Abstract
We investigated the host range of Cassytha filiformis L. in the heath forests using six 50-metre transects. Sixteen shrubs and tree species were infected by C. filiformis vines, including two exotic Acacia species. This paper also examined the density and vigour of C. filiformis when infecting the two most preferred and common hosts, the heath native Dillenia suffruticosa (Griff. ex Hook. f. and Thomson) Martelli, and the invasive Acacia mangium Willd. The results suggested that C. filiformis has higher vigour when infecting native hosts than in exotic A. mangium albeit being not statistically significant. The long thread-like stems of parasite were present at relatively high density when infecting A. mangium, regardless of the host conditions. We also assessed the functionality of the haustoria on both D. suffruticosa and A. mangium using histological methods. It was found that C. filiformis can establish a true haustorial endophytic connection with studied hosts. Under controlled conditions, C. filiformis pose as a possible candidate for a biological control agent of A. mangium to curtail the fast spreading of this introduced species in tropical Borneo.
Keywords: Cassytha filiformis, Hemiparasites, Heath Forests, Haustoria, Host Selectivity
Highlights.
The investigation of the host selectivity of Cassytha filiformis in the heath forests using six 50-meter transects revealed that sixteen shrubs and tree species were infected by the parasitic vines, including two exotic Acacia species.
C. filiformis exhibited higher vigour when infecting native hosts compared to exotic A. mangium and demonstrated relatively high density when infecting A. mangium, irrespective of host conditions.
Using histological methods, C. filiformis can establish a true haustorial endophytic connection with A. mangium and D. suffruticosa.
INTRODUCTION
Throughout the course of evolutionary transitions, about 1% of angiosperms (Westwood et al. 2010) have adapted parasitism by acquiring resources from other plants via specialised organs of a morphological and physiological function called haustoria (Kuijt 1969; Yoshida et al. 2016; Teixeira-Costa & Davis 2021). Parasitic plants are often categorised by the extent of their host dependence (Heide-Jørgensen 2008). Facultative parasites are known to survive without a host for a certain period but would obtain their supply of water and/or nutrients when the opportunity arises. Alternatively, there are those that must require a host to live which are referred to as obligate parasitic plants. These plants are also recognised for their ability to photosynthesise (hemiparasites) or entirely non-chlorophyllous (holoparasites) (Musselman & Press 1995).
In terms of host preference, except for a few other specialists, most parasitic plants have a broad host range, especially when occurring in their natural habitat (Nickrent 2002). However, host specificity and the choice of hosts to infect ultimately depend on its accessibility and ability to locate hosts by selectively spreading towards or away from hosts, or by selectively penetrating host tissues upon contact through haustoria (Callaway & Pennings 1998; Runyon et al. 2006; Marquardt & Pennings 2010; Facelli et al. 2020).
Cassytha of the subfamily Cassythoideae is the only parasitic genus in the Lauraceae family (Awang et al. 2018). Cassytha filiformis Mill. is the sole pantropical species with wide global distribution in Asia, Africa, and tropical and subtropical America (Sastri 1962). It is a perennial hemiparasitic vine that infects its hosts by attaching to their stems. The generalist Cassytha has a relatively large and well-documented host range (Zhang et al. 2022). Despite the availability of hosts in the field, the obligate C. filiformis strands are often seen parasitising on only certain host species thus demonstrating the parasites’ preferential behaviour as highlighted by Koch et al. (2004) and Facelli et al. (2020). A common trait among generalists, the varying level of infection is also an indication of the mechanism of either active parasitism or a possible resistance on hosts (Kelly 1992) which could be examined by investigating the host stem histology and its anatomical response to the penetrating haustoria (Zhang et al. 2022). For instance, in a study by Facelli et al. (2020), Acacia myrtifolia was reported to exhibit resistance against the infection of Cassytha pubescens despite the presence of a firmly attached haustorium due to the lack of developed vascular connections. Under histological methods, the thickening cortical tissue of native species A. myrtifolia was observed thus preventing the parasite from forming true functional haustoria.
Cassytha are often seen along the coasts, sprawling on various host species at beaches around the world (Furuhashi et al. 2016). This is also a typical occurrence in Brunei where C. filiformis is abundant along the coasts (Rosli 2014; Tennakoon et al. 2016). Other than the preliminary list of hosts from an opportunistic field survey by Tennakoon et al. (2016), the study of host specificity in C. filiformis is lacking in Southeast Asia.
Despite accounting for 1% of the country’s forest cover, most of tropical Brunei’s coastlines are covered by a characteristic forest type known as heath forest. Bornean heath forests, locally referred to as Kerangas which means an area where rice cannot grow in the native Iban language (Davies & Salim 1999; Jambul et al. 2020), are mainly attributed to the highly acidic and low nutrient soils, and often inhabited by plant species with unique adaptive features (Newbery 1991; Wong et al. 2015; Hattori et al. 2019).
Tropical heath forests, especially those in Borneo, are susceptible to drastic environmental changes and anthropogenic activities (Din et al. 2015; Jambul et al. 2020). Similarly in Brunei, drastic changes in the ecosystem in the last 30 years have altered the soil properties causing this unique forest to be sensitive to degradation, fire, and habitat fragmentation (Zoletto & Cicuzza 2022). This is further exacerbated by the subsequent growth of the invasive and exotic Acacia species (Jaafar et al. 2016; Tuah et al. 2020) resulting in the secondary development of the now-threatened tropical heath (Kerangas) forest.
Much of the current host, C. filiformis studies looked into areas of its bioactivity (e.g., Abubacker et al. 2005; Armenia et al. 2015; Agbodjento et al. 2020; Umedum et al. 2020), physiology (e.g., Mukhtar et al. 2010; Mahadevan & Jayasuriya 2013; Balasubramanian et al. 2014; Furuhashi et al. 2021) and phylogeny (Wu et al. 2017; Zhang et al. 2020), while there are only few that discussed the effect of the stem hemiparasite on different hosts of a particular ecosystem (Kokubugata & Yokota 2012; Prider et al. 2009; Cai et al. 2020).
We present the first study on the host selectivity of C. filiformis in the threatened tropical Bornean heath forests. We examined:
Host range parasitised by C. filiformis using the transect method.
The impact of infection on hosts’ vigour relative to the density and vigour of the hemiparasite stem strands.
The anatomy of the haustorial interface of selected hosts to determine its functionality.
MATERIALS AND METHODS
Study Site
The study was conducted in the secondary heath (Kerangas) forests along the coastal highway (from 4°57’59.99°N, 114°52’33.531°E to 4°59’6.22°N, 114°54’1.472°E), within ca. 5 km off the coast of Brunei Darussalam from July to August 2021. Heath forests in Borneo are characterised by aseasonal lowland tropical rainforests that develop predominately on podzolised, highly acidic, sandy soils with relatively low macronutrients (Ghazoul & Sheil 2010; Jaafar et al. 2016; Ibrahim et al. 2020). Brunei has a tropical equatorial climate with average temperatures of 25.5°C and 28.9°C during the night and day throughout the year and total rainfall of 3815.1 mm in 2021 (Brunei Meteorological Service, unpublished data).
In the study area, the secondary heath forests are inhabited by a co-occurring composition of native species, such as Buchanania arborescens, Callophyllum inophyllum, Dillenia suffruticosa, Elaeocarpus mastersii, Melastoma malabathricum and Ploiarium alternifolium, and the invasive species of Acacia mangium, A. auriculiformis and A. holosericea (Tuah et al. 2020). C. filiformis are also observed infecting certain host plants. These species exist within the vicinities of settlements and urban developments (Fig. 1; see also Jambul et al. 2020).
Figure 1.
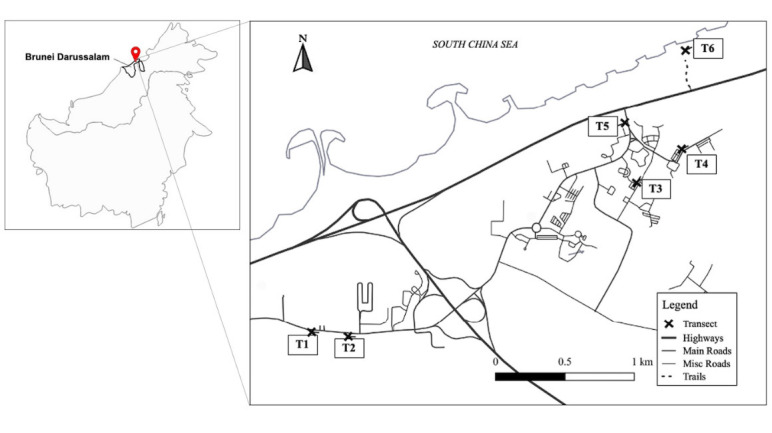
Map of Borneo Island (left) and the locations of the six transect surveys (T1 to T6) within the coastal heath forests of Brunei Darussalam (right).
Field Transect Survey
We established six 3 m × 50 m belt transects in July 2021 with ca. 0.5 m–1.0 m from the edge of the tropical heath forests. Within each transect, every individual of woody dicot species (i.e., shrubs and trees) with a height ca. 0.5 m and taller was recorded as “frequency of observation”, based on the methods employed by Kokubugata and Yokota (2012). The voucher specimens of the observed plants within the transect areas were collected for identification and confirmation at the Brunei National Herbarium (BRUN). Voucher specimens were deposited in the Universiti Brunei Darussalam Herbarium. To study the impact of infections on the two host plants with the highest frequencies of observations within these transects were selected, which are Acacia mangium Willd and Dillenia suffruticosa (Griff. ex Hook. f. and Thomson) Martelli.
