Abstract
Effective cognitive performance often requires the allocation of additional neural resources (i.e. blood-oxygen-level-dependent [BOLD] activation) as task demands increase, and this demand-related modulation is affected by amyloid-beta deposition and normal aging. The present study investigated these complex relationships between amyloid, modulation, and cognitive function (i.e. fluid ability). Participants from the Dallas Lifespan Brain Study (DLBS, n = 252, ages 50–89) completed a semantic judgment task during functional magnetic resonance imaging (fMRI) where the judgments differed in classification difficulty. Amyloid burden was assessed via positron emission tomography (PET) using 18F-florbetapir. A quadratic relationship between amyloid standardized value uptake ratios (SUVRs) and BOLD modulation was observed such that modulation was weaker in those with moderately elevated SUVRs (e.g. just reaching amyloid-positivity), whereas those with very high SUVRs (e.g. SUVR > 1.5) showed strong modulation. Greater modulation was related to better fluid ability, and this relationship was strongest in younger participants and those with lower amyloid burden. These results support the theory that effective demand-related modulation contributes to healthy cognitive aging, especially in the transition from middle age to older adulthood, whereas high modulation may be dysfunctional in those with substantial amyloid deposition.
Keywords: aging, fluid intelligence, fMRI, PET, semantic
Introduction
As a task becomes more cognitively challenging, additional brain resources are needed to maintain strong performance. Indeed, functional magnetic resonance imaging (fMRI) studies have found that adults have improved performance on a wide range of tasks when they increase activation of primary task regions in response to increased demands (Mattay Venkata et al. 2006; Elman et al. 2014; Bauer et al. 2015; Kennedy et al. 2018). This effect, which we label demand-related modulation, involves a relative increase in activity for task-positive regions or decrease in activity for task-negative regions as task demands increase. This ability to modulate the blood-oxygen-level-dependent (BOLD) response declines linearly across the adult lifespan (Kennedy et al. 2015). More specifically, older adults often show increased activation relative to younger adults at low levels of task difficulty (i.e. working memory load), but then fail to ramp up activation to the same extent when demands increase (Mattay Venkata et al. 2006; Cappell et al. 2010; Bauer et al. 2015). The compensation-related utilization of neural circuits hypothesis (CRUNCH, Reuter-Lorenz and Cappell 2008) posits that older adults likely recruit additional neural resources to maintain youthlike performance when a task is easier, but then hit a resource ceiling as the task becomes more challenging resulting in lower activation and worse performance relative to younger adults (e.g. Cappell et al. 2010). Of course, older adults vary considerably in their demand-related modulation, and the above evidence suggests that the ability to effectively modulate neural activation in response to a challenging task is fundamental to achieving good cognitive performance in demanding situations. Research on factors that either support or undermine effective modulation is necessary to understand this aspect of healthy cognitive aging. In the current study, we sought to determine the extent that amyloid-beta accumulation is related to individual differences in modulation in older adults, and, to extend research in this field, we investigated whether the commonly observed positive association between modulation and cognition is age-dependent (i.e. stronger earlier or later in older adulthood).
Neurological accumulation of amyloid-beta has a notable potential to disrupt demand-based modulation. Amyloid-beta is a misfolded protein and a key initiator of Alzheimer’s disease (Hardy and Higgins 1992; Selkoe and Hardy 2016), because it promotes neurotoxic tauopathy (Jin et al. 2011; Jack et al. 2013). Amyloid burden in cognitively normal older adults has been linked to hyperactivation of hippocampal, entorhinal, and parietal cortex (Edelman et al. 2017; Elman et al. 2014; Huijbers et al. 2015; Marks et al. 2017), and hyperconnectivity in the default mode and frontoparietal networks (Ben-Nejma et al. 2019; Hahn et al. 2019; Moffat et al. 2022; Quevenco et al. 2020; Schultz et al. 2017; see also Ingala et al. 2021). Rodent research has demonstrated that amyloid has a nonlinear dose-dependent effect on hippocampal activity with low levels of amyloid promoting increased long-term potentiation and synaptic facilitation via increased presynaptic vesicle release, and substantial amyloid accumulation causing a collapse in potentiation and facilitation (Puzzo et al. 2008; Abramov et al. 2009). Their collapse is largely due to NMDA and AMPA receptor dysfunction (for a review, see Palop and Mucke 2010). In line with this neurobiological account, hippocampal activation in humans follows a nonlinear trend across the stages of Alzheimer’s pathology. Those with mild cognitive impairment, an intermediate stage between normal cognitive aging and dementia, show hyperactivation of hippocampus relative to cognitive unimpaired individuals, whereas those with Alzheimer’s disease show hypoactivation (Dickerson et al. 2005; Celone et al. 2006; Sperling et al. 2010). The Harvard Aging Study found a similar trend in functional connectivity of the default mode and salience networks for those with only amyloid deposition versus those with both amyloid and tauopathy (Schultz et al. 2017). These studies generally observed that hypoactivation was related to impaired task performance, suggesting that it is detrimental (Dickerson et al. 2005; Celone et al. 2006).
