Abstract
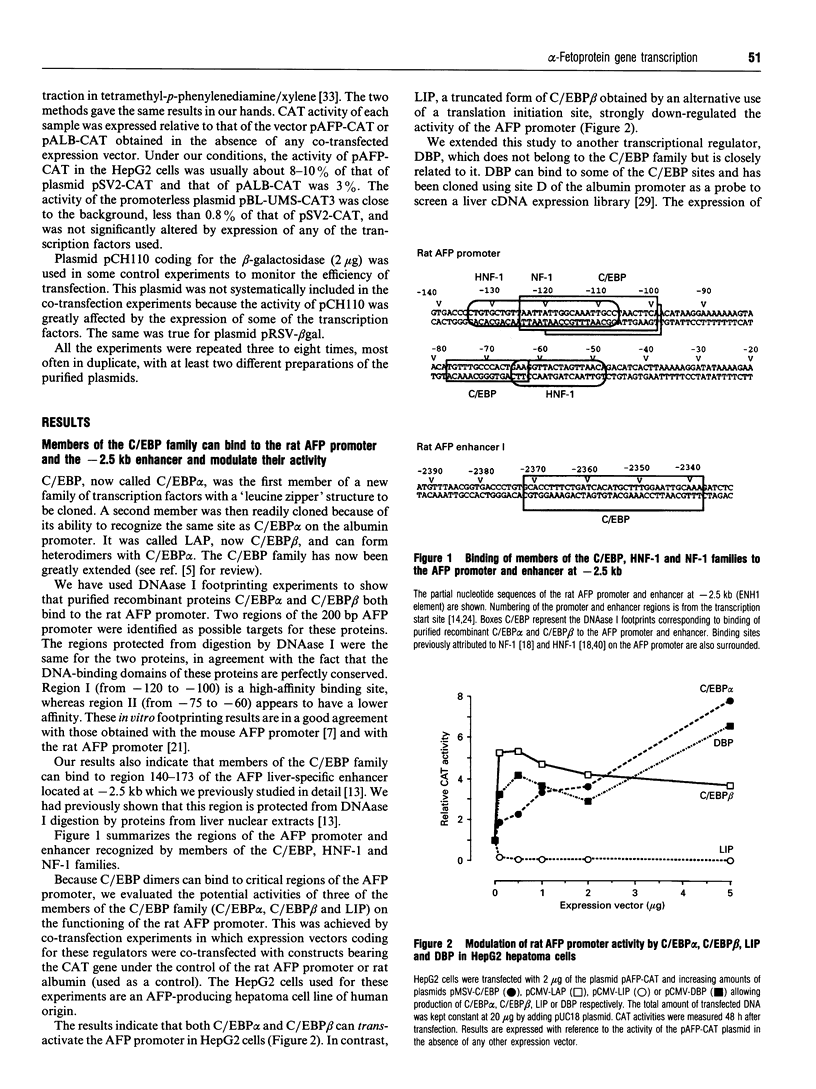
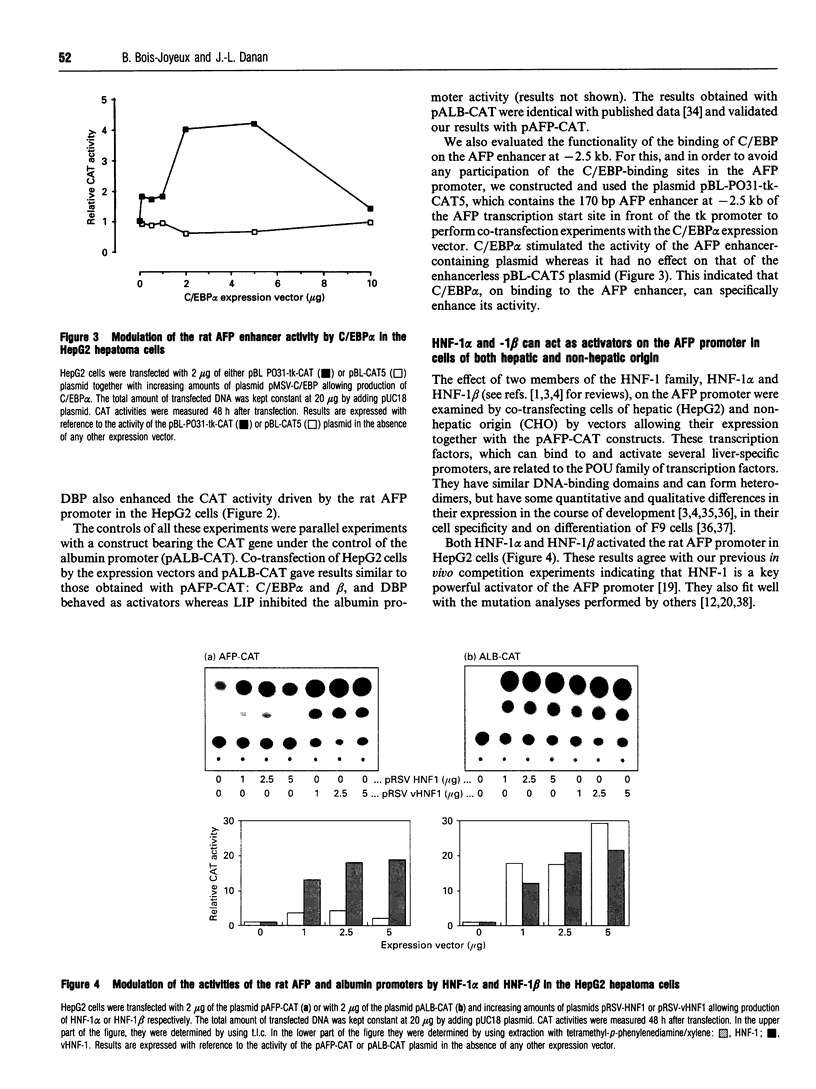
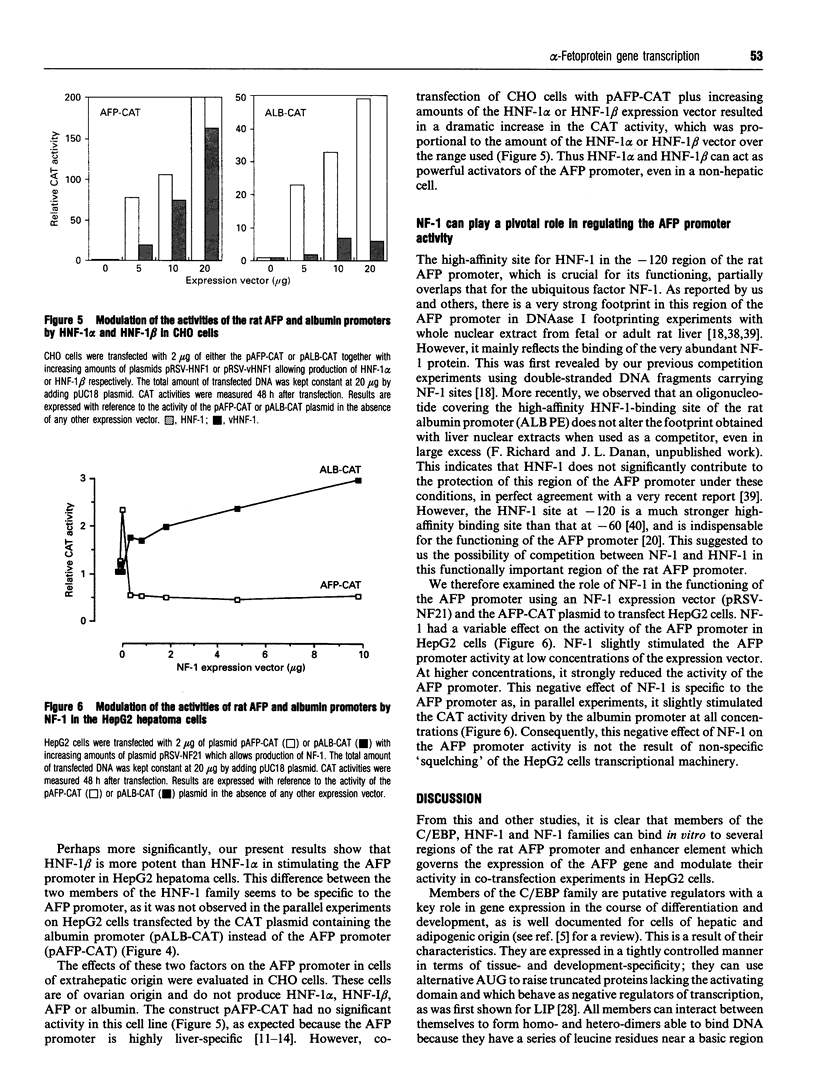
The promoter of the rat alpha-fetoprotein (AFP) gene, which makes the expression of the developmentally regulated AFP gene specific to the liver, is a putative target for transcription factors of the CAAT/enhancer-binding protein (C/EBP), hepatocyte nuclear factor-1 (HNF-1) and nuclear factor-1 (NF-1) families. We have evaluated the influence of these factors on the activity of the AFP promoter by transfection of HepG2 hepatoma cells with the appropriate expression vector plus a CAT plasmid under the control of the AFP promoter. A similar plasmid bearing the rat albumin promoter was used as a control. C/EBP alpha, C/EBP beta and D-binding protein (DBP) acted as trans-activators on the AFP promoter, whereas liver inhibitory protein (LIP), a truncated form of C/EBP beta, was a potent negative regulator of the promoter. C/EBP alpha also bound to and stimulated the activity of the AFP enhancer at -2.5 kb. Interestingly, HNF-1 beta was found to be more potent than HNF-1 alpha in activating the AFP promoter. This effect was specific, as it did not occur with the rat albumin promoter. HNF-1 beta, which is produced earlier than HNF-1 alpha during liver development, would thus have the greater influence on the AFP promoter in early development. Both HNF-1s allowed expression of the AFP promoter in cells of nonhepatic origin. Overexpression of NF-1 induced a specific decrease in the activity of the AFP promoter. This strongly suggests that competition between NF-1 and HNF-1 for binding to their overlapping binding sites on the AFP promoter is critical for modulating its activity. Thus changing combinations of these trans-acting factors may tightly modulate the AFP promoter activity in the course of liver development and carcinogenesis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angrand P. O., Rousset J. P., Weiss M. C. Cell phenotype, binding affinity and promoter structure modulate transactivation by HNF1 and LAP. J Cell Sci. 1992 Dec;103(Pt 4):1083–1092. doi: 10.1242/jcs.103.4.1083. [DOI] [PubMed] [Google Scholar]