Acacia mangium Willd. (hereafter Acacia) is a fast-growing leguminous tree species native to Australia and was introduced to Brunei in the late 1980s to mitigate soil erosion and as a timber plantation tree species (Osunkoya & Damit 2005; Ismail & Metali 2014; Jambul et al. 2020). It was then learnt that Acacia trees thrive in disturbed heath forests, especially since their seed dormancy is well-adapted to the recurring fire episodes and possesses the ability to fix nitrogen directly from the atmosphere (Jambul et al. 2020; Tuah et al. 2020). Osunkoya and Damit (2005) reported that Acacia could easily outcompete native plants such as Melastoma beccarianum under disturbed and degraded conditions, which eventually transform these habitats into nearly monospecific stands. Dillenia suffruticosa (Griff. ex Hook. f. and Thomson) Martelli. (hereafter Dillenia) is an important native pioneer shrub that may significantly impact the secondary succession of tropical forests (Rosli 2014). It is commonly distributed in disturbed areas, especially along roadsides and forest edges. Laboratory investigations have shown that Dillenia has anti-fungal, anti-bacterial and anti-cancer properties (Muliawan 2008; Armania et al. 2013; Goh et al. 2017).
The visual assessment of the host plants’ vigour and Cassytha cover were classified according to Prider et al. (2009). The vigour of Cassytha on each shrub was scored as “high” (actively growing, green stems), “low” (stems are partly dead and no active growth visible) or “dead” (no green stems). In our investigation, Cassytha cover was qualitatively scored as low, medium, high, and very high density. Low density infections covered <25% of the host where Cassytha was usually present as a few stems only, and medium density infections covered <50% of the host plant. High density infections covered <75% of the host, with Cassytha growing in entwined auto-parasitising strands to dense coiling mats. Very high density of Cassytha entailed the host plant being almost completely shrouded by the parasite, which can seem to deprive the hosts of sunlight.
Hosts’ growth condition or vigour was qualitatively scored as good, fair, poor and dead. “Good” hosts are when more than 90% of the individual plant is still alive where all or most of the leaves are green and intact. “Fair” host plants are 50% to 90% alive where some stems or leaves of hosts are dead or discoloured. Host plants that are mostly (<50%) dead or discoloured are scored as “poor”. Hosts are considered “dead” when all leaves are dead or discoloured. Cassytha infection was scored as present only when haustoria were observed on the plants within the transect areas. Chi-squared tests for independence were used to determine if there was a significant association between: (1) Cassytha vigour (i.e., High, Low, Dead) and its hosts; (2) Cassytha density (i.e., Very High, High, Medium, Low) and its hosts; (3) Cassytha density and the conditions of Acacia (i.e. Good, Fair, Poor, Dead); and (4) Cassytha density and the conditions of Dillenia (i.e., Good, Fair, Poor, Dead). Statistical analysis was conducted using R statistical programme version 4.1.3 RStudio (R Core Team 2022).
Haustorial Anatomy
Developing and attached mature haustoria on the selected hosts, Acacia and Dillenia, were fixed in an ethanol and xylene series as described in Tennakoon and Cameron (2006) and embedded in wax blocks with the haustorial interface arranged longitudinally. Using a microtome (Shandon Finesse ME+ Thermo Electron Corporation, Cheshire, UK), 10 μm–20 μm thick sections were prepared and placed onto glass slides. The thickness of the sections was based on the hardness of the host stems. Young and soft host stems were preferable in this experiment to ease the microtome process. Waxed sections were de-waxed and rehydrated prior to staining with 1% Toluidine Blue. Histological sections were examined under a light microscope (Leica DM2500 Microsystems CMS GmbH, Wetzlar, Germany). Images were acquired using a digital camera (Olympus DP73, Tokyo, Japan) using CellSens imaging software (Version 1.9, Olympus, Tokyo, Japan).
RESULTS
Host-Parasite Associations
A total of 336 individual dicotyledonous plants (see Appendix) were sampled from the six transect areas, where 99 individuals (29.5%) were found infected (Table 1). A total of 17 species from 16 genera and 15 families were recorded as host species. Buchanania arborescens, Dillenia suffruticosa, Elaeocarpus aff. mastersii, Nepenthes gracilis, Pouteria obovata, Psychotria sarmentosa, Rhodomyrtus tormentosa and Timonius flavescens were the native heath or Kerangas species identified (Coode et al. 1996; Tuah et al. 2020). Two invasive, introduced plant species, Acacia mangium and Acacia auriculiformis, were common and frequently observed within the study sites. Other than these two, the host species in Table 1 are native to Brunei (Coode et al. 1996; Zamri & Slik 2018; Tuah et al. 2020) and they are common to secondary forests of Brunei (Coode et al. 1996).
Table 1.
Summary of host plants from the six 3 m × 50 m transect surveys. The family and species names are arranged according to the frequencies of observations.
| Host plants | Frequency of observationa | |
|---|---|---|
|
| ||
| Family | Species | |
| Dilleniaceae | Dillenia suffruticosa (Griff. ex Hook.f. and Thomson) Martellib | 25 |
| Fabaceae | Acacia mangium Willdc | 19 |
| Fabaceae | Acacia auriculiformis A. Cunn. ex Benthc | 16 |
| Melastomataceae | Melastoma malabathricum L. | 10 |
| Euphorbiaceae | Endospermum diadenum (Miq.) Airy Shaw | 5 |
| Nepenthaceae | Nepenthes gracilis Korth.b | 5 |
| Lamiaceae | Vitex pinnata L. | 4 |
| Elaeocarpaceae | Elaeocarpus aff. mastersii Kingb | 3 |
| Anacardiaceae | Buchanania arborescens (Blume) Blumeb | 2 |
| Malvaceae | Commersonia batramia (L.) Merr. | 2 |
| Rubiaceae | Timonius flavescens (Jacq.) Bakerb | 2 |
| Casuarinaceae | Casuarina equisetifolia L. | 1 |
| Euphorbiaceae | Macaranga tanarius (L.) Müll.Arg. | 1 |
| Myrtaceae | Rhodomyrtus tomentosa (Aiton) Hassk.b | 1 |
| Myrtaceae | Syzygium acuminatissimum (Blume) DC.b | 1 |
| Rubiaceae | Psychotria sarmentosa Blumeb | 1 |
| Sapotaceae | Pouteria obovata (R.Br.) Baehnib | 1 |
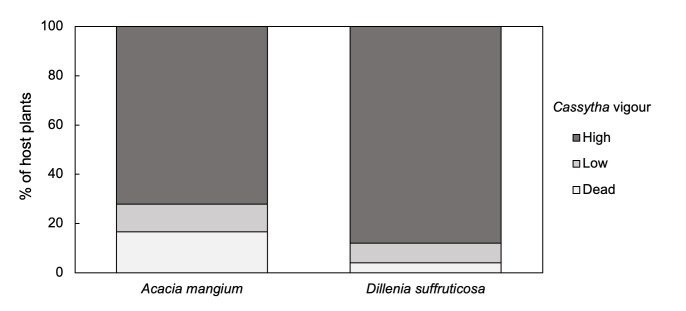
Dillenia and Acacia were the two host plants with the highest frequency of observations (Table 1) and were selected to assess the in-situ effect of the Cassytha infection. The vigour of Cassytha while infecting the selected hosts was assessed in Fig. 2. Cassytha stems had higher vigour, i.e., better health while infecting the native Dillenia than that in Acacia, with more than 80% growing healthily in the former. It was found that there was higher mortality in Cassytha when infecting Acacia (16.7%) than that with Dillenia (4.0%). Chi-squared test was used to determine if there was a significant association between Cassytha vigour and the hosts. There was not a statistically significant association between the two variables (χ2(2, N = 43) = 2.24, p = 0.32).
Figure 2.
Impact of increasing Cassytha filiformis vigour on the two host species Acacia mangium and Dillenia suffruticosa. The vigour of Cassytha on each shrub was scored as “high” (actively growing, green stems), “low” (stems are partly dead and no active growth visible) or “dead” (no green stems).
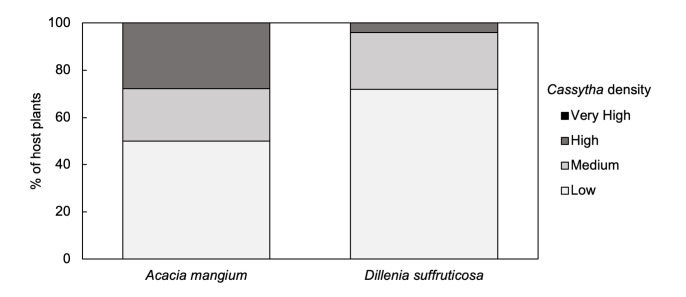
The percentage of both host plants infected by various Cassytha densities is represented in Fig. 3. Despite the healthy growth of Cassytha on Dillenia, there was a higher infection density in the introduced species, with 27.8% and 22.2% of Acacia infected by high density and medium density of Cassytha, respectively. About 72% of Dillenia were infected by low density of Cassytha. None of the Dillenia and Acacia were infected by the very high density of Cassytha. Chi-squared test was also used to determine if there was a significant association between the increasing Cassytha density and the hosts. There was not a statistically significant association between the two variables (χ2 (2, N = 43) = 5.06, p = 0.08).
Figure 3.
Impact of increasing C. filiformis densities on the two host species A. mangium and D. suffruticosa. Cassytha cover was qualitatively scored as low, medium, high and very high density. Low density infections covered <25% of the host where Cassytha was usually present as a few stems only, and medium density infections covered <50% of the host plant. High density infections covered <75% of the host, with Cassytha growing in entwined auto-parasitising strands to dense coiling mats. Very high density of Cassytha entailed the host plant being almost completely covered by the parasite.
Fig. 4 illustrates the health conditions or vigour of Acacia mangium and Dillenia suffruticosa with respect to the density of C. filiformis infection. In general, the virulence of Cassytha was high when host plants were healthy. However, the hemiparasite did not parasitise on Dillenia of lower vigour. Their preference was rather indifferent when infecting the introduced species where “poor” Acacia plants were parasitised by Cassytha. Chi-squared test was run to determine if there was a significant association between the increasing Cassytha densities and the growth conditions of hosts. There were no statistically significant associations between the two variables for both Acacia and Dillenia i.e., (χ2 (6, N = 94) = 11.69, p = 0.07) and (χ2 (3, N = 75) = 4.78, p = 0.19), respectively.