With the advent of radiotracers that bind to amyloid-beta, it became possible to examine potential nonlinear effects of amyloid on human BOLD activity and modulation in vivo. In Kennedy et al. (2018), participants completed an fMRI n-back working memory task and amyloid was examined with 18F-florbetapir positron emission tomography (PET) imaging. Amyloid burden demonstrated a quadratic relationship to demand-related modulation in bilateral basal ganglia and the cerebellum such that those with modestly elevated amyloid-beta levels displayed increased modulation relative to those with no evidence of amyloid, and this trend eventually reversed as those with the highest burden showed reduced modulation. Greater modulation was related to better working memory and executive function. Plausibly, similar to CRUNCH findings (Cappell et al. 2010; Bauer et al. 2015), additional neural resources are allocated to maintain performance despite low levels of amyloid, but the neurotoxic effect of substantial amyloid burden may be so overwhelming that it impedes effective modulation. Quadratic relationships between amyloid burden and activation of bilateral angular and medial frontal gyri have also been observed for a spatial judgments task (Foster et al. 2018), and amyloid positivity has been linked to reduced demand-related modulation in a study examining cognitive control load with a single/dual-task paradigm (Oh et al. 2016).
In the present study, we sought to expand on research showing effects of amyloid burden on modulation and BOLD-signal (Foster et al. 2018; Kennedy et al. 2018) in a sample of older adults from the DLBS (ages 50–89). Participants completed an fMRI semantic judgment task involving living/nonliving judgments that differed in difficulty. Amyloid burden was estimated using AV-45 18F-florbetapir PET imaging. We hypothesized that, like Kennedy et al. (2018) and Foster et al. (2018), amyloid burden would have a quadratic relationship with demand-related modulation. Those studies observed significant effects of amyloid in basal ganglia, cerebellum, middle temporal gyrus, angular gyrus, and anterior cingulate/medial frontal gyrus; we predicted that similar regions would be implicated here, though this semantic judgment task produces particularly strong modulation in middle temporal and angular gyri and lateral prefrontal cortex (Noonan et al. 2013).
BOLD modulation has been linked to greater working memory, executive function, and reasoning (Rieck et al. 2017; Kennedy et al. 2018), and we predicted that its effect would extend to general fluid ability, a more comprehensive and fundamental cognitive measure that declines with age (Park et al. 2002; Salthouse et al. 2008). Finally, we examined whether age, amyloid, and modulation would interact in the prediction of fluid ability, to better understand whether the optimal pattern of modulation differs along these variables. A key unanswered question that we wanted to address is whether amyloid-related differences in modulation have a greater association with cognition earlier or later in older adulthood. Plausibly, amyloid-related modulation reduction would predict worse cognition in late middle age, as that could indicate early neurological decline. However, modulation could also reasonably support cognitive function in the oldest participants; as demand-related modulation declines with age (Cappell et al. 2010; Bauer et al. 2015; Kennedy et al. 2015), those who maintain it into very old age might be considered “super agers” in terms of neural function.
Materials and methods
Participants
All DLBS participants who were initially tested between 2008–2013 (n = 464) were included in the current study if they completed a PET-amyloid scan, were missing data on no more than one cognitive test used in this study, and were at least age 50; one participant with very poor MRI signal in the medial temporal lobe was excluded from analysis. This resulted in a final sample size of 252 right-handed participants, ages 50–89 (M = 68.26, SD = 10.01). These participants were recruited from the Dallas community using media advertisements and flyers. Age 50 was used as an inclusionary cutoff to ensure findings reflected cognitive aging in later life and for consistency with prior related research (Foster et al. 2018; Kennedy et al. 2018). Cognitive, MRI, and PET data were collected in separate sessions, and demographic data for this sample are reported in Table 1. Participants were native English speakers, well-educated (M = 15.32 years of education, SD = 2.20), and had a Mini-Mental State Examination score of at least 26. Exclusion criteria for the DLBS included: major psychiatric or neurological disorder within past 3 years, cancer treatment or surgery within past year, coronary bypass within past year, history of substance abuse, recreational drug use in past six months, history of central nervous systems disease or brain injury, corrected vision poorer than 20/30, unable to undergo MRI scanning due to contraindications, and taking sedatives, benzodiazepines, or antipsychotics. All participants provided written informed consent in accordance with the University of Texas at Dallas and the University of Texas Southwestern (UTSW) Medical Center Institutional Review Boards.
Table 1.
