | −0.6 (0.2) | −2.3 (0.3) | −1.5 (0.1) | −2.5 (0.2) | −2.7 (0.2) |
| Functional capacity | −0.3 (0.1) | −0.6 (0.1) | −2.5 (0.2) | −1.4 (0.1) | −2.2 (0.1) | −2.6 (0.1) | −0.4 (0.2) | −0.6 (0.2) | −2.5 (0.2) | −1.6 (0.1) | −2.5 (0.2) | −2.8 (0.2) |
| Discomfort | −0.2 (0.1) | −0.6 (0.2) | −2.4 (0.2) | −1.6 (0.1) | −2.2 (0.1) | −2.7 (0.1) | −0.4 (0.2) | −0.5 (0.2) | −2.7 (0.3) | −1.8 (0.2) | −2.5 (0.2) | −2.7 (0.2) |
| Sleep disturbance | −0.4 (0.2) | −0.7 (0.2) | −1.9 (0.2) | −0.9 (0.1) | −1.4 (0.1) | −1.9 (0.1) | −0.2 (0.2) | 0.1 (0.2) | −1.5 (0.3) | −1.3 (0.2) | −1.9 (0.2) | −2.0 (0.2) |
| Coping | −0.2 (0.1) | −0.7 (0.1) | −2.2 (0.2) | −1.1 (0.1) | −1.6 (0.1) | −2.1 (0.1) | −0.1 (0.2) | −0.3 (0.2) | −2.1 (0.2) | −1.1 (0.2) | −2.0 (0.2) | −2.2 (0.2) |
| Anxiety, fear and uncertainty | −0.1 (0.2) | −0.4 (0.1) | −1.2 (0.2) | −0.7 (0.1) | −0.8 (0.1) | −1.0 (0.1) | 0.1 (0.2) | 0.2 (0.2) | −0.8 (0.2) | −0.6 (0.1) | −1.1 (0.2) | −1.2 (0.2) |
| Embarrassment and/or shame | −0.1 (0.1) | −0.2 (0.2) | −1.6 (0.2) | −1.0 (0.1) | −1.4 (0.1) | −1.6 (0.1) | 0.1 (0.2) | −0.2 (0.2) | −1.6 (0.3) | −1.2 (0.2) | −1.9 (0.2) | −2.0 (0.2) |
| Social participation | −0.1 (0.1) | −0.4 (0.2) | −1.6 (0.2) | −1.0 (0.1) | −1.4 (0.1) | −1.6 (0.1) | −0.3 (0.2) | −0.5 (0.2) | −1.7 (0.3) | −1.1 (0.1) | −1.7 (0.2) | −1.7 (0.2) |
| Depression | 0.0 (0.1) | 0.0 (0.1) | −0.7 (0.1) | −0.4 (0.1) | −0.4 (0.1) | −0.6 (0.1) | 0.3 (0.1) | 0.1 (0.2) | −0.6 (0.2) | −0.7 (0.1) | −1.0 (0.1) | −1.0 (0.2) |
Randomized set. PsAID-12 scores range from 0 to 10; higher scores indicate a worse status.
bDMARD: biologic DMARD; CfB: change from baseline; MI: multiple imputation; PsAID-12: 12-item PsA Impact of Disease; Q4W: every 4 weeks; TNFi-IR: inadequate response or intolerance to TNF inhibitors.
Although BE OPTIMAL was not powered for statistical comparisons between patients in the adalimumab reference arm and bimekizumab-randomized patients, improvements in PsAID-12 total and single-item domain scores were of a similar magnitude across treatment arms; mean (SE) PsAID-12 total and single-item domain scores for the adalimumab reference arm are presented in Supplementary Table 2, available at Rheumatology online.
Results for the pooled population of bDMARD-naïve and TNFi-IR patients to Week 16 are reported in Supplementary Fig. 2, available at Rheumatology online.