- Bernier D., Thomassin H., Allard D., Guertin M., Hamel D., Blaquière M., Beauchemin M., LaRue H., Estable-Puig M., Bélanger L. Functional analysis of developmentally regulated chromatin-hypersensitive domains carrying the alpha 1-fetoprotein gene promoter and the albumin/alpha 1-fetoprotein intergenic enhancer. Mol Cell Biol. 1993 Mar;13(3):1619–1633. doi: 10.1128/mcb.13.3.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkenmeier E. H., Gwynn B., Howard S., Jerry J., Gordon J. I., Landschulz W. H., McKnight S. L. Tissue-specific expression, developmental regulation, and genetic mapping of the gene encoding CCAAT/enhancer binding protein. Genes Dev. 1989 Aug;3(8):1146–1156. doi: 10.1101/gad.3.8.1146. [DOI] [PubMed] [Google Scholar]
- Blumenfeld M., Maury M., Chouard T., Yaniv M., Condamine H. Hepatic nuclear factor 1 (HNF1) shows a wider distribution than products of its known target genes in developing mouse. Development. 1991 Oct;113(2):589–599. doi: 10.1242/dev.113.2.589. [DOI] [PubMed] [Google Scholar]
- Boshart M., Klüppel M., Schmidt A., Schütz G., Luckow B. Reporter constructs with low background activity utilizing the cat gene. Gene. 1992 Jan 2;110(1):129–130. doi: 10.1016/0378-1119(92)90456-y. [DOI] [PubMed] [Google Scholar]
- Brüggemeier U., Rogge L., Winnacker E. L., Beato M. Nuclear factor I acts as a transcription factor on the MMTV promoter but competes with steroid hormone receptors for DNA binding. EMBO J. 1990 Jul;9(7):2233–2239. doi: 10.1002/j.1460-2075.1990.tb07393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camper S. A., Tilghman S. M. Postnatal repression of the alpha-fetoprotein gene is enhancer independent. Genes Dev. 1989 Apr;3(4):537–546. doi: 10.1101/gad.3.4.537. [DOI] [PubMed] [Google Scholar]
- Cereghini S., Ott M. O., Power S., Maury M. Expression patterns of vHNF1 and HNF1 homeoproteins in early postimplantation embryos suggest distinct and sequential developmental roles. Development. 1992 Nov;116(3):783–797. doi: 10.1242/dev.116.3.783. [DOI] [PubMed] [Google Scholar]
- Chouard T., Blumenfeld M., Bach I., Vandekerckhove J., Cereghini S., Yaniv M. A distal dimerization domain is essential for DNA-binding by the atypical HNF1 homeodomain. Nucleic Acids Res. 1990 Oct 11;18(19):5853–5863. doi: 10.1093/nar/18.19.5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtois G., Baumhueter S., Crabtree G. R. Purified hepatocyte nuclear factor 1 interacts with a family of hepatocyte-specific promoters. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7937–7941. doi: 10.1073/pnas.85.21.7937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Simone V., Cortese R. Transcriptional regulation of liver-specific gene expression. Curr Opin Cell Biol. 1991 Dec;3(6):960–965. doi: 10.1016/0955-0674(91)90114-e. [DOI] [PubMed] [Google Scholar]
- Descombes P., Chojkier M., Lichtsteiner S., Falvey E., Schibler U. LAP, a novel member of the C/EBP gene family, encodes a liver-enriched transcriptional activator protein. Genes Dev. 1990 Sep;4(9):1541–1551. doi: 10.1101/gad.4.9.1541. [DOI] [PubMed] [Google Scholar]
- Descombes P., Schibler U. A liver-enriched transcriptional activator protein, LAP, and a transcriptional inhibitory protein, LIP, are translated from the same mRNA. Cell. 1991 Nov 1;67(3):569–579. doi: 10.1016/0092-8674(91)90531-3. [DOI] [PubMed] [Google Scholar]
- Dziadek M. A., Andrews G. K. Tissue specificity of alpha-fetoprotein messenger RNA expression during mouse embryogenesis. EMBO J. 1983;2(4):549–554. doi: 10.1002/j.1460-2075.1983.tb01461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerman M. H., Godbout R., Ingram R. S., Tilghman S. M. Tissue-specific transcription of the mouse alpha-fetoprotein gene promoter is dependent on HNF-1. Mol Cell Biol. 1989 Oct;9(10):4204–4212. doi: 10.1128/mcb.9.10.4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman A. D., Landschulz W. H., McKnight S. L. CCAAT/enhancer binding protein activates the promoter of the serum albumin gene in cultured hepatoma cells. Genes Dev. 1989 Sep;3(9):1314–1322. doi: 10.1101/gad.3.9.1314. [DOI] [PubMed] [Google Scholar]
- Godbout R., Ingram R. S., Tilghman S. M. Fine-structure mapping of the three mouse alpha-fetoprotein gene enhancers. Mol Cell Biol. 1988 Mar;8(3):1169–1178. doi: 10.1128/mcb.8.3.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregori C., Kahn A., Pichard A. L. Competition between transcription factors HNF1 and HNF3, and alternative cell-specific activation by DBP and C/EBP contribute to the regulation of the liver-specific aldolase B promoter. Nucleic Acids Res. 1993 Feb 25;21(4):897–903. doi: 10.1093/nar/21.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronostajski R. M. Analysis of nuclear factor I binding to DNA using degenerate oligonucleotides. Nucleic Acids Res. 1986 Nov 25;14(22):9117–9132. doi: 10.1093/nar/14.22.9117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruss C., Sogo J. M. Chromatin replication. Bioessays. 1992 Jan;14(1):1–8. doi: 10.1002/bies.950140102. [DOI] [PubMed] [Google Scholar]