Demographic and descriptive statistics.
| Mean (SD) | |
|---|---|
| Age (y) | 68.26 (10.01) |
| Male/Female (% Female) | 101/151 (59.9%) |
| Education (y) | 15.32 (2.20) |
| MMSE | 28.27 (1.23) |
| Judgment RT (low demand) | 986.51 (136.53) |
| Judgment RT (high demand) | 1244.52 (175.67) |
| Global SUVR | 1.09 (.15) |
| APOEε4 carrier status n (%) | 58 (23.0%) |
n = 252. Abbreviations: MMSE, Mini-Mental State Examination; RT, response time (median RT in ms); SUVR, standardized uptake value ratio.
Cognitive measures
A measure of general cognition, fluid ability, was computed as the average of standardized scores from six cognitive tasks assessing processing speed, working memory, and reasoning. Processing speed tasks included WAIS-III Digit Symbol (Wechsler 1997) and Digit Comparison (Hedden et al. 2002; adapted from Salthouse and Babcock 1991). Working memory tasks included WAIS-III Letter-Number Sequencing (Wechsler 1997) and Operation Span (Turner and Engle 1989). Reasoning tasks included Educational Testing Service Letter Sets (Ekstrom et al. 1976) and Raven’s Progressive Matrices (Raven 1938). For each of these three subcomponents, missing data were imputed using expectation–maximization implemented in SPSS ver 29.0. The overall fluid ability construct had high internal reliability (Cronbach’s α = 0.81).
Functional magnetic resonance imaging task
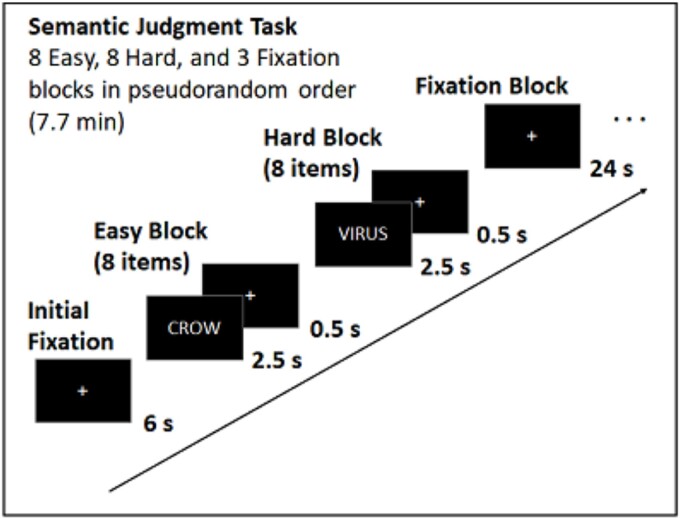
For the in-scanner semantic judgment task (Fig. 1), participants viewed 128 English nouns presented in 16 blocks of eight items and made a judgment as to whether each item denoted something living (right index) or nonliving (middle finger) via button box. Eight of the item blocks were “low demand” and included concrete objects (e.g. dog, table) that had an unambiguous living/nonliving categorization. The other eight item blocks were “high demand” as items included characteristics of both living and nonliving things, resulting in more ambiguous categorization (e.g. ghost, virus) and requiring a longer judgment time (Table 1). Response times were our main measure of behavioral performance for this task as classification accuracy was near ceiling for low demand items (M = 0.96, SD = 0.08) and could not be determined for high demand items as those items were selected to have ambiguous classification. Block-order was pseudorandom and within each block item order was randomized. Each item was displayed for 2500 ms followed by a 500 ms fixation cross. Total scan time was 7.7 min and included a 6 s fixation interval before the first block and three 24 s fixation blocks. Stimuli were programmed in E-prime (Psychology Software Tools, Pittsburgh, PA, USA) and presented using a mirror attached to the head coil.
Fig. 1.
Task diagram for the fMRI semantic judgment task.
Magnetic resonance imaging acquisition
All participants were scanned at UTSW on a single 3 T Philips Achieva scanner (Philips Medical Systems, Best, The Netherlands) equipped with an 8-channel head coil. Anatomical data were acquired with a T1-weighted MP-RAGE sequence with the following parameters: 160 sagittal slices, 1 × 1 × 1 mm3 voxels, 204 × 256 × 160 matrix, TR = 8.1 ms, TE = 3.7 ms, flip-angle = 12°. Functional MRI data were acquired using a T2*-weighted echo-planar imaging sequence with full brain coverage and 43 interleaved axial slices per volume acquired parallel to the AC-PC line and with the following parameters: SENSE = 2, 3.4 × 3.4 × 3.5 mm voxels, 64 × 64 × 43 matrix, FOV = 220 × 220 mm, TR = 2 s, TE = 25 ms, flip angle = 80°. The first five dummy scans were discarded at the beginning of scanning for T1 stabilization. Raw imaging data were converted to Neuroimaging Informatics Technology Initiative (NIFTI) format using r2agui.