Single-item domains
Improvements in pain, fatigue and skin problems, the most impacted single-item domains at baseline, were consistent with those reported for the total score (Table 2). Rapid improvements were observed for bimekizumab-randomized patients at Week 4; these improvements continued to Week 16 and were sustained to Week 52/40, with placebo-randomized patients who switched to bimekizumab at Week 16 achieving similar mean (SE) CfB to bimekizumab-randomized patients. At 1 year, mean (SE) CfB ranged from –2.6 (0.3) to –2.9 (0.2) for pain, –1.9 (0.2) to –2.4 (0.2) for fatigue, and –2.9 (0.2) to –3.7 (0.2) for skin problems across all patients in both studies. Findings across all other single-item domains followed a similar trend, with smaller improvements in those with lower baseline scores, and were comparable between study populations. (Table 2, Fig. 2).
Figure 2.
PsAID-12 single-item domain mean scores at baseline and Weeks 16 and 52/40 [MI]. Randomized set. bDMARD: biologic DMARD; MI: multiple imputation; PsAID-12: 12-item PsA Impact of Disease; Q4W: every 4 weeks; TNFi-IR: inadequate response or intolerance to TNF inhibitors
Pooled results for single-item domain mean scores, including pain, fatigue, and skin problems, to Week 16 are reported in Supplementary Fig. 3, available at Rheumatology online.
Clinically meaningful within-patient improvement
Total score
A numerically greater proportion of bimekizumab-randomized patients achieved clinically meaningful within-patient improvement in PsAID-12 total score at Weeks 4 and 16 compared with placebo patients (Fig. 3). At Week 4, this improvement was achieved by 20.3% bimekizumab and 2.5% placebo bDMARD-naïve patients, and 21.2% bimekizumab and 0% placebo TNFi-IR patients. The proportion of patients randomized to bimekizumab that reported clinically meaningful levels of improvement increased to Week 16 in both studies: 36.8% bimekizumab and 10.1% placebo bDMARD-naïve patients, and 49.5% bimekizumab and 5.0% placebo TNFi-IR patients.
Figure 3.
PsAID-12 clinically meaningful within-patient improvements at Weeks 4, 16 and 52/40 [NRI]. Randomized set in patients with a total or item score of ≥3 at baseline; shown are the proportion of patients achieving this threshold (decrease of ≥3 from baseline). NRI: non-responder imputation; PsAID-12: 12-item PsA Impact of Disease; Q4W: every 4 weeks
Proportions of patients achieving clinically meaningful within-patient improvement were sustained for bimekizumab-randomized patients to week 52/40, with similar proportions of placebo/bimekizumab patients achieving this threshold following the switch to bimekizumab: 49.0% bimekizumab and 44.4% placebo/bimekizumab bDMARD-naïve patients (Week 52), and 48.5% bimekizumab and 40.6% placebo/bimekizumab TNFi-IR patients (Week 40).
The proportion of patients achieving clinically meaningful within-patient improvement among the pooled population of bDMARD-naïve and TNFi-IR patients to Week 16 are reported in Supplementary Fig. 4, available at Rheumatology online.
Single-item domains
Compared with placebo, a numerically greater proportion of bimekizumab-randomized patients achieved clinically meaningful within-patient improvement across all single-item domains at Week 4, with proportions increasing to Week 16 in both bDMARD-naïve and TNFi-IR patient populations (Fig. 3). The proportion of bimekizumab-randomized patients achieving clinically meaningful within-patient improvement further increased to Week 52/40, with similar levels of improvement observed in placebo/bimekizumab patients at this timepoint.
The highest proportions of patients achieving clinically meaningful within-patient improvement were consistently observed in the skin problems domain. At Week 4, around half of bimekizumab-randomized patients across studies achieved clinically meaningful within-patient improvement in skin problems; this figure rose to just under 70% at Week 16, with around 20% of placebo patients achieving such improvement at the same timepoint. At Week 52/40, this threshold was achieved by ∼70% of all patients, regardless of initial randomization group or study population (bDMARD-naïve bimekizumab 72.3%, placebo/bimekizumab 69.2%; TNFi-IR bimekizumab 75.0%, placebo/bimekizumab 67.0%).