- Guertin M., LaRue H., Bernier D., Wrange O., Chevrette M., Gingras M. C., Bélanger L. Enhancer and promoter elements directing activation and glucocorticoid repression of the alpha 1-fetoprotein gene in hepatocytes. Mol Cell Biol. 1988 Apr;8(4):1398–1407. doi: 10.1128/mcb.8.4.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heard J. M., Herbomel P., Ott M. O., Mottura-Rollier A., Weiss M., Yaniv M. Determinants of rat albumin promoter tissue specificity analyzed by an improved transient expression system. Mol Cell Biol. 1987 Jul;7(7):2425–2434. doi: 10.1128/mcb.7.7.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson D. A., Rowader K. E., Stevens K., Jiang C., Milos P., Zaret K. S. Modulation of liver-specific transcription by interactions between hepatocyte nuclear factor 3 and nuclear factor 1 binding DNA in close apposition. Mol Cell Biol. 1993 Apr;13(4):2401–2410. doi: 10.1128/mcb.13.4.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jose-Estanyol M., Danan J. L. A liver-specific factor and nuclear factor I bind to the rat alpha-fetoprotein promoter. J Biol Chem. 1988 Aug 5;263(22):10865–10871. [PubMed] [Google Scholar]
- Jose-Estanyol M., Poliard A., Foiret D., Danan J. L. A common liver-specific factor binds to the rat albumin and alpha-foetoprotein promoters in vitro and acts as a positive trans-acting factor in vivo. Eur J Biochem. 1989 May 15;181(3):761–766. doi: 10.1111/j.1432-1033.1989.tb14789.x. [DOI] [PubMed] [Google Scholar]
- Kuo C. J., Mendel D. B., Hansen L. P., Crabtree G. R. Independent regulation of HNF-1 alpha and HNF-1 beta by retinoic acid in F9 teratocarcinoma cells. EMBO J. 1991 Aug;10(8):2231–2236. doi: 10.1002/j.1460-2075.1991.tb07759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai E., Darnell J. E., Jr Transcriptional control in hepatocytes: a window on development. Trends Biochem Sci. 1991 Nov;16(11):427–430. doi: 10.1016/0968-0004(91)90169-v. [DOI] [PubMed] [Google Scholar]