Functional magnetic resonance imaging data processing
All participants’ functional images were preprocessed and analyzed using SPM12 (Wellcome Centre for Human Neuroimaging, London, UK), along with additional AFNI (National Institute of Mental Health: Scientific and Statistical Computing Core, MD, USA) and FSL (the Analysis Group, Oxford, UK) functions using in-lab custom scripts. Preprocessing started with motion correction using six motion regressors that were used as covariates of no interest. Functional images were then normalized to standard MNI space (ICBM152) and resampled into 3 × 3 × 3 mm3 voxels using each participant’s T1-weighted structural image as a co-registration intermediary. Images were then smoothed using an isotropic 8 mm full width at half maximum Gaussian kernel. Each participant’s BOLD signal for each task condition (low demand, high demand, or fixation) was modeled as a block convolved with a canonical hemodynamic response function. An AR(1) term was used to correct for time-series autocorrelations. Activation in the high and low demand conditions were contrasted with fixation, and our contrast of interest, high demand—low demand, reflected the level of demand-related modulation.
Positron emission tomography acquisition
Participants were injected with an ~370 MBq (10 mCi) bolus of 18F-AV-45 (18F-florbetapir; Avid Radiopharmaceutical/Eli Lilly) 30 min prior to scanning in a Siemens ECAT HR+ PET scanner (Siemens, Munich, Germany) at UTSW. At the start of scanning, a 2 min scout was acquired to ensure full brain coverage and that there was no planar rotation. Fifty minutes after injection, PET data were collected using a dynamic emission acquisition including two 5-min frames. Immediately after, an internal rod source transmissions scan was performed for 7 min. This transmission image was reconstructed using back-projection and a 6 mm full width at half maximum Gaussian filter with four iterations, 16 subsets, and a 3 mm full width at half maximum ramp filter.
Positron emission tomography preprocessing
First, each participant’s T1-weighted anatomical scan (MP-RAGE) was processed using Freesurfer ver. 5.3 (Martinos Center for Biomedical Imaging, MA, USA). These Freesurfer-parcellated images were manually edited, when necessary, by a well-trained team and quality was verified by a separate research lab. Next, the PET data were registered to the participant’s T1 scan using FSL’s flirt command with 12 degrees of freedom, a mutual information cost function, and trilinear interpolation; the average interval between PET-amyloid and MRI scanning was 0.31 years (SD = 0.35). Freesurfer’s Desikan–Killiany atlas (Desikan et al. 2006) was then used to create eight subject-specific bilateral regions of interest (ROIs): anterior and posterior cingulate, dorsolateral prefrontal, lateral parietal, lateral temporal, orbitofrontal, lateral occipital, and precuneus. Tracer counts were extracted from each of these eight regions, and then standardized value uptake ratios (SUVRs) were computed using whole cerebellum as the reference. These eight SUVRs were then averaged to form a global cortical SUVR that reflected amyloid burden across the vast majority of cortex, excluding sensory and motor cortex. These regions are the standardly utilized PET regions in the field (Rodrigue et al. 2012; Farrell et al. 2018) as they represent where amyloid typically accumulates in those without dementia (Braak and Braak 1991).
Statistical analyses
All fMRI analyses were performed in SPM ver. 12 (Functional Imaging Laboratory, London, UK) with ROI results extracted using marsbar ver 0.45. Prior to testing our hypotheses, we characterized the effect of task demands on BOLD activation as well as the effect of age on demand-related modulation using the age factor from the following model. Next, the association between amyloid deposition and demand-related modulation was examined using a voxel-wise linear regression (F-test), with modulation to semantic judgment demands (high demand—low demand) as the contrast of interest, an intercept, and mean-centered age, mean-centered global SUVR, and the mean-centered quadratic effect of SUVR (SUVR2) entered as factors. Similar to previous research linking amyloid to demand-related modulation (Foster et al. 2018; Kennedy et al. 2018), all statistical maps were thresholded using a voxel-wise P < 0.005; note that clusters were smaller in the current project and did not survive the cluster extent-based thresholding in the Statistical Non-Parametric Mapping toolbox (SnPM; https://warwick.ac.uk) that the previous studies implemented. In line with those studies, the quadratic effect of SUVR on modulation was of primary interest. Linear effects of SUVR were also examined in a separate SPM model including an intercept, age, and linear SUVR, but no SUVR2 factor. The P < 0.005 thresholded SUVR2 map from the previous model was used as an exclusionary mask, though it was later determined that no voxel showed both a significant linear and quadratic SUVR effect.