Disease severity states from patient-reported impact
Total score
Using the previously reported disease severity state thresholds [15], 85.1% bDMARD-naïve and 85.7% TNFi-IR placebo-randomized patients were experiencing high or moderate disease impact at baseline, based on reporting a PsAID-12 total score of >1.95. Among bimekizumab-randomized patients, 82.4% bDMARD-naïve and 87.2% TNFi-IR patients were experiencing high or moderate disease impact at baseline.
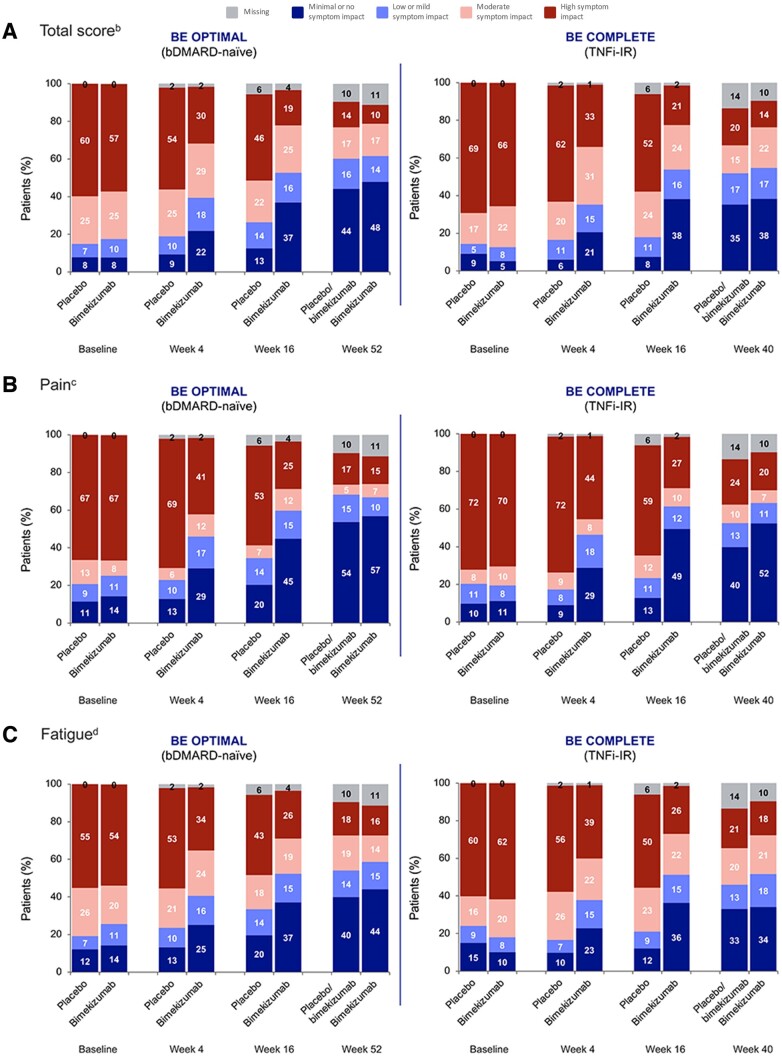
Across both studies, greater proportions of bimekizumab-randomized patients reported a favourable status (defined here as minimal or no symptom impact, based on reporting a PsAID-12 total score of ≤1.15) at Weeks 4 and 16, as compared with placebo-randomized patients. At Week 4, 21.8% of bimekizumab-randomized bDMARD-naïve patients exhibited minimal or no symptom impact, compared with 9.3% of placebo-randomized patients. At Week 16, the proportion of bimekizumab-randomized patients reporting favourable status increased to 36.9%, while the proportion of placebo-randomized bDMARD-naïve patients remained stable and lower (12.5%). Similar results were observed for TNFi-IR patients at Week 4 (bimekizumab 20.6%, placebo 6.0%) and Week 16 (bimekizumab 38.2%, placebo 7.5%). At Week 52/40, similar rates of bimekizumab (bDMARD-naïve 47.8%; TNFi-IR 38.2%) and placebo/bimekizumab (44.1%; 35.3%) patients were experiencing minimal or no symptom impact based on their PsAID-12 total score being ≤1.15 (Fig. 4A).