- Landschulz W. H., Johnson P. F., Adashi E. Y., Graves B. J., McKnight S. L. Isolation of a recombinant copy of the gene encoding C/EBP. Genes Dev. 1988 Jul;2(7):786–800. doi: 10.1101/gad.2.7.786. [DOI] [PubMed] [Google Scholar]
- Luckow B., Schütz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res. 1987 Jul 10;15(13):5490–5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVey J. H., Michaelides K., Hansen L. P., Ferguson-Smith M., Tilghman S., Krumlauf R., Tuddenham E. G. A G-->A substitution in an HNF I binding site in the human alpha-fetoprotein gene is associated with hereditary persistence of alpha-fetoprotein (HPAFP). Hum Mol Genet. 1993 Apr;2(4):379–384. doi: 10.1093/hmg/2.4.379. [DOI] [PubMed] [Google Scholar]
- Moorman A. F., De Boer P. A., Evans D., Charles R., Lamers W. H. Expression patterns of mRNAs for alpha-fetoprotein and albumin in the developing rat: the ontogenesis of hepatocyte heterogeneity. Histochem J. 1990 Dec;22(12):653–660. doi: 10.1007/BF01047449. [DOI] [PubMed] [Google Scholar]
- Mueller C. R., Maire P., Schibler U. DBP, a liver-enriched transcriptional activator, is expressed late in ontogeny and its tissue specificity is determined posttranscriptionally. Cell. 1990 Apr 20;61(2):279–291. doi: 10.1016/0092-8674(90)90808-r. [DOI] [PubMed] [Google Scholar]
- Nahon J. L., Danan J. L., Poiret M., Tratner I., Jose-Estanyol M., Sala-Trepat J. M. The rat alpha-fetoprotein and albumin genes. Transcriptional control and comparison of the sequence organization and promoter region. J Biol Chem. 1987 Sep 15;262(26):12479–12487. [PubMed] [Google Scholar]
- Nahon J. L. The regulation of albumin and alpha-fetoprotein gene expression in mammals. Biochimie. 1987 May;69(5):445–459. doi: 10.1016/0300-9084(87)90082-4. [DOI] [PubMed] [Google Scholar]
- Paonessa G., Gounari F., Frank R., Cortese R. Purification of a NF1-like DNA-binding protein from rat liver and cloning of the corresponding cDNA. EMBO J. 1988 Oct;7(10):3115–3123. doi: 10.1002/j.1460-2075.1988.tb03178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliard A., Bakkali L., Poiret M., Foiret D., Danan J. L. Regulation of the rat alpha-fetoprotein gene expression in liver. Both the promoter region and an enhancer element are liver-specific and negatively modulated by dexamethasone. J Biol Chem. 1990 Feb 5;265(4):2137–2141. [PubMed] [Google Scholar]