Finally, we evaluated whether demand-related modulation was related to cognitive function via a linear regression computed in R statistics (R Core Team, Vienna, Austria; lm package). Fluid ability (Z-scored) was the dependent variable and age, SUVR, modulation, and corresponding interaction terms were entered as mean-centered predictors. Post hoc analysis of significant interactions was performed by examining simple slopes as well as using the Johnson-Neyman technique (interactions package), which uses 95% confidence intervals to determine at what moderator values (e.g. age range) a simple slope is significant (Preacher et al. 2006). Ninety-five percent confidence intervals are reported for betas, and median response times on the judgment task were evaluated due to high distribution skewness.
Results
Behavioral and BOLD differences based on task demands and age
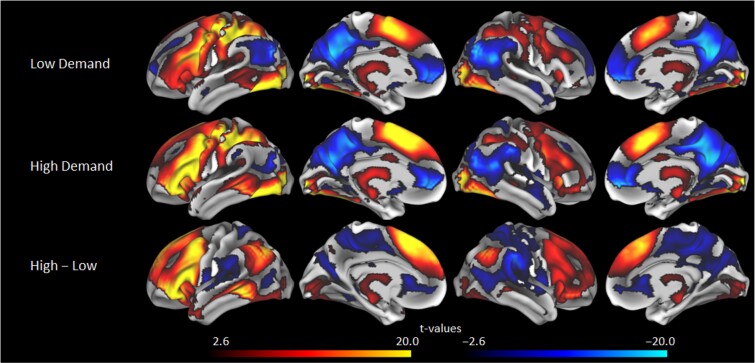
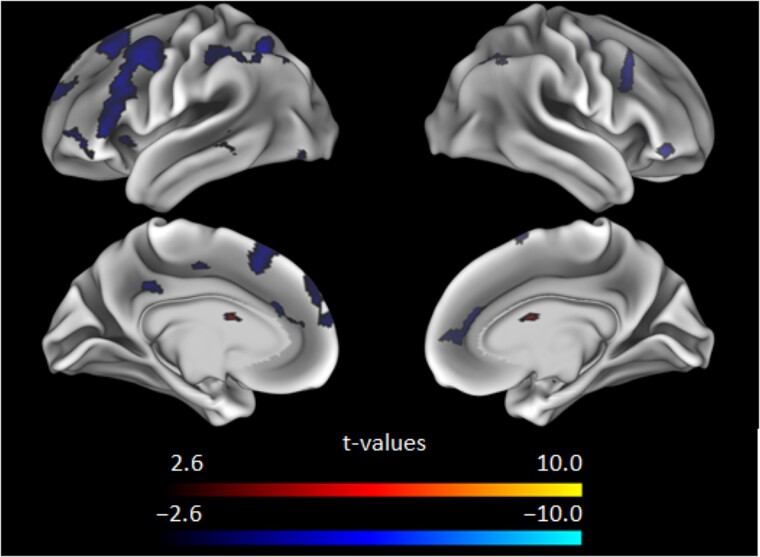
For the in-scanner semantic judgment task, participants had longer median response times for the high demand condition (M = 1244.52 ms, SD = 175.67) versus the low demand condition (M = 986.51, SD = 136.53), t(250) = 33.97, P < 0.001, d = 2.14, suggesting the demand manipulation was effective; note that one participant did not have RT data due to a technical error during data collection. Low demand blocks elicited activation in key regions of the canonical semantic network (Binder et al. 2009; Hoffman and Morcom 2018), with task-positive regions including inferior and middle frontal gyri, dorsomedial prefrontal cortex, most of lateral parietal, middle and superior temporal gyri, lateral occipital and fusiform gyri and task-negative regions including key default mode network regions such as precuneus and posterior cingulate (Fig. 2); note that although the semantic network is left-lateralized in younger adults, bilateral portions of these highlighted areas were activated here as in prior work showing increased bilaterality with age (Kennedy et al. 2015; Hoffman and Morcom 2018; Hennessee et al. 2022). High demand blocks evidenced similar areas of activation, but with increased activation extent and intensity (Fig. 2), which also exemplifies the overall effect of demand-related modulation (high—low demand) to patterns of activation (Fig. 2). Age had an almost exclusively negative relationship to demand-related modulation with most regions that displayed significant modulation being affected, and with the strongest peaks localized in left middle and superior frontal gyri, dorsomedial prefrontal, bilateral caudate, right superior parietal lobule, right inferior frontal gyrus, and left inferior and middle temporal gyri, with additional significant effects in left inferior frontal gyrus and most of left lateral parietal (Fig. 3). Age was related to longer RTs for the low-demand (r = 0.31, P < 0.001) and high-demand (r = 0.23, P < 0.001) conditions, elevated amyloid SUVR (r = 0.27, P < 0.001), and lower fluid ability (r = −.52, P < 0.001).
Fig. 2.