Figure 4.
Disease severity states by visit for PsAID-12 (A) total score, (B) pain, (C) fatigue and (D) skin problems [OCa]. Randomized set. Percentages may not sum to 100 as a result of rounding. BE OPTIMAL bimekizumab n = 431, placebo/bimekizumab n = 281 and BE COMPLETE bimekizumab n = 267, placebo/bimekizumab n = 133. aData are OC including missing categories. bRemission: ≤1.15, Mild: >1.15 to ≤1.95, Moderate: >1.95 to ≤3.6, High: >3.6; cRemission: ≤2, Low: 3, Moderate: 4, High: ≥5. dRemission: ≤1, Low: 2, Moderate: 3 or 4, High: ≥5. bDMARD: biologic DMARD; OC: observed case; PsAID-12: 12-item PsA Impact of Disease; TNFi-IR: inadequate response or intolerance to TNF inhibitors
Figure 4.
Continued.
Single-item domains
Trends were comparable for pain, fatigue and skin problems; greater proportions of bimekizumab-randomized patients in both studies had minimal or no symptom impact compared with placebo-randomized patients at Weeks 4 and 16 (Fig. 4B–D). The shift from moderate or high symptom impact to minimal or no symptom impact was sustained to Week 52/40, with similar proportions of patients in both bimekizumab and placebo/bimekizumab groups achieving minimal pain (bDMARD-naïve bimekizumab 56.8%, placebo/bimekizumab 53.7%; TNFi-IR bimekizumab 52.4%, placebo/bimekizumab 39.8%), fatigue (bDMARD-naïve bimekizumab 44.1%, placebo/bimekizumab 39.9%; TNFi-IR bimekizumab 34.1%, placebo/bimekizumab 33.1%) and skin problems (bDMARD-naïve bimekizumab 64.0%, placebo/bimekizumab 61.2%; TNFi-IR bimekizumab 59.9%, placebo/bimekizumab 54.1%).
Disease activity levels for all other single-item domains for which disease severity thresholds have been identified were similar to those observed for pain, fatigue, skin problems and total score, and are reported in Supplementary Fig. 5, available at Rheumatology online.
Discussion
Bimekizumab treatment reduced the impact PsA had on multiple aspects of patients’ lives, as assessed by the PsAID-12 questionnaire. The results were consistent across the BE OPTIMAL and BE COMPLETE studies, indicating improvement irrespective of prior biologic use. Rapid improvements in PsAID-12 total and all single-item domain scores were observed with bimekizumab as early as Week 4, and continued to improve to Week 16, as compared with placebo. The analysis reported here used recently published patient impact thresholds of PsAID-12 total score and single-item domains to evaluate and provide clinical meaningfulness of the impact of bimekizumab on the symptoms and HRQoL of bDMARD-naïve or TNFi-IR patients with active PsA up to 1 year [15]. Importantly, close to half of these patients with long-standing, active disease were able to reach states of minimal or no symptom impact at 1 year; these findings will be important when discussing expectations with patients in a shared decision-making approach.
Greatest improvements were observed in the single-item domains with the highest baseline scores, indicating patients reported these symptoms (pain, fatigue and skin problems) to be the most impactful. Baseline scores were also high for work and/or leisure activities, functional capacity and discomfort, demonstrating the considerable impact on patients’ HRQoL beyond just the symptoms of PsA. The magnitude of improvement with bimekizumab treatment for these single-item domains was similar. Mean baseline anxiety, fear and uncertainty, and depression domain scores indicated BE OPTIMAL and BE COMPLETE patients were not experiencing these psychosocial symptoms at baseline to as great an extent relative to physical symptoms. This was likely as a result of the study exclusion criteria. However, the proportions of patients with a baseline score of ≥3 in the anxiety, fear and uncertainty, and depression domains achieving clinically meaningful within-patient improvement were similar to those of the more widely impacted single-item domains. The proportions achieving this improvement threshold were similar across the bDMARD-naïve and TNFi-IR populations, and trends were consistent across all items, including pain, fatigue and skin problems, each of which represents a key symptomatic feature of PsA that is detrimental to patient well-being.