Figure 4.
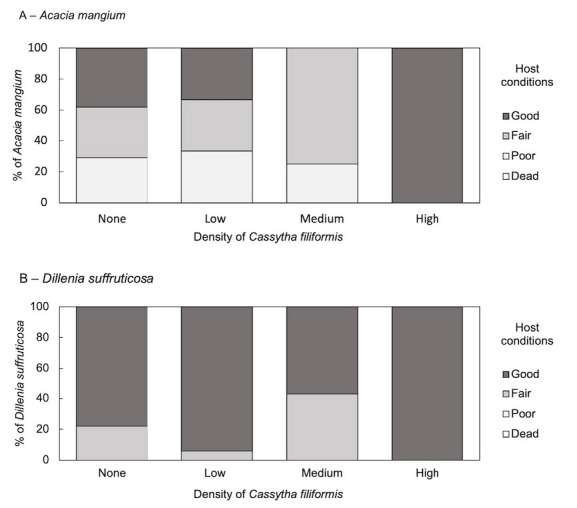
Frequency histograms of the proportions of: (A) A. mangium, and (B) D. suffruticosa, in different growth conditions when infected by C. filiformis of increasing density levels. Hosts’ growth condition or vigour was qualitatively scored as good, fair, poor and dead. “Good” hosts are when more than 90% of the individual plant is still alive where all or most of the leaves are green and intact. “Fair” host plants are 50% to 90% alive where some stem or leaves of hosts are dead or discoloured. Host plants that are mostly (<50%) dead or discoloured are scored as “poor”. Hosts are considered “dead” when all leaves are dead or discoloured.
Histology of Haustoria Formation
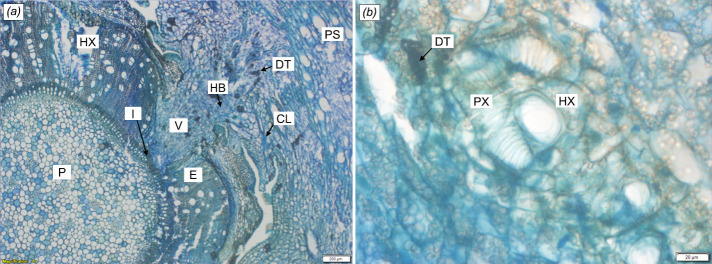
Sections that were complete (intact) and best represent the behaviour of the haustoria are presented in Figs. 5 and 6. The haustorial endophytes had successfully reached and penetrated the vasculature of the host stems of D. suffruticosa (Fig. 5). The haustoria of C. filiformis appeared to grow into the host tissue in a wedge-like shape endophyte (E), mostly in direct contact with the host xylem (HX) (Fig. 5a). The presence of vascular core (V) was visible in middle section of the endophyte with the relatively high observation of xylem tissues. Another section of the same host – parasite (PX) association has shown direct luminal contact (Fig. 5b, PX, HX) with host xylem within the vascular core of the haustoria. Few cells of the endophytes were darkly stained, thus creating dense tissue (DT) in Fig. 5a. High nucleic-structures of hyaline body (HB) were present in the endophyte.
Figure 5.
Detailed anatomy of the haustorial interface of Cassytha filiformis with Dillenia suffruticosa at (a) ×4 magnification, and (b) at ×40 magnification, highlighting direct lumen-to-lumen xylem connections between the xylems of the host (HX) and parasite (PX). H = haustoria; P = host stem pith; PS = parasite stem; E = endophyte; HX = host xylem; PX = parasite xylem; I = interface between host and parasite; V = vascular core; DT = darkly stained tissue; CL = collapsed layer; HB = hyaline body.
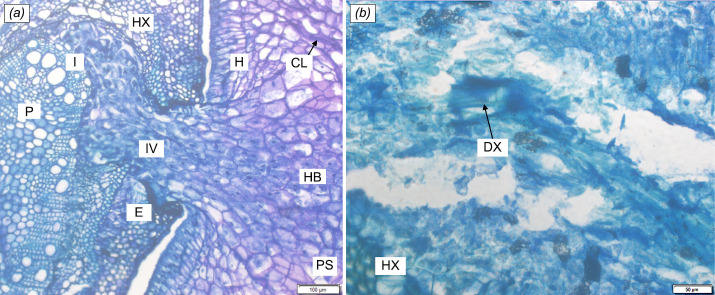
Figure 6.
Detailed anatomy of the haustorial interface of Cassytha filiformis with Acacia mangium at (a) ×10 magnification (b) ×20 magnification, particularly a section of the haustorial endophyte. H = haustoria; P = host stem pith; PS = parasite stem; DX = differentiated xylem; E = endophyte; HX = host xylem; I = interface between host and parasite; IV = initial vascular core formation; CL = collapsed layer; HB = hyaline body.
As with the haustoria of C. filiformis on A. mangium in Fig. 6, the endophyte seemed to have spread around the host vascular structure creating an ellipsoidal flattened disc increasing the surface area of contact (Fig. 6a, I). The mass differentiating parenchyma cells running through the middle section of the endophyte indicate the initial development of the vascular core (Fig. 6a, IV). While the initiation of vascular core is yet to be present in Fig. 6a, differentiated xylem (DX) within the endophyte is evident in a different histological section (Fig. 6b). The presence of HB is also visible. The wedge-like endophytic growth of the parasite within the host tissue is also observed in other haustorial sections. This may be due to the relative thickness of D. suffruticosa stems, i.e., ca. 1.5 cm in comparison to the stems of A. mangium (ca. 0.5 cm–1.0 cm).
DISCUSSION
This study has shown the wide host specificity range of the hemiparasitic C. filiformis, thus exhibiting its generalist nature. This is evident in their unselective behaviour in infecting various host species, including the previously unrecorded grasses and fern species. The two most common host species for C. filiformis were A. mangium and D. suffruticosa. Although the parasite showed a slight preference for Dillenia, Cassytha thrives to high densities on both Acacia and Dillenia.
The results also demonstrated that under very high Cassytha density, a good Acacia stand exists. This may be because of the in-situ nature of the study where the age of the infection was not considered. The healthy Acacia was perhaps just newly infected, and the negative physiological effect of the infection was not apparent yet. Since every individual plant of height ca. 0.5 m and taller was recorded for this investigation, the age of the host plants is also highly variable. This potentially affects how the hosts respond to the parasitic infection. Nonetheless, host susceptibility to infection and the virulence of the parasite were greater in the introduced host than in the native host. This is a similar pattern observed in the parasitism of Cassytha pubescens on Leptospermum myrsinoides and Cytisus scoparius, a native and introduced species to Australia (Prider et al. 2009).
The soils of the threatened Kerangas forest are high in nitrogen and have always been negatively affected by Acacia trees which are invasive nitrogen-fixing legumes in Brunei (Tuah et al. 2020). A study by Yusoff et al. (2019) reported that there was a significantly higher concentration of total N in leaf litters in an Acacia-invaded Kerangas forest, suggesting that the invasive Acacia has further decreased the naturally poor soil nutrients. Non-fixers parasitic plants are likely to infect nitrogen-fixing hosts (Press et al. 1993; Seel & Press 1993) because hosts with high nutrient content such as legumes are often preferred (Matthies 1996; Pate & Bell 2000; Pennings & Callaway 2002) thus making Acacia spp. the highly favoured candidates as hosts. Recent nutrient studies on Cassytha hosts by Rosli et al. (unpublished data) have shown that D. suffruticosa has a similar amount of total N content (14.12 mg/g) to that of A. mangium (14.71 mg/g). The total N content of D. suffruticosa was also found to be higher than in the native pioneer, Melastoma malabathricum (12.59 mg/g).
The preference for hosts with relatively high nitrogen content is attributed to the lack of means to perform active uptake of such nutrients. Thus, hemiparasites like Cassytha opt to take organic nitrogen and other mineral nutrients that are diverted from the host xylem sap via the haustoria, to promote growth and increase their own biomass. Another reason for Cassytha’s acquisition of host-derived organic nitrogen elements is that they potentially lack the symbioses for nitrogenase enzyme production which is essential in biological nitrogen fixation. However, this assumption warrants further confirmation.
It is imperative to note that nitrogen-rich plants have reduced growth performance and are more vulnerable to parasitic infections which can further impair their stressed conditions (Kelly 1992; Gehring & Whitham 1992; Jeschke et al. 1994; Matthies 1996; Jeschke & Hilpert 1997; Pennings & Simpson 2008). Once infected, the host performance worsens as parasites thrive with the nutrients obtained from the hosts (Prider et al. 2009). It is evident in this study where C. filiformis also infected A. mangium at “poor” condition.
Bioactivity compounds attributed to the host-parasite dynamics also play a role in host specificity, specifically in the attachment process. The induction of chemical molecular signals, germination stimulants and haustoria-inducing factors are some examples of the products (Okubamichael et al. 2011; Yoshida et al. 2016). However, further investigations involving studies of bioactive compounds are required to confirm this potential cause.
Studies on host preference also reported that there are plant traits that appeared to be manipulated to demonstrate that they directly affect parasite preferences or performance (Kelly 1992; Pennings & Simpson 2008; Marquardt & Pennings 2010). This may account for Dillenia being one of the highest infected host plants in this study. This is also evident in the high percentage of Dillenia infection by a low density of Cassytha. C. filiformis are reported to prefer woody host plants with soft, thin barks and periderm and those with low and much-branched (Werth et al. 1979; Buriyo et al. 2015); both physical traits that are present in Dillenia as a pioneer, woody shrub that tends to grow in dense thickets. This indicates that Dillenia presents as a more accessible host to Cassytha by acquiring the required metabolites without investing much effort in heavy infestations.