Activation t-values for low demand semantic judgments (top) and high demand judgments (middle), as well as demand-based modulation (high—Low demand; bottom) with a voxel-wise threshold of P < 0.005.
Fig. 3.
Effect of age t-values on demand-based modulation (high—Low demand) with a voxel-wise threshold of P < 0.005. Note that age almost exclusively has a negative relationship to modulation.
Association of amyloid burden with blood-oxygen-level-dependent modulation
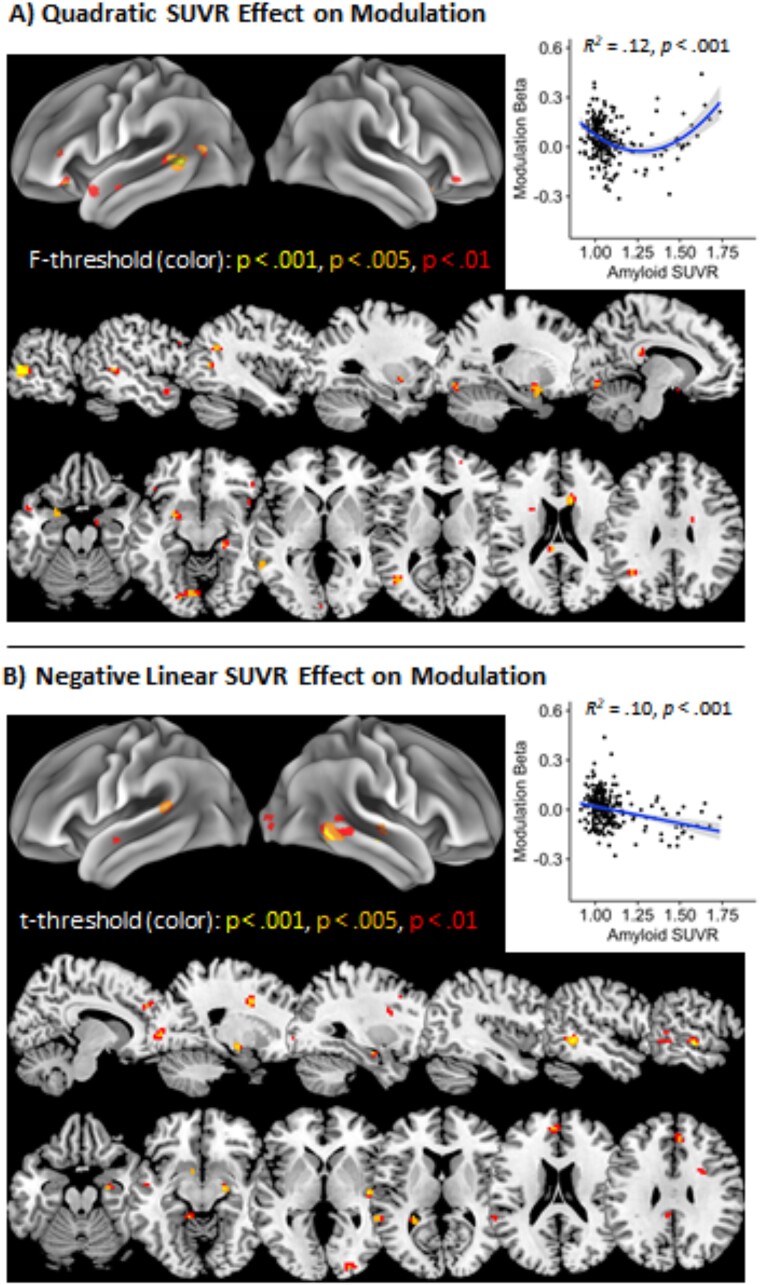
Nonlinear associations between amyloid SUVR and demand-related modulation were examined using an SPM model with an intercept included and age, SUVR, and SUVR2 entered as factors. For the key SUVR2 effect, significant clusters were identified in left middle temporal gyrus, right caudate, left putamen, and left posterior middle temporal/inferior occipital/angular gyri at the temporo-parieto-occipital (TPO) junction (Table 2, Fig. 4A). The left middle temporal and left putamen clusters were predominantly task-positive with greater activation for the hard condition relative to the easy condition, though the TPO junction and to a lesser extent the right caudate clusters were task-negative with more negative scores as task demands increased. The quadratic effect of SUVR was due to modulation decreasing with modest amounts of amyloid SUVR, then increasing again for those with high SUVR (see Fig. 4A, bottom panel). Furthermore, the degree of modulation here—in a mask of all voxels showing the significant quadratic effect of SUVR—was strongly related to activity in these regions for high demand judgments (r = 0.43, P < 0.001), but not to activity for low demand judgments (r = −.01, P = 0.824), suggesting that this demand-related modulation primarily reflects increased activation to high demand items.
Table 2.