These results, which reflect the patient perspective, support published improvements in overall clinical efficacy and safety demonstrated by bimekizumab in PsA [18, 19]. Furthermore, the findings reported here are consistent with improvements observed in other established patient-reported outcome measures assessing the spectrum of PsA manifestations, including the Patient’s Assessment of Arthritis Pain (PtAAP), Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue, HAQ-DI and SF-36 Physical Component Summary [16, 17].
Collectively, these results provide additional evidence for the longer-term efficacy of bimekizumab in reducing the burden of PsA-related symptoms, including pain, fatigue and skin problems, thereby improving overall patient HRQoL.
Strengths
A key strength of this analysis is the inclusion of two patient populations, showing the relevance of bimekizumab treatment to patients who are bDMARD-naive as well as those who have exhibited inadequate response or intolerance to prior TNFi therapy. The use of a side-by-side analysis to demonstrate the similarity of results across both studies is also a strength of this analysis. Furthermore, pooling the two study populations to Week 16, after which assessment time points and study designs differed, allowed for a robust analysis using a larger group of patients with active PsA; an additional strength, particularly given the similarity to the side-by-side analysis.
This study is, to the best of the authors’ knowledge, the first in PsA to incorporate the PsAID-12 questionnaire, a psychometrically-validated disease-specific fit-for-purpose measure, on top of standard generic measures, to assess patient-relevant symptoms and the impact of receiving bimekizumab for the treatment of PsA. Further, the PsAID-12 questionnaire, comprising 12 single-item domains, covers a broad spectrum of symptoms that impact patient HRQoL, making the measure suitable to holistically assess disease impact from the patient perspective. All results reported in this manuscript are from a tool informed by patients, thereby offering an insight into the effect of bimekizumab treatment across various aspects and timepoints of patients’ lives, capturing immediate changes at Week 4, short-term improvements at Week 16 and longer-term responses up to 1 year.
Limitations
The findings from this analysis will require confirmation with complex, non-study populations including different demographic groups, such as gender and age-based groups [23, 24]. Furthermore, longer-term data will need to be assessed to demonstrate how these patient-reported outcome results are sustained in the context of a chronic disease requiring lifelong treatment.
There likely exist differences between the study populations and the real-world clinical population, which may be more heterogeneous in terms of disease manifestations, comorbidities, disease severity and prior treatment experience [25]; these differences may influence baseline scores, in turn impacting the generalizability of the results. For example, the psychosocial single-item domain scores reported at baseline may not be reflective of the real-world clinical population due to the BE OPTIMAL and BE COMPLETE inclusion criteria, which stipulated that patients with major depression were ineligible to participate. Similarly, high baseline scores were reported across the physical single-item domains, perhaps due to study inclusion criteria for moderate to severe PsA, and these may contribute to the high levels of responsiveness observed in the current analysis [26]. As such, real-world evidence data reported in future publications could further demonstrate how both physical and psychosocial items are impacted by bimekizumab treatment by capturing patient experiences in routine clinical practice.
Conclusion
Treatment with bimekizumab resulted in rapid and sustained clinically meaningful improvements in most PsAID-12 items, capturing relevant outcomes up to 1 year. Improvements were similar in both bDMARD-naïve and TNFi-IR patients with active PsA, demonstrating consistent responses to dual inhibition of IL-17A and IL-17F. These improvements should be further confirmed in future publications with longer-term study and real-world data.
Supplementary Material
Acknowledgements
The authors thank the patients, the investigators and their teams who took part in this study. The authors also acknowledge Heather Edens, PhD, UCB Pharma, Smyrna, Georgia, USA for publication coordination and Aditi Mehta, MSc, Costello Medical, UK, for medical writing and editorial assistance based on the authors’ input and direction. This study was funded by UCB Pharma.