Another possible explanation for the preferential behaviour may be attributed to the availability of more suitable resources which they acquire through the direct lumen-to-lumen linkages of the endophytes of A. mangium and D. suffruticosa: Cassytha associations. Through light microscopy investigations, this study was able to demonstrate such connections in the Dillenia-Cassytha association. This could not be captured in the Acacia-Cassytha sections, despite the proximity of the endophyte to the host vascular structure and the presence of the differentiating xylems. Thus, to further confirm this observation, we suggest utilising fluorescent trackers to the host root or sampling the xylem and phloem of the host and parasite and comparing the solute compositions (i.e., sugars, organic acids or amino acids) via isotope labelling (Tennakoon et al. 1997; Hibberd & Jeschke 2001; Jiang 2004; Tennakoon & Cameron 2006; Facelli et al. 2020).
Host tolerance to Cassytha infection may contribute to the reduced impacts of the parasites (Prider et al. 2009), however resistance was not observed in this study since no cases of pseudo-haustorial connections were encountered. It is also important to note that the field survey conducted in this in situ study did not determine if Cassytha was also connected to other surrounding hosts that could have been supporting its growth.
The outcomes of this study suggest that C. filiformis is indifferent to the hosts they parasitise, irrespective of whether hosts are native or exotic hosts. This confirms that generalist parasites are able to infect hosts which have not co-evolve to adopt a resistance or defence strategy (Koch et al. 2004; Cirocco et al. 2016). However, based on the results which highlight the higher density of C. filiformis on the invasive A. mangium, C. filiformis could be considered an important biological controlling agent under well-controlled conditions to reduce further spread of alien invasive A. mangium in tropical Southeast Asia. This concurs with the biotic resistance hypothesis where parasitic plants may be candidates for “a cost-effective environmentally sustainable component of invasion management scheme” (Těšitel et al. 2020). Generally, species that are used for biological control have high host specificity to ensure that only the targeted species is affected by the introduction of the species into a system (Myers & Bazely 2003). In the case of the secondary heath forest, most tree stands consist of the fast-growing Acacia species, and those infected are often in poor conditions based on field observations.
The next question should investigate this parasitism’s effects on the hosts’ physiology. The physiological impacts of parasites on invasive species have a greater effect on host health, biomass, and fecundity than on native hosts (Prider et al. 2009; Cirocco et al. 2016; 2018). Physiological studies such as photosynthetic activities and nutrient analysis on this host-parasitic association would be able to explain the extent of the impact of parasitism on these hosts within this unique site.
CONCLUSION
C. filiformis exhibited low host-specificity with its wide range of hosts, irrespective of their nativity to the tropical heath habitat. This is illustrated in the well-established haustorial structures in both A. mangium and D. suffruticosa. However, employing better histological techniques, such as scanning electron microscopy (SEM), may illustrate detailed anatomical evidence to prove successful haustorial connections. Previous studies conducted on C. filiformis in Brunei suggested that the hemiparasitic vine has the potential to act as a biocontrol agent against invasive species. The outcome of this investigation has shown that even with high Cassytha vigour, infected hosts can still thrive and did not specifically fulfil the previous statement. This could also be a possible inkling of a co-existing behaviour of Cassytha to certain hosts. This would entail an intricate look at resistance genes in the host genomes. However, in the existing state of the heath forests in Brunei where natives are threatened to be outcompeted by the monodominant A. mangium, C. filiformis is a good candidate for a potential biocontrol agent. This is feasible under controlled conditions by careful monitoring and ensuring that the spread of the hemiparasitic vines is limited to the invasive Acacia species only.
Furthermore, there are several determining factors and experimental modifications to this study that could be included to further test the impact of Cassytha infection on these hosts such as host biomass and the environmental conditions, for example, ex-situ and greenhouse experiments where the growth of the parasites and their hosts are monitored. Nonetheless, the findings also indicate that Cassytha can still be used to reduce the spread of exotic weeds and invasive plants.
ACKNOWLEDGEMENTS
This study was funded by Universiti Brunei Darussalam’s research grants (UBD/RSCH/1.13/FICBF(b)/2020/025 and IBER/RP22/MG/2020/007). The authors thank the Brunei Darussalam Meteorological Department, Ministry of Transport and Infocommunications for weather data, the Brunei National Herbarium (BRUN) for the identification of host species, and the Forestry Department, Ministry of Primary Resources and Tourism, Brunei Darussalam for entry and collection permits ([99]/JPH/UDN/17PT.1). We are grateful to the Environmental and Life Sciences of Faculty of Science, UBD, the Institute for Biodiversity and Environmental Research, and the Botanical Research Centre (BRC) for their support in this research. We thank Abdul Muiz Hj Abdul Razak, Nurul ‘Aqilah Assyahirah Hj Abd Aziz, Amanda Liew Wei Yi, Ak Abd Qayyum Pg Zamanulalam Omarali Mohamed and Amira binti Hj Abd Rashid for their assistance with field and lab work.
APPENDIX
List of 336 dicotyledonous individual plants that were sampled during the field survey at the six 50-m transects, noted with the host plants’ vigour and Cassytha density.
| Site | Individual | Infected (/) | Host Plant Vigour | Cassytha Density | Genus | Species |
|---|---|---|---|---|---|---|
| T1 | 1 | X | Good | - | Acacia | mangium |
| T1 | 2 | X | Good | - | Acacia | mangium |
| T1 | 3 | X | Good | - | Acacia | mangium |
| T1 | 4 | X | Good | - | Buchanania | arborescens |
| T1 | 5 | X | Good | - | Acacia | mangium |
| T1 | 6 | X | Good | - | Acacia | mangium |
| T1 | 7 | X | Good | - | Acacia | mangium |
| T1 | 8 | X | Good | - | Acacia | mangium |
| T1 | 9 | X | Good | - | Acacia | auriculiformis |
| T1 | 10 | X | Good | - | Acacia | auriculiformis |
| T1 | 11 | X | Good | - | Acacia | mangium |
| T1 | 12 | X | Good | - | Acacia | auriculiformis |
| T1 | 13 | X | Good | - | Acacia | mangium |
| T1 | 14 | / | Good | High | Acacia | auriculiformis |
| T1 | 15 | / | Good | High | Buchanania | arborescens |
| T1 | 16 | / | Good | Low | Syzygium | acuminatissimum |
| T1 | 17 | X | Good | - | Syzygium | acuminatissimum |
| T1 | 18 | X | Good | - | Callophylum | inophyllum |
| T1 | 19 | X | Good | - | Acacia | mangium |
| T1 | 20 | / | Good | High | Acacia | mangium |
| T1 | 21 | / | Good | Low | Dilennia | suffruticosa |
| T1 | 22 | / | Good | Low | Dilennia | suffruticosa |
| T1 | 23 | X | Good | - | Acacia | mangium |
| T1 | 24 | / | Good | High | Acacia | mangium |
| T1 | 25 | / | Good | Low | Timonius | flavescens |
| T1 | 26 | / | Good | Low | Acacia | auriculiformis |
| T1 | 27 | X | Good | - | Syzygium | sp. |
| T1 | 28 | / | Good | High | Acacia | mangium |
| T1 | 29 | / | Good | High | Acacia | mangium |
| T1 | 30 | / | Good | Low | Pouteria | obovata |
| T1 | 31 | / | Good | Low | Endospermum | Diodenum |
| T1 | 32 | X | Good | - | Endospermum | Diodenum |
| T1 | 33 | / | Good | Medium | Endospermum | Diodenum |
| T1 | 34 | / | Good | Low | Acacia | mangium |
| T1 | 35 | X | Good | - | Acacia | mangium |
| T1 | 36 | / | Good | High | Elaeocarpus | mastersii |