Cluster peaks for quadratic and linear effect of amyloid burden on BOLD modulation.
| Cluster Label | k | x | y | z | Peak F | Peak p uncorr | Cluster q FDR-cor |
|---|---|---|---|---|---|---|---|
| Quadratic SUVR Effect | |||||||
| L middle temporal gyrus, L superior temporal gyrus | 50 | –60 | −49 | 5 | 18.58 | <.001 | .808 |
| R caudate | 15 | 12 | 14 | 17 | 14.71 | <.001 | .808 |
| L putamen, L entorhinal | 24 | −21 | 5 | −16 | 10.86 | .001 | .808 |
| L middle temporal, L inferior occipital, L angular, L middle occipital gyri | 10 | −42 | −64 | 8 | 10.41 | .001 | .808 |
| Cluster Label | k | x | y | z | Peak t | Peak p uncorr | Cluster q FDR-cor |
| Linear SUVR Effect | |||||||
| brain stem | 37 | 0 | −40 | −22 | 4.07 | <.001 | .279 |
| R middle temporal gyrus, R inferior temporal gyrus | 34 | 54 | −49 | −4 | −3.54 | <.001 | .807 |
| R superior temporal gyrus, R middle temporal gyrus | 14 | 63 | −19 | −4 | −3.27 | .001 | .807 |
| R hippocampus | 18 | 24 | −10 | −13 | −3.24 | .001 | .807 |
| R anterior cingulate | 11 | 18 | 5 | 38 | −3.18 | .001 | .807 |
Only clusters with at least 10 voxels are reported for conciseness. Abbreviations: k, cluster extent; corr, corrected; L, left; R, right; uncorr, uncorrected; SUVR, standardized uptake value ratio.
Fig. 4.
Nonlinear and linear associations between BOLD modulation and amyloid SUVR. (A) Quadratic effect of amyloid SUVR on demand-based modulation (high—Low demand) at multiple F-value thresholds in left sagittal and axial slices (bottom) and with average modulation beta weights across the full P < 0.005 map plotted (top-right). (B) Negative linear effect of amyloid SUVR on demand-based modulation (high—Low demand) at multiple t-value thresholds in right sagittal and axial slices (bottom) and with average modulation beta weights across the full P < 0.005 map plotted (top-right). Scatter plots include 95% confidence intervals for each trend in shaded gray.
There was also a predominantly negative linear relationship between SUVR and modulation, which notably showed no spatial overlap with the quadratic effect of SUVR, with significant negative clusters in right inferior, middle, and superior temporal gyri, right anterior cingulate, and right hippocampus, and a significant positive cluster in the brainstem (Fig. 4B). These regions were more likely to be task-negative—i.e. middle and superior temporal gyri—with negative modulation scores (high demand < low demand), although right middle cingulate showed task-positive activation. As shown in the Fig. 4B scatterplot, the negative relationship between SUVR and modulation here typically reflected more negative modulation scores in those with substantial amyloid burden.
Associations of amyloid burden and blood-oxygen-level-dependent modulation to general cognitive function
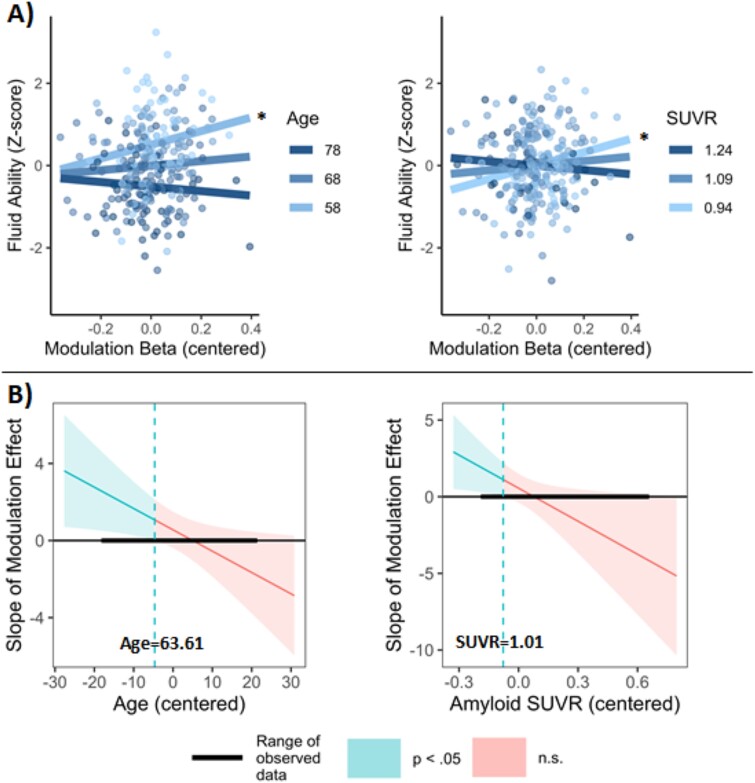
To test the hypothesis that demand-related modulation predicts cognitive function, and that amyloid and/or age may moderate this relationship, a linear regression was computed with fluid ability as the dependent variable and age, amyloid SUVR, the beta-weight for modulation, and the full set of interaction terms as predictors (Note that adding a quadratic SUVR factor to the model did not significantly improve model fit, F(8,236) = 0.98, P = 0.454, so only linear SUVR was included.). The three-way interaction of age × SUVR × modulation was not statistically significant (b = 0.48, CI = [−0.06, 1.03], P = 0.083), but significant interactions were observed for age × modulation (b = −0.11, CI = [−0.21, −0.01], P = 0.027) and SUVR × modulation (b = −7.19, CI = [−13.71, −0.67], P = 0.031). A main effect of age was also observed (b = −0.05, CI = [−0.06, −0.04], P < 0.001), though main effects of SUVR (b = −0.003, CI = [−0.94, 0.93], P = 0.996) and modulation (b = 0.56, CI = [−0.39, 1.50], P = 0.247) were not significant. The significant two-way interactions were characterized by modulation showing a positive association with fluid ability that was strongest in those who were younger and who had lower amyloid burden (Fig. 5A). More specifically, examination of confidence bands developed using the Johnson-Neyman technique revealed that modulation had a significant positive relationship to fluid ability in those ages 50–63.61 and in those with an amyloid SUVR below 1.01 (Fig. 5B). In a supplemental analysis of modulation in regions showing a negative linear effect of SUVR on modulation (Fig. 4B), a largely similar relationship was observed between modulation and fluid ability, albeit with a significant modulation × age × SUVR interaction, b = 1.23, CI = [0.14, 2.33], P = 0.028 (Supplementary Material).