Contributor Information
Laure Gossec, Sorbonne Université, INSERM, Institut Pierre Louis d'Epidémiologie et de Santé Publique, Paris, France; AP-HP, Pitié Salpêtrière Hospital, Rheumatology Department, Paris, France.
Ana-Maria Orbai, Division of Rheumatology, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Maarten de Wit, Patient Research Partner, Stichting Tools, Amsterdam, The Netherlands.
Laura C Coates, Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Diseases, University of Oxford and Oxford Biomedical Research Centre, Oxford University Hospitals NHS Trust, Oxford, UK.
Alexis Ogdie, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA.
Barbara Ink, UCB Pharma, Slough, UK.
Jason Coarse, UCB Pharma, Morrisville, NC, USA.
Jérémy Lambert, UCB Pharma, Colombes, France.
Vanessa Taieb, UCB Pharma, Colombes, France.
Dafna D Gladman, Schroeder Arthritis Institute, Krembil Research Institute, Toronto Western Hospital, University Health Network, University of Toronto, ON, Canada.
Supplementary material
Supplementary material is available at Rheumatology online.
Data availability
Data from this manuscript may be requested by qualified researchers 6 months after product approval in the US and/or Europe, or global development is discontinued, and 18 months after study completion. Investigators may request access to anonymised individual patient data and redacted study documents which may include: raw datasets, analysis-ready datasets, study protocol, blank case report form, annotated case report form, statistical analysis plan, dataset specifications and clinical study report. Prior to use of the data, proposals need to be approved by an independent review panel at www.Vivli.org and a signed data sharing agreement will need to be executed. All documents are available in English only, for a prespecified time, typically 12 months, on a password protected portal.
Contribution statement
Substantial contributions to study conception and design: L.G., A.M.O., M.d.W., L.C.C., A.O., B.I., J.C., J.L., V.T., D.D.G.; substantial contributions to analysis and interpretation of the data: L.G., A.M.O., M.d.W., L.C.C., A.O., B.I., J.C., J.L., V.T., D.D.G.; drafting the article or revising it critically for important intellectual content: L.G., A.M.O., M.d.W., L.C.C., A.O., B.I., J.C., J.L., V.T., D.D.G.; final approval of the version of the article to be published: L.G., A.M.O., M.d.W., L.C.C., A.O., B.I., J.C., J.L., V.T., D.D.G.
Funding
This study was sponsored by UCB Pharma. Support for third-party writing assistance for this article, provided by Aditi Mehta, MSc, Costello Medical UK, was funded by UCB Pharma in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Disclosure statement: L.G.: Grants from AbbVie, Biogen, Lilly, Novartis, Sandoz and UCB Pharma; personal fees from AbbVie, Amgen, BMS, Celltrion, Galapagos, Janssen, Lilly, MSD, Novartis, Pfizer, Sandoz and UCB Pharma. A.M.O.: Research grants to Johns Hopkins University from AbbVie, Amgen and Janssen; consulting fees from BMS, Janssen, Sanofi and UCB Pharma. M.d.W.: Stichting Tools has received fees for lectures or consultancy provided by MdW from Celgene, Eli Lilly, Janssen-Cilag, Pfizer and UCB Pharma. L.C.C.: Grants/research support from AbbVie, Amgen, Celgene, Eli Lilly, Gilead, Janssen, Novartis, Pfizer and UCB Pharma; consultant for AbbVie, Amgen, BMS, Boehringer Ingelheim, Celgene, Domain, Eli Lilly, Galapagos, Gilead, Janssen, Moonlake Pharma, Novartis, Pfizer and UCB Pharma; speaking fees from AbbVie, Amgen, Biogen, Celgene, Eli Lilly, Galapagos, Gilead, GSK, Janssen, medac, Novartis, Pfizer and UCB Pharma. A.O.: Grant/research support from AbbVie, Amgen, Janssen, Novartis and Pfizer; consultant for AbbVie, Amgen, BMS, Celgene, CorEvitas, Eli Lilly, GSK, Gilead, Janssen, Novartis, Pfizer, Takeda and UCB Pharma. B.I.: Employee of UCB Pharma; shareholder of AbbVie, GSK and UCB Pharma. J.C., J.L., V.T.: Employee and shareholder of UCB Pharma. D.D.G.: Consultant and/or received grant support from AbbVie, Amgen, BMS, Eli Lilly, Gilead, Galapagos, Janssen, Novartis, Pfizer and UCB Pharma.