| T1 | 37 | / | Good | Medium | Elaeocarpus | mastersii |
| T1 | 38 | / | Good | Medium | Acacia | auriculiformis |
| T1 | 39 | / | Good | High | Elaeocarpus | mastersii |
| T1 | 40 | / | Good | High | Acacia | mangium |
| T1 | 41 | / | Good | Low | Endospermum | diadenum |
| T1 | 42 | / | Good | Low | Endospermum | diadenum |
| T1 | 43 | X | Good | - | Elaeocarpus | mastersii |
| T1 | 44 | X | Good | - | Endospermum | diadenum |
| T1 | 45 | X | Good | - | Endospermum | diadenum |
| T1 | 46 | X | Good | - | Acacia | auriculiformis |
| T1 | 47 | X | Good | - | Endospermum | diadenum |
| T1 | 48 | X | Good | - | Endospermum | diadenum |
| T1 | 49 | X | Good | - | Endospermum | diadenum |
| T1 | 50 | X | Good | - | Elaeocarpus | mastersii |
| T1 | 51 | / | Good | - | Timonius | flavescens |
| T1 | 52 | X | Good | - | Acacia | mangium |
| T1 | 53 | X | Good | - | Pouteria | obovata |
| T1 | 54 | X | Good | - | Pouteria | obovata |
| T1 | 55 | X | Good | - | Endospermum | diadenum |
| T1 | 56 | X | Good | - | Dillenia | suffruticosa |
| T1 | 57 | X | Good | - | Pouteria | obovata |
| T1 | 58 | X | Good | - | Licania | splendens |
| T1 | 59 | X | Good | - | Elaocarpus | mastersii |
| T1 | 60 | X | Good | - | Pouteria | obovata |
| T1 | 61 | X | Good | - | Buchanania | arborescens |
| T1 | 62 | X | Good | - | Acacia | mangium |
| T1 | 63 | X | Good | - | Licania | splendens |
| T1 | 64 | X | Good | - | Dilennia | suffruticosa |
| T2 | 1 | X | Poor | - | Acacia | mangium |
| T2 | 2 | X | Poor | - | Acacia | mangium |
| T2 | 3 | X | Poor | - | Acacia | mangium |
| T2 | 4 | X | Poor | - | Acacia | mangium |
| T2 | 5 | X | Poor | - | Acacia | mangium |
| T2 | 6 | X | Fair | - | Acacia | mangium |
| T2 | 7 | X | Fair | - | Acacia | mangium |
| T2 | 8 | X | Good | - | Syzygium | incarnatum |
| T2 | 9 | X | Good | - | Acacia | mangium |
| T2 | 10 | X | Good | - | Maranthes | corymbosa |
| T2 | 11 | X | Poor | - | Acacia | mangium |
| T2 | 12 | X | Poor | - | Acacia | mangium |
| T2 | 13 | X | Fair | - | Acacia | mangium |
| T2 | 14 | X | Fair | - | Acacia | mangium |
| T2 | 15 | X | Poor | - | Acacia | mangium |
| T2 | 16 | X | Poor | - | Acacia | mangium |
| T2 | 17 | X | Poor | - | Acacia | mangium |
| T2 | 18 | / | Poor | Low | Acacia | mangium |
| T2 | 19 | X | Fair | - | Acacia | mangium |
| T2 | 20 | X | Good | - | Picrophloeus | belukar |
| T2 | 21 | X | Poor | - | Acacia | mangium |
| T2 | 22 | X | Poor | - | Acacia | mangium |
| T2 | 23 | / | Poor | Medium | Acacia | mangium |
| T2 | 24 | / | Poor | Low | Acacia | mangium |
| T2 | 25 | / | Fair | Medium | Acacia | mangium |
| T2 | 26 | / | Fair | Medium | Acacia | mangium |
| T2 | 27 | / | Fair | Medium | Acacia | mangium |
| T2 | 28 | / | Fair | Low | Acacia | mangium |
| T2 | 29 | X | Fair | - | Acacia | mangium |
| T2 | 30 | X | Fair | - | Acacia | mangium |
| T2 | 31 | / | Poor | Low | Acacia | mangium |
| T2 | 32 | X | Poor | - | Acacia | mangium |
| T2 | 33 | X | Poor | - | Acacia | mangium |
| T2 | 34 | X | Fair | - | Acacia | mangium |
| T2 | 35 | X | Fair | - | Acacia | mangium |
| T2 | 36 | X | Fair | - | Acacia | mangium |
| T2 | 37 | X | Fair | - | Acacia | mangium |
| T2 | 38 | X | Fair | - | Acacia | mangium |
| T2 | 39 | X | Fair | - | Acacia | mangium |
| T2 | 40 | X | Fair | - | Acacia | mangium |
| T2 | 41 | X | Poor | - | Acacia | mangium |
| T2 | 42 | X | Poor | - | Acacia | mangium |
| T2 | 43 | X | Good | - | Acacia | mangium |
| T2 | 44 | X | Good | - | Acacia | mangium |
| T2 | 45 | X | Fair | - | Acacia | mangium |
| T2 | 46 | X | Good | - | Acacia | auriculiformis |
| T2 | 47 | X | Good | - | Acacia | mangium |
| T2 | 48 | X | Fair | - | Acacia | mangium |
| T2 | 49 | X | Fair | - | Acacia | mangium |
| T2 | 50 | X | Poor | - | Acacia | mangium |
| T2 | 51 | X | Good | - | Elaeocarpus | mastersii |
| T2 | 52 | / | Fair | Medium | Acacia | auriculiformis |
| T2 | 53 | / | Good | Low | Melastoma | malabathricum |
| T2 | 54 | X | Poor | - | Acacia | mangium |
| T2 | 55 | X | Fair | - | Acacia | mangium |
| T2 | 56 | X | Fair | - | Pternandra | coerulescens |
| T2 | 57 | X | Good | - | Buchanania | arborescens |
| T2 | 58 | X | Good | - | Acacia | mangium |
| T2 | 59 | / | Fair | Low | Psychotria | sarmentosa |
| T2 | 60 | X | Fair | - | Acacia | mangium |
| T2 | 61 | X | Fair | - | Acacia | mangium |
| T2 | 62 | X | Fair | - | Acacia | mangium |
| T2 | 63 | X | Fair | - | Timonius | flavescens |
| T2 | 64 | X | Fair | - | Psychotria | sarmentosa |
| T2 | 65 | X | Fair | - | Acacia | mangium |
| T2 | 66 | / | Fair | Low | Timonius | flavescens |
| T2 | 67 | X | Fair | - | Acacia | mangium |
| T2 | 68 | X | Fair | - | Melastoma | malabathricum |
| T2 | 69 | X | Fair | - | Melastoma | malabathricum |
| T2 | 70 | X | Good | - | Endospermum | diadenum |
| T2 | 71 | / | Good | Low | Buchanania | arborescens |
| T2 | 72 | X | Good | - | Acacia | mangium |
| T2 | 73 | X | Good | - | Timonius | flavescens |
| T2 | 74 | X | Good | - | Dillenia | suffruticosa |
| T2 | 75 | X | Fair | - | Acacia | mangium |
| T2 | 76 | X | Good | - | Timonius | flavescens |
| T2 | 77 | X | Good | - | Timonius | flavescens |
| T2 | 78 | / | Good | Low | Melastoma | malabathricum |
| T2 | 79 | X | Fair | - | Melastoma | malabathricum |
| T2 | 80 | X | Good | - | Timonius | flavescens |
| T2 | 81 | X | Good | - | Melastoma | malabathricum |
| T3 | 1 | X | Good | - | Dillenia | suffruticosa |
| T3 | 2 | / | Fair | Low | Vitex | pinnata |
| T3 | 3 | / | Good | Medium | Dillenia | suffruticosa |
| T3 | 4 | / | Good | Very high | Melastoma | malabathricum |
| T3 | 5 | / | Good | Medium | Melastoma | malabathricum |
| T3 | 6 | / | Fair | Low | Comersonia | batramia |
| T3 | 7 | X | Good | - | Melastoma | malabathricum |
| T3 | 8 | X | Good | - | Melastoma | malabathricum |
| T3 | 9 | X | Good | - | Melastoma | malabathricum |
| T3 | 10 | X | Fair | - | Comersonia | batramia |
| T3 | 11 | / | Good | Very high | Comersonia | batramia |
| T3 | 12 | / | Good | Medium | Macaranga | tanarius |
| T3 | 13 | X | Fair | - | Comersonia | batramia |
| T3 | 14 | X | Good | - | Dillenia | suffruticosa |
| T3 | 15 | / | Good | Low | Acacia | mangium |
| T3 | 16 | X | Good | - | Melastoma | malabathricum |
| T3 | 17 | X | Good | - | Dillenia | suffruticosa |
| T3 | 18 | / | Good | Low | Melastoma | malabathricum |
| T3 | 19 | X | Good | - | Melastoma | malabathricum |
| T3 | 20 | X | Good | - | Melastoma | malabathricum |
| T3 | 21 | X | Good | - | Melastoma | malabathricum |
| T3 | 22 | X | Good | - | Melastoma | malabathricum |
| T3 | 23 | X | Good | - | Melastoma | malabathricum |
| T3 | 24 | X | Good | - | Melastoma | malabathricum |
| T3 | 25 | X | Good | - | Vitex | pinnata |
| T3 | 26 | X | Good | - | Acacia | auriculiformis |
| T3 | 27 | X | Good | - | Acacia | auriculiformis |
| T3 | 28 | X | Good | - | Melastoma | malabathricum |
| T3 | 29 | X | Good | - | Melastoma | malabathricum |
| T3 | 30 | X | Good | - | Melastoma | malabathricum |
| T3 | 31 | X | Good | - | Melastoma | malabathricum |
| T3 | 32 | X | Good | - | Melastoma | malabathricum |
| T3 | 33 | X | Good | - | Dillenia | suffruticosa |
| T3 | 34 | / | Good | Low | Melastoma | malabathricum |
| T3 | 35 | X | Good | - | Dillenia | suffruticosa |
| T3 | 36 | X | Good | - | Dillenia | suffruticosa |
| T3 | 37 | X | Good | - | Dillenia | suffruticosa |
| T3 | 38 | X | Good | - | Dillenia | suffruticosa |
| T3 | 39 | X | Good | - | Dillenia | suffruticosa |
| T3 | 40 | X | Good | - | Dillenia | suffruticosa |
| T3 | 41 | X | Good | - | Dillenia | suffruticosa |
| T3 | 42 | X | Good | - | Dillenia | suffruticosa |
| T3 | 43 | X | Good | - | Dillenia | suffruticosa |
| T3 | 44 | X | Good | - | Acacia | mangium |
| T3 | 45 | X | Good | - | Vitex | pinnata |
| T3 | 46 | / | Good | High | Nepenthes | gracilis |
| T3 | 47 | / | Good | High | Nepenthes | gracilis |
| T3 | 48 | / | Good | High | Nepenthes | gracilis |