- Sala-Trepat J. M., Dever J., Sargent T. D., Thomas K., Sell S., Bonner J. Changes in expression of albumin and alpha-fetoprotein genes during rat liver development and neoplasia. Biochemistry. 1979 May 29;18(11):2167–2178. doi: 10.1021/bi00578a006. [DOI] [PubMed] [Google Scholar]
- Schild C., Claret F. X., Wahli W., Wolffe A. P. A nucleosome-dependent static loop potentiates estrogen-regulated transcription from the Xenopus vitellogenin B1 promoter in vitro. EMBO J. 1993 Feb;12(2):423–433. doi: 10.1002/j.1460-2075.1993.tb05674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seed B., Sheen J. Y. A simple phase-extraction assay for chloramphenicol acyltransferase activity. Gene. 1988 Jul 30;67(2):271–277. doi: 10.1016/0378-1119(88)90403-9. [DOI] [PubMed] [Google Scholar]
- Sell S., Becker F. F. alpha-Fetoprotein. J Natl Cancer Inst. 1978 Jan;60(1):19–26. doi: 10.1093/jnci/60.1.19. [DOI] [PubMed] [Google Scholar]
- Thomassin H., Hamel D., Bernier D., Guertin M., Belanger L. Molecular cloning of two C/EBP-related proteins that bind to the promoter and the enhancer of the alpha 1-fetoprotein gene. Further analysis of C/EBP beta and C/EBP gamma. Nucleic Acids Res. 1992 Jun 25;20(12):3091–3098. doi: 10.1093/nar/20.12.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacher J., Tilghman S. M. Dominant negative regulation of the mouse alpha-fetoprotein gene in adult liver. Science. 1990 Dec 21;250(4988):1732–1735. doi: 10.1126/science.1702902. [DOI] [PubMed] [Google Scholar]
- Watanabe K., Saito A., Tamaoki T. Cell-specific enhancer activity in a far upstream region of the human alpha-fetoprotein gene. J Biol Chem. 1987 Apr 5;262(10):4812–4818. [PubMed] [Google Scholar]
- Wen P., Groupp E. R., Buzard G., Crawford N., Locker J. Enhancer, repressor, and promoter specificities combine to regulate the rat alpha-fetoprotein gene. DNA Cell Biol. 1991 Sep;10(7):525–536. doi: 10.1089/dna.1991.10.525. [DOI] [PubMed] [Google Scholar]
- Zhang D. E., Ge X., Rabek J. P., Papaconstantinou J. Functional analysis of the trans-acting factor binding sites of the mouse alpha-fetoprotein proximal promoter by site-directed mutagenesis. J Biol Chem. 1991 Nov 5;266(31):21179–21185. [PubMed] [Google Scholar]