Consent for publication: All the results presented in this article are in aggregate form, and no personally identifiable information was used for this study.
References
- 1. Veale DJ, Fearon U.. The pathogenesis of psoriatic arthritis. Lancet 2018;391:2273–84. [DOI] [PubMed] [Google Scholar]
- 2. Gladman DD, Antoni C, Mease P, Clegg DO, Nash P.. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis 2005;64(Suppl 2):ii14–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mease PJ, Armstrong AW.. Managing patients with psoriatic disease: the diagnosis and pharmacologic treatment of psoriatic arthritis in patients with psoriasis. Drugs 2014;74:423–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ritchlin CT, Colbert RA, Gladman DD.. Psoriatic arthritis. New Engl J Med 2017;376:957–70. [DOI] [PubMed] [Google Scholar]
- 5. Gudu T, Gossec L.. Quality of life in psoriatic arthritis. Expert Rev Clin Immunol 2018;14:405–17. [DOI] [PubMed] [Google Scholar]
- 6. Husted JA, Gladman DD, Farewell VT, Cook RJ.. Health-related quality of life of patients with psoriatic arthritis: a comparison with patients with rheumatoid arthritis. Arthritis Care Res 2001;45:151–8. [DOI] [PubMed] [Google Scholar]
- 7. Salaffi F, Carotti M, Gasparini S, Intorcia M, Grassi W.. The health-related quality of life in rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis: a comparison with a selected sample of healthy people. Health Qual Life Outcomes 2009;7:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Husni ME, Merola JF, Davin S.. The psychosocial burden of psoriatic arthritis. Semin Arthritis Rheum 2017;47:351–60. [DOI] [PubMed] [Google Scholar]
- 9. Husted JA, Tom BD, Schentag CT, Farewell VT, Gladman DD.. Occurrence and correlates of fatigue in psoriatic arthritis. Ann Rheum Dis 2009;68:1553–8. [DOI] [PubMed] [Google Scholar]
- 10. Ogdie A, Michaud K, Nowak M. et al. Patient’s experience of psoriatic arthritis: a conceptual model based on qualitative interviews. RMD Open 2020;6:e001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Coates LC, Ritchlin CT, Kavanaugh AF.. GRAPPA treatment recommendations: an update from the GRAPPA 2013 annual meeting. J Rheumatol 2014;41:1237–9. [DOI] [PubMed] [Google Scholar]
- 12. Leung Y-Y, Orbai A-M, Hojgaard P. et al. OMERACT Filter 2.1 instrument selection for physical function domain in psoriatic arthritis: provisional endorsement for HAQ-DI and SF-36 PF. Semin Arthritis Rheum 2021;51:1117–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Orbai A-M, Holland R, Leung YY. et al. PsAID12 provisionally endorsed at OMERACT 2018 as core outcome measure to assess psoriatic arthritis-specific health-related quality of life in clinical trials. J Rheumatol 2019;46:990–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Wit MPT, Kvien TK, Gossec L.. Patient participation as an integral part of patient-reported outcomes development ensures the representation of the patient voice: a case study from the field of rheumatology. RMD Open 2015;1:e000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gossec L, Orbai A-M, Coates LC. et al. Validity and score interpretation of the 12-item Psoriatic Arthritis Impact of Disease: an analysis of pooled data from two phase 3 trials of bimekizumab in patients with psoriatic arthritis. RMD Open 2024;10:e003548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ritchlin CT, Coates LC, McInnes IB. et al. Bimekizumab treatment in biologic DMARD-naïve patients with active psoriatic arthritis: 52-week efficacy and safety results from the phase III, randomised, placebo-controlled, active reference BE OPTIMAL study. Ann Rheum Dis 2023;82:1404–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Coates LC, Landewé R, McInnes IB. et al. Bimekizumab treatment in patients with active psoriatic arthritis and prior inadequate response to tumour necrosis factor inhibitors: 52-week safety and efficacy from the phase III BE COMPLETE study and its open-label extension BE VITAL. RMD Open 2024;10:e003855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McInnes IB, Asahina A, Coates LC. et al. Bimekizumab in patients with psoriatic arthritis, naive to biologic treatment: a randomised, double-blind, placebo-controlled, phase 3 trial (BE OPTIMAL). Lancet 2023;401:25–37. [DOI] [PubMed] [Google Scholar]