| T3 | 49 | / | Good | Medium | Nepenthes | gracilis |
| T3 | 50 | X | Good | - | Nepenthes | gracilis |
| T3 | 51 | / | Good | Low | Nepenthes | gracilis |
| T3 | 52 | / | Medium | Rhodomyrtus | tomentosa | |
| T4 | 1 | / | Good | Low | Acacia | auriculiformis |
| T4 | 2 | X | Good | - | Glochidion | littorale |
| T4 | 3 | / | Fair | Medium | Acacia | auriculiformis |
| T4 | 4 | X | Good | - | Melastoma | malabathricum |
| T4 | 5 | X | Good | - | Vitex | pinnata |
| T4 | 6 | X | Good | - | Dillenia | suffruticosa |
| T4 | 7 | X | Good | - | Acacia | mangium |
| T4 | 8 | / | Good | Low | Acacia | mangium |
| T4 | 9 | X | Good | - | Vitex | pinnata |
| T4 | 10 | X | Good | - | Vitex | pinnata |
| T4 | 11 | / | Good | High | Vitex | pinnata |
| T4 | 12 | / | Fair | High | Vitex | pinnata |
| T4 | 13 | / | Good | Low | Vitex | pinnata |
| T4 | 14 | X | Good | - | Acacia | mangium |
| T4 | 15 | X | Good | - | Endospermum | diadenum |
| T4 | 16 | X | Good | - | Dillenia | suffruticosa |
| T4 | 17 | X | Good | - | Acacia | mangium |
| T4 | 18 | X | Fair | - | Vitex | pinnata |
| T4 | 19 | X | Good | - | Melastoma | malabathricum |
| T4 | 20 | / | Good | Low | Melastoma | malabathricum |
| T4 | 21 | X | Good | - | Acacia | mangium |
| T4 | 22 | X | Good | - | Melastoma | malabathricum |
| T4 | 23 | / | Fair | Low | Endospermum | diadenum |
| T4 | 24 | X | Good | - | Acacia | mangium |
| T4 | 25 | X | Good | - | Cocos | nucifera |
| T5 | 1 | / | Good | High | Melastoma | malabathricum |
| T5 | 2 | / | Good | Low | Acacia | auriculiformis |
| T5 | 3 | X | Good | - | Acacia | auriculiformis |
| T5 | 4 | X | Good | - | Acacia | auriculiformis |
| T5 | 5 | X | Good | - | Acacia | auriculiformis |
| T5 | 6 | X | Good | - | Alpinia | aquatica |
| T5 | 7 | X | Good | - | Melastoma | malabathricum |
| T5 | 8 | X | Good | - | Melastoma | malabathricum |
| T5 | 9 | X | Good | - | Dilennia | suffruticosa |
| T5 | 10 | X | Good | - | Dilennia | suffruticosa |
| T5 | 11 | X | Good | - | Dilennia | suffruticosa |
| T5 | 12 | X | Good | - | Melastoma | malabathricum |
| T5 | 13 | X | Good | - | Dilennia | suffruticosa |
| T5 | 14 | X | Good | - | Melastoma | malabathricum |
| T5 | 15 | X | Good | - | Melastoma | malabathricum |
| T5 | 16 | X | Good | - | Melastoma | malabathricum |
| T5 | 17 | X | Good | - | Elaeocarpus | aff. mastersii |
| T5 | 18 | X | Good | - | Melastoma | malabathricum |
| T5 | 19 | X | Good | - | Melastoma | malabathricum |
| T5 | 20 | X | Good | - | Dilennia | suffruticosa |
| T5 | 21 | X | Good | - | Melastoma | malabathricum |
| T5 | 22 | X | Good | - | Melastoma | malabathricum |
| T5 | 23 | X | Good | - | Acacia | auriculiformis |
| T5 | 24 | X | Good | - | Melastoma | malabathricum |
| T5 | 25 | X | Good | - | Melastoma | malabathricum |
| T5 | 26 | X | Good | - | Melastoma | malabathricum |
| T5 | 27 | / | Good | Low | Dilennia | suffruticosa |
| T5 | 28 | / | Good | Medium | Acacia | auriculiformis |
| T5 | 29 | X | Good | - | Elaeocarpus | marginatus |
| T6 | 1 | X | Fair | - | Acacia | auriculiformis |
| T6 | 2 | X | Fair | - | Acacia | auriculiformis |
| T6 | 3 | / | Good | Low | Casuarina | equisetifolia |
| T6 | 4 | X | Good | - | Melastoma | malabathricum |
| T6 | 5 | X | Good | - | Dilennia | suffruticosa |
| T6 | 6 | X | Fair | - | Acacia | auriculiformis |
| T6 | 7 | X | Poor | - | Acacia | mangium |
| T6 | 8 | X | Fair | - | Acacia | mangium |
| T6 | 9 | X | Fair | - | Acacia | auriculiformis |
| T6 | 10 | X | Good | - | Dilennia | suffruticosa |
| T6 | 11 | X | Good | - | Dilennia | suffruticosa |
| T6 | 12 | X | Good | - | Dilennia | suffruticosa |
| T6 | 13 | X | Good | - | Dilennia | suffruticosa |
| T6 | 14 | X | Good | - | Dilennia | suffruticosa |
| T6 | 15 | X | Fair | - | Acacia | mangium |
| T6 | 16 | X | Good | - | Acacia | mangium |
| T6 | 17 | X | Good | - | Dilennia | suffruticosa |
| T6 | 18 | / | Fair | Low | Dilennia | suffruticosa |
| T6 | 19 | / | Fair | High | Acacia | auriculiformis |
| T6 | 20 | / | Fair | Low | Acacia | mangium |
| T6 | 21 | / | Fair | Medium | Dilennia | suffruticosa |
| T6 | 22 | / | Good | Medium | Melastoma | malabathricum |
| T6 | 23 | / | Fair | Low | Acacia | mangium |
| T6 | 24 | / | Fair | High | Acacia | auriculiformis |
| T6 | 25 | / | Fair | High | Acacia | auriculiformis |
| T6 | 26 | / | Good | Medium | Melastoma | malabathricum |
| T6 | 27 | / | Good | Low | Dilennia | suffruticosa |
| T6 | 28 | / | Fair | High | Acacia | auriculiformis |
| T6 | 29 | / | Good | Low | Dilennia | suffruticosa |
| T6 | 30 | / | Fair | V.High | Acacia | auriculiformis |
| T6 | 31 | / | Good | V.High | Acacia | auriculiformis |
| T6 | 32 | / | Fair | Medium | Acacia | auriculiformis |
| T6 | 33 | / | Fair | Low | Acacia | auriculiformis |
| T6 | 34 | X | Fair | - | Dilennia | suffruticosa |
| T6 | 35 | X | Good | - | Melastoma | malabathricum |
| T6 | 36 | / | Fair | Medium | Dilennia | suffruticosa |
| T6 | 37 | / | Good | Low | Dilennia | suffruticosa |
| T6 | 38 | / | Good | h | Acacia | auriculiformis |
| T6 | 39 | / | Good | Low | Dilennia | suffruticosa |
| T6 | 40 | / | Fair | Low | Dilennia | suffruticosa |
| T6 | 41 | X | Fair | - | Dilennia | suffruticosa |
| T6 | 42 | X | Fair | - | Dilennia | suffruticosa |
| T6 | 43 | / | Good | Low | Dilennia | suffruticosa |
| T6 | 44 | / | Good | Low | Dilennia | suffruticosa |
| T6 | 45 | / | Good | Low | Dilennia | suffruticosa |
| T6 | 46 | / | Good | Medium | Dilennia | suffruticosa |
| T6 | 47 | X | Good | - | Dilennia | suffruticosa |
| T6 | 48 | / | Good | Low | Dilennia | suffruticosa |
| T6 | 49 | X | Fair | - | Dilennia | suffruticosa |
| T6 | 50 | / | Good | Low | Dilennia | suffruticosa |
| T6 | 51 | X | Good | - | Dilennia | suffruticosa |
| T6 | 52 | / | Good | Low | Dilennia | suffruticosa |
| T6 | 53 | X | Fair | - | Dilennia | suffruticosa |
| T6 | 54 | X | Good | - | Dilennia | suffruticosa |
| T6 | 55 | X | Fair | - | Dilennia | suffruticosa |
| T6 | 56 | X | Fair | - | Dilennia | suffruticosa |
| T6 | 57 | X | Good | - | Dilennia | suffruticosa |
| T6 | 58 | X | Good | - | Dilennia | suffruticosa |
| T6 | 59 | X | Fair | - | Dilennia | suffruticosa |
| T6 | 60 | X | Good | - | Dilennia | suffruticosa |
| T6 | 61 | X | Good | - | Dilennia | suffruticosa |
| T6 | 62 | X | Good | - | Dilennia | suffruticosa |
| T6 | 63 | X | Fair | - | Dilennia | suffruticosa |
| T6 | 64 | / | Good | Medium | Dilennia | suffruticosa |
| T6 | 65 | / | Good | Low | Dilennia | suffruticosa |
| T6 | 66 | X | Good | - | Dilennia | suffruticosa |
| T6 | 67 | X | Fair | - | Dilennia | suffruticosa |
| T6 | 68 | X | Fair | - | Dilennia | suffruticosa |
| T6 | 69 | X | Good | - | Dilennia | suffruticosa |
| T6 | 70 | / | Good | Low | Dilennia | suffruticosa |
| T6 | 71 | / | Good | High | Dilennia | suffruticosa |
| T6 | 72 | X | Good | - | Dilennia | suffruticosa |
| T6 | 73 | / | Good | Low | Dilennia | suffruticosa |
| T6 | 74 | / | Fair | Medium | Acacia | mangium |
| T6 | 75 | X | Good | - | Acacia | mangium |
| T6 | 76 | X | Good | - | Acacia | mangium |
| T6 | 77 | X | Good | - | Dilennia | suffruticosa |
| T6 | 78 | X | Fair | - | Acacia | mangium |
| T6 | 79 | / | Good | Medium | Dilennia | suffruticosa |
| T6 | 80 | X | Good | - | Acacia | mangium |
| T6 | 81 | X | Good | - | Casuarina | equisetifolia |
| T6 | 82 | X | Good | - | Casuarina | equisetifolia |
| T6 | 83 | / | Good | Medium | Casuarina | equisetifolia |
| T6 | 84 | X | Good | - | Casuarina | equisetifolia |
| T6 | 85 | X | Good | - | Casuarina | equisetifolia |
Footnotes
AUTHORS’ CONTRIBUTIONS: Roshanizah Rosli: Conceptualised the research and designed experiments, collected the data, managed the grants, performed the experiments, conducted statistical analysis, wrote the manuscript and participated in manuscript revisions. Muhammad Yusran S. M. Yaakub: Collected the data and performed the experiments.
Nur Aqilah H. Zainal Ariffin: Collected the data and performed the experiments. Kushan U. Tennakoon: Supervised students, edited the paper and participated in manuscript revisions.
Faizah Metali: Conceptualised the research and designed experiments, supervised students, managed the grants, conducted statistical analysis, edited the manuscript and participated in manuscript revisions.
All authors approved the final version of the manuscript.
ETHICAL STATEMENT: No animal or human subjects were used in this work.
REFERENCES
- Abubacker MN, Prince M, Hariharan Y. Histochemical and biochemical studies of parasite–host interaction of Cassytha filiformis Linn. and Zizyphus jujuba Lamk. Current Science. 2005;89(12):2156–2159. [Google Scholar]
- Agbodjento E, Klotoé JR, Sacramento TI, Dougnon TV, Déguenon E, Agbankpé J, Fabiyi K, Assogba P, Hounkanrin MP, Akotegnon R, Dougnon TJ, Atègbo JM. Larval cytotoxic and subacute toxicity of Gardenia ternifolia, Rourea coccinea, and Cassytha filiformis used in traditional medicine of Benin (West Africa) Journal of Toxicology. 20202020:8843575. doi: 10.1155/2020/8843575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armania N, Yazan LS, Musa SN, Ismail IS, Foo JB, Chan KW, Noreen H, Hisyam AH, Zulfahmi S, Ismail M. Dillenia suffruticosa exhibited antioxidant and cytotoxic activity through induction of apoptosis and G2/M cell cycle arrest. Journal of Ethnopharmacology. 2013;146(2):525–535. doi: 10.1016/j.jep.2013.01.017. [DOI] [PubMed] [Google Scholar]
- Armenia N, Gustinanda D, Nur Salasa G, Yuliandra Y. Acute and delayed toxicity study of Cassytha filiformis defatted ethanolic extract. World Journal of Pharmacy and Pharmaceutical Sciences. 2015;4(10):155–162. [Google Scholar]
- Awang K, Conran JG, Waycott M. Cuticular and ultrastructure characters on Cassytha L (Lauraceae) stem. 2018. [accessed on 16 January 2022]. https://www.researchgate.net/publication/325870821_Cuticular_and_Ultrastructure_Characters_on_Cassytha_L_Lauraceae_Stem/citations .
- Balasubramanian D, Lingakuma K, Arunachalam A. Characterization of anatomical and physiological adaptations in Cassytha filiformis L.: An advanced obligate hemiparasite on Morinda tinctoria Roxb. Taiwania. 2014;59:98–105. doi: 10.6165/tai.2014.59.98. [DOI] [Google Scholar]
- Buriyo AS, Kasuga L, Moshi HN, Nene WA. Ecological distribution and abundance of the parasitic weed, Cassytha filiformis L. (Lauraceae) in major cashew, Anacardium occidentale L. growing regions in Tanzania. International Journal of Basic and Applied Sciences. 2015;5(3):109–116. [Google Scholar]
- Cai H, Lu H, Tian Y, Liu Z, Huang Y, Jian S. Effects of invasive plants on the health of forest ecosystems on small tropical coral islands. Ecological Indicators. 2020;117:106656. doi: 10.1016/j.ecolind.2020.106656. [DOI] [Google Scholar]
- Callaway RM, Pennings SC. Impact of a parasitic plant on the zonation of two salt marsh perennials. Oecologia. 1998;114(1):100–105. doi: 10.1007/s004420050425. [DOI] [PubMed] [Google Scholar]
- Cirocco RM, Facelli JM, Watling JR. Does light influence the relationship between a native stem hemiparasite and a native or introduced host? Annals of Botany. 2016;117(3):521–531. doi: 10.1093/aob/mcv193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirocco RM, Facelli JM, Watling JR. A native parasitic plant affects the performance of an introduced host regardless of environmental variation across field sites. Functional Plant Biology. 2018;45(11):1128–1137. doi: 10.1071/FP17358. [DOI] [PubMed] [Google Scholar]
- Coode MJE, Dransfield J, Forman LL, Kirkup DW, Said IM. In: A checklist of the flowering plants and gymnosperms of Brunei Darussalam. Begawan Bandar Seri., editor. Brunei Darussalam: Ministry of Industry and Primary Resources; 1996. [Google Scholar]
- Davies SJ, Salim KA. Forests and trees of Brunei Darussalam. In: Wong KM, Kamariah AS, editors. Chapter 2: The rainforests of Brunei. Brunei Darussalam: Universiti Brunei Darussalam; 1999. pp. 15–34. [Google Scholar]
- Din H, Metali F, Sukri RS. Tree diversity and community composition of the Tutong white sands, Brunei Darussalam: A rare tropical heath forest ecosystem. International Journal of Ecology. 20152015:807876. doi: 10.1155/2015/807876. [DOI] [Google Scholar]
- Facelli E, Wynn N, Tsang HT, Watling JR, Facelli JM. Defence responses of native and invasive plants to the native generalist vine parasite Cassytha pubescens –anatomical and functional studies. Australian Journal of Botany. 2020;68(4):300–309. doi: 10.1071/BT19136. [DOI] [Google Scholar]
- Furuhashi T, Nakamura T, Iwase K. Analysis of metabolites in stem parasitic plant interactions: Interaction of Cuscuta–Momordica versus Cassytha– Ipomoea. Plants. 2016;5(4):421. doi: 10.3390/Fplants5040043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuhashi K, Iwase K, Furuhashi T. Role of light and plant hormones in stem parasitic plant (Cuscuta and Cassytha) twining and haustoria induction. Photochemistry and Photobiology. 2021;97:1054–1062. doi: 10.1111/php.13441. [DOI] [PubMed] [Google Scholar]
- Gehring CA, Whitham TG. Reduced mycorrhizae on Juniperus monosperma with mistletoe: The influence of environmental stress and tree gender on a plant parasite and a plant-fungal mutualism. Oecologia. 1992;89(2):298–303. doi: 10.1007/BF00317231. [DOI] [PubMed] [Google Scholar]
- Ghazoul J, Sheil D. Tropical rain forest ecology, diversity, and conservation. Oxford; Oxford University Press; 2010. [Google Scholar]
- Goh MP, Basri AM, Yasin H, Taha H, Ahmad N. Ethnobotanical review and pharmacological properties of selected medicinal plants in Brunei Darussalam: Litsea elliptica, Dillenia suffruticosa, Dillenia excelsa, Aidia racemosa, Vitex pinnata and Senna alata. Asian Pacific Journal of Tropical Biomedicine. 2017;7(2):173–180. doi: 10.1016/j.apjtb.2016.11.026. [DOI] [Google Scholar]
- Hattori D, Kenzo T, Shirahama T, Harada Y, Kendawang J, Ninomiya I, Sakurai K. Degradation of soil nutrients and slow recovery of biomass following shifting cultivation in the heath forests of Sarawak, Malaysia. Forest Ecology and Management. 2019;432:467–477. doi: 10.1016/j.foreco.2018.09.051. [DOI] [Google Scholar]
- Heide-Jørgensen HS. Parasitic flowering plants. Brill. 2008 doi: 10.1163/ej.9789004167506.i-438. [DOI] [Google Scholar]
- Hibberd JM, Jeschke WD. Solute flux into parasitic plants. Journal of Experimental Botany. 2001;52(363):2043–2049. doi: 10.1093/jexbot/52.363.2043. [DOI] [PubMed] [Google Scholar]
- Ibrahim MH, Sukri RS, Tennakoon KU, Quang-Vuong L, Metali F. Photosynthetic responses of invasive Acacia mangium and co-existing native heath forest species to elevated temperature and CO2 concentrations. Journal of Sustainable Forestry. 2020;40:1–21. doi: 10.1080/10549811.2020.1792317. [DOI] [Google Scholar]
- Ismail N, Metali F. Allelopathic effects of invasive Acacia mangium on germination and growth of local paddy varieties. Journal of Agronomy. 2014;13:158–168. doi: 10.3923/ja.2014.158.168. [DOI] [Google Scholar]
- Jaafar SM, Sukri RS, Proches S. An investigation of soil physico-chemical variables across different lowland forest ecosystems of Brunei Darussalam. Malaysian Journal of Science. 2016;35(2):151–168. doi: 10.22452/mjs.vol35no2.6. [DOI] [Google Scholar]
- Jambul R, Limin A, Ali AN, Slik F. Invasive Acacia mangium dominance as an indicator for heath forest disturbance. Environmental and Sustainability Indicators. 2020;8:100059. doi: 10.1016/j.indic.2020.100059. [DOI] [Google Scholar]
- Jeschke WD, Hilpert A. Sink-stimulated photosynthesis and sink-dependent increase in nitrate uptake: Nitrogen and carbon relations of the parasitic association Cuscuta reflexa–Ricinus communis. Plant, Cell and Environment. 1997;20:47–56. [Google Scholar]
- Jeschke WD, Baumel P, Rath N, Czygan FC, Proksch P. Modeling of the flows and partitioning of carbon and nitrogen in the holoparasite Cuscuta reflexa Roxb and its host Lupinus albus L. 2. Flows between host and parasite and within the parasitized host. Journal of Experimental Botany. 1994;45:801–812. [Google Scholar]
- Jiang F. PhD diss. University of Würzbürg; 2004. Water, mineral nutrient and hormone flows and exchanges in the hemiparasitic association between root hemiparasite Rhinanthus minor and the host Hordeum vulgare. [Google Scholar]
- Kelly CK. Resource choice in Cuscuta europaea. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:12194–12197. doi: 10.1073/pnas.89.24.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch AM, Binder C, Sanders IR. Does the generalist parasitic plant Cuscuta campestris selectively forage in heterogeneous plant communities? The New Phytologist. 2004;162(1):147–155. doi: 10.1046/j.1469-8137.2004.00999.x. [DOI] [Google Scholar]