- 19. Merola JF, Landewé R, McInnes IB. et al. Bimekizumab in patients with active psoriatic arthritis and previous inadequate response or intolerance to tumour necrosis factor-alpha inhibitors: a randomised, double-blind, placebo-controlled, phase 3 trial (BE COMPLETE). Lancet 2023;401:38–48. [DOI] [PubMed] [Google Scholar]
- 20. Coates L, Landewé RBM, Mcinnes I. et al. POS0231 Sustained efficacy and safety of bimekizumab in patients with active psoriatic arthritis and prior inadequate response to tumour necrosis factor inhibitors: results from the phase 3 be complete study and its open-label extension up to 1 year. Ann Rheum Dis 2023;82:346–7. [Google Scholar]
- 21. Gossec L, de Wit M, Kiltz U. et al. ; EULAR PsAID Taskforce. A patient-derived and patient-reported outcome measure for assessing psoriatic arthritis: elaboration and preliminary validation of the Psoriatic Arthritis Impact of Disease (PsAID) questionnaire, a 13-country EULAR initiative. Ann Rheum Dis 2014;73:1012–9. [DOI] [PubMed] [Google Scholar]
- 22. Wild D, Grove A, Martin M. et al. ; ISPOR Task Force for Translation and Cultural Adaptation. Principles of good practice for the translation and cultural adaptation process for Patient-Reported Outcomes (PRO) measures: report of the ISPOR Task Force for Translation and Cultural Adaptation. Value Health 2005;8:94–104. [DOI] [PubMed] [Google Scholar]
- 23. Gossec L, Theander E, Chakravarty SD. et al. Response to treatment in psoriatic arthritis, the effect of age: analysis of patients receiving ustekinumab in the PsABio real-world study. Arthritis Res Ther 2023;25:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Højgaard P, Ballegaard C, Cordtz R. et al. Gender differences in biologic treatment outcomes—a study of 1750 patients with psoriatic arthritis using Danish Health Care Registers. Rheumatology 2018;57:1651–60. [DOI] [PubMed] [Google Scholar]
- 25. Walsh JA, Ogdie A, Michaud K. et al. Impact of key manifestations of psoriatic arthritis on patient quality of life, functional status, and work productivity: findings from a real-world study in the United States and Europe. Joint Bone Spine 2023;90:105534. [DOI] [PubMed] [Google Scholar]
- 26. Karmacharya P, Stull C, Stephens-Shields A. et al. Responsiveness and minimum clinically important difference in patient-reported outcome measures among patients with psoriatic arthritis: a prospective cohort study. Arthritis Care Res 2023;75:2182–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from this manuscript may be requested by qualified researchers 6 months after product approval in the US and/or Europe, or global development is discontinued, and 18 months after study completion. Investigators may request access to anonymised individual patient data and redacted study documents which may include: raw datasets, analysis-ready datasets, study protocol, blank case report form, annotated case report form, statistical analysis plan, dataset specifications and clinical study report. Prior to use of the data, proposals need to be approved by an independent review panel at www.Vivli.org and a signed data sharing agreement will need to be executed. All documents are available in English only, for a prespecified time, typically 12 months, on a password protected portal.