- Kokubugata G, Yokota M. Host specificity of Cassytha filiformis and C. pergracilis (Lauraceae) in the Ryukyu Archipelago. Bulletin of the National Museum of Nature and Science, Series B (Botany) 2012;38:47–53. [Google Scholar]
- Kuijt J. The biology of parasitic flowering plants. Berkeley, CA: University of California Press; 1969. [Google Scholar]
- Mahadevan N, Jayasuriya KMGG. Water-impermeable fruits of the parasitic angiosperm Cassytha filiformis (Lauraceae): confirmation of physical dormancy in Magnoliidae and evolutionary considerations. Australian Journal of Botany. 2013;61(4):322–329. doi: 10.1071/BT12275. [DOI] [Google Scholar]
- Mukhtar I, Khokhar I, Mushtaq S. First report on Cassytha filiformis L. (Lauraceae), a parasitic weeds from Lahore, Pakistan. Pakistan Journal of Weed Science Research. 2010;16(4):451–457. [Google Scholar]
- Marquardt ES, Pennings SC. Constraints on host use by a parasitic plant. Oecologia. 2010;164(1):177–184. doi: 10.1007/s00442-010-1664-7. [DOI] [PubMed] [Google Scholar]
- Musselman LJ, Press MC. Introduction to parasitic plants. In: Press MC, Graves JD, editors. Parasitic plants. London, UK: Chapman and Hall; 1995. pp. 1–13. [Google Scholar]
- Matthies D. Interactions between the root hemiparasite Melampyrum arvense and mixtures of host plants: Heterotrophic benefit and parasite-mediated competition. Oikos. 1996;75:118–124. [Google Scholar]
- Muliawan SY. Effect of Dillenia suffruticosa extract on dengue virus type 2 replication. Universa Medicina. 2008;27:1–5. doi: 10.18051/UnivMed.2008.v27.1-5. [DOI] [Google Scholar]
- Myers J, Bazely D. Ecology and control of introduced plants. Cambridge, UK: Cambridge University Press; 2003. [DOI] [Google Scholar]
- Newbery DM. Floristic variation within Kerangas (Heath) forest: Re-evaluation of data from Sarawak and Brunei. Vegetatio. 1991;96(1):43–86. doi: 10.1007/BF00031653. [DOI] [Google Scholar]
- Nickrent DL. Parasitic plants of the world. In: López-Sáez JA, Catalán P, Sáez L, editors. Parasitic plants of the Iberian Peninsula and Balearic Islands. Southern Illinois University; 2002. pp. 7–27. [Google Scholar]
- Okubamichael DY, Griffiths ME, Ward D. Host specificity, nutrient and water dynamics of the mistletoe Viscum rotundifolium and its potential host species in the Kalahari of South Africa. Journal of Arid Environments. 2011;75:898–902. doi: 10.1016/j.jaridenv.2011.04.026. [DOI] [Google Scholar]
- Osunkoya O, Damit N. Population dynamics of the invasive Acacias in Brunei Darussalam using matrix modelling. Journal of Physical Therapy Science. 2005;16(2):115–126. [Google Scholar]
- Pate JS, Bell TL. Host associations of the introduced annual root hemiparasite Parentucellia viscosa in agricultural and bushland settings in Western Australia. Annals of Botany. 2000;85:203–213. [Google Scholar]
- Pennings SC, Callaway RM. Parasitic plants: Parallels and contrasts with herbivores. Oecologia. 2002;131:479–489. doi: 10.1007/s00442-002-0923-7. [DOI] [PubMed] [Google Scholar]
- Pennings S, Simpson J. Like herbivores, parasitic plants are limited by host nitrogen content. Plant Ecology. 2008;196:245–250. [Google Scholar]
- Press MC, Parsons AN, Mackay AW, Vincent CA, Cochrane V, Seel WE. Gas-exchange characteristics and nitrogen relations of two Mediterranean root hemiparasites – Bartsia trixago and Parentucellia viscosa. Oecologia. 1993;95:145–151. doi: 10.1007/BF00649518. [DOI] [PubMed] [Google Scholar]
- Prider J, Watling J, Facelli JM. Impacts of a native parasitic plant on an introduced and a native host species: Implications for the control of an invasive weed. Annals of Botany. 2009;103:107–115. doi: 10.1093/aob/mcn214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2022. [accessed 10 October 2022]. https://www.R-project.org/ [Google Scholar]
- Rosli R. MSc diss. Universiti Brunei Darussalam; 2014. Biology and physiology of the hemiparasitic Cassytha filiformis L. [Google Scholar]
- Runyon JB, Mescher MC, De Moreas CM. Volatile chemical cues guide host location and host selection by parasitic plants. Science. 2006;313:1964–1967. doi: 10.1126/science.1131371. [DOI] [PubMed] [Google Scholar]
- Sastri BN. The wealth of India–raw materials. New Delhi: CSIR; 1962. [Google Scholar]
- Seel WE, Press MC. Influence of the host on 3 sub-Arctic annual facultative root hemiparasites. 1. Growth, mineral accumulation and aboveground dry-matter partitioning. New Phytologist. 1993;125:131–138. doi: 10.1111/j.1469-8137.1993.tb03871.x. [DOI] [PubMed] [Google Scholar]
- Teixeira-Costa L, Davis CC. Life history, diversity, and distribution in parasitic flowering plants. Plant Physiology. 2021;187(1):32–51. doi: 10.1093/plphys/kiab279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennakoon KU, Cameron DD. The anatomy of Santalum album (Sandalwood) haustoria. Canadian Journal of Botany. 2006;84:1608–1616. doi: 10.1139/b06-118. [DOI] [Google Scholar]
- Tennakoon KU, Pate JS, Arthur D. Ecophysiological aspects of the woody root hemiparasite Santalum acuminatum (R. Br.) A. DC and its common hosts in South Western Australia. Annals of Botany. 1997;80:245–256. doi: 10.1006/anbo.1997.0432. [DOI] [Google Scholar]
- Tennakoon KU, Rosli R, Le QV. Biology of aerial parasitic vines in Brunei Darussalam: Cuscuta and Cassytha. Scientiana Bruneiana. 2016;15:58–64. doi: 10.46537/scibru.v15i0.24. [DOI] [Google Scholar]
- Těšitel J, Cirocco RM, Facelli JM, Watling JR. Native parasitic plants: Biological control for plant invasions? Applied Vegetation Science. 2020;23(3):464–469. [Google Scholar]
- Tuah WH, Tennakoon KU, Jaafar SM, Sukri RS. Post-fire impacts on tree diversity in coastal heath forests of Brunei Darussalam. Scientia Bruneiana. 2020;19(1):19–32. doi: 10.46537/scibru.v19i1.109. [DOI] [Google Scholar]
- Umedum NL, Anarado C, Anarado C, Chukwubueze F, Anarado I. Phytochemical and Antimicrobial analysis of leaves of Bridelia micrantha, Cassytha filiformis, Euphorbia hirta and Securinega virosa. Journal of Pharmacognosy and Phytochemistry. 2020;9(3):581–587. [Google Scholar]
- Werth CR, Pusateri WP, Eshbaugh WH, Wilson TK. Field observations on the natural history of Cassytha filiformis L. (Lauraceae) in the Bahamas. In: Musselman LJ, Worsham AD, Eplee RE, editors. Proceeding of the 2nd International Symposium on Parasitic Weeds. North Carolina State University; Raleigh, North Carolina: 1979. pp. 94–102. [Google Scholar]
- Westwood JH, Yoder JI, Timko MP, dePamphilis CW. The evolution of parasitism in plants. Trends in Plant Science. 2010;15(4):227–235. doi: 10.1016/j.tplants.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Wong KM, Ahmad J, Low Y, Kalat MA. Rainforest plants and flowers of Brunei Darussalam. Brunei Darussalam: Forestry Department, Ministry of Industry and Primary Resources; 2015. pp. 150–151. [Google Scholar]
- Wu CS, Wang TJ, Wu CW, Wang YN, Chaw SM. Plastome evolution in the sole hemiparasitic genus laurel dodder (Cassytha) and insights into the plastid phylogenomics of Lauraceae. Genome Biology and Evolution. 2017;9(10):2604–2614. doi: 10.1093/gbe/evx177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Cui S, Ichihashi Y, Shirasu K. The haustorium, a specialized invasive organ in parasitic plants. Annual Review of Plant Biology. 2016;67:643–667. doi: 10.1146/annurev-arplant-043015-111702. [DOI] [PubMed] [Google Scholar]
- Yusoff A, Tennakoon KU, Jaafar S, Zaman DNAN, Sukri RS. Effects of Acacia invasion on leaf litter nutrient and soil properties of coastal Kerangas forests in Brunei Darussalam. Scientia Bruneiana. 2019;18:1–10. doi: 10.46537/scibru.v18i1.87. [DOI] [Google Scholar]
- Zamri A, Slik JWF. Checklist of seedplant holdings of the UBD Herbarium (UBDH), with 234 new plant records for Brunei Darussalam. Scientia Bruneiana. 2018;17(1):6–122. [Google Scholar]
- Zhang C, Ma H, Sanchez-Puerta MV, Li L, Xiao J, Liu Z, Ci X, Li J. Horizontal gene transfer has impacted cox1 gene evolution in Cassytha filiformis. Journal of Molecular Evolution. 2020;88(4):361–371. doi: 10.1007/s00239-020-09937-1. [DOI] [PubMed] [Google Scholar]
- Zhang H, Florentine S, Tennakoon KU. The angiosperm stem hemiparasitic genus Cassytha (Lauraceae) and its host interactions: A review. Frontiers in Plant Science. 2022;13:864110. doi: 10.3389/fpls.2022.864110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoletto B, Cicuzza D. Heath forest in tropical Southeast Asia: Its ecology and conservation risk. Imperiled: The Encyclopedia of Conservation. (2022):114–128. doi: 10.1016/B978-0-12-821139-7.00235-X. [DOI] [Google Scholar]