Fig. 5.
(A) Moderation effects of age and amyloid SUVR on the relationship between modulation and fluid ability. Moderator simple slopes were estimated at their mean +/− 1 SD, and modulation beta weights (high—Low demand) were extracted from the primary mask of the P < 0.005 SUVR2 effect. Asterisks indicate significant slopes: *, P < 0.05. (B) Conditional effect of modulation on fluid ability at different values of age and amyloid SUVR. Confidence bands estimated using the Johnson-Neyman procedure indicated the SUVR and age values at which modulation had a significant or nonsignificant effect. The effect of modulation was significant at ages 50–63.61 and in those with an SUVR below 1.01.
Discussion
In this study of 252 cognitively normal older adults, we report two major findings. First, amyloid-beta deposition was related to demand-related modulation on a semantic judgment task with several regions showing a quadratic effect of amyloid and others showing a linear negative effect. Second, greater modulation was associated with higher fluid intelligence, similar to other studies (Rieck et al. 2017; Kennedy et al. 2018), but, importantly, modulation interacted with age and amyloid such that modulation was positively related to cognition in younger participants and those with minimal amyloid burden. Paradoxically, modulation in the very old and those with substantial amyloid burden was statistically unrelated to cognition, suggesting that modulation may be less beneficial when neural resources are taxed by age and amyloid.
The current findings indicated that amyloid impacts demand-related modulation in several brain regions that also show markedly reduced modulation in older age and are involved in semantic processing (Binder et al. 2009). Quadratic effects of amyloid burden on modulation were observed in predominantly task-positive regions including left middle temporal gyrus, right caudate, left putamen, and the left TPO junction, a region at the posterior end of the Sylvian fissure where several high-level functions and sensory details are integrated (De Benedictis et al. 2014). This quadratic relationship was characterized by amyloid having a negative relationship to modulation in those with a youthlike low SUVR, then this relationship reversing as those with a high SUVR showed a strong modulation response. We note that these findings were significant at a voxel-based threshold of P < 0.005, but did not survive cluster-extent based thresholding. To partially address this limitation, the activation maps in Fig. 4 display the voxelwise p-values at three different thresholds (color-coded) to more fully represent which regions evidenced the most statistically robust effects of amyloid on modulation, namely those centered in the bilateral middle temporal gyri, right caudate, and portions of the brain stem (see also Table 2). Importantly, these findings closely mirrored effects of amyloid observed in Foster et al. (2018) and Kennedy et al. (2018). Kennedy et al. also found quadratic effects of amyloid deposition on BOLD modulation in the basal ganglia (i.e. caudate and putamen) for an n-back task, as well as in cerebellum. Foster et al. observed quadratic effects of amyloid on BOLD-signal to hard spatial distance judgments in similar left middle temporal and left TPO junction clusters, as well as an additional medial frontal cluster. Although additional research is needed, these findings suggest that activity in left middle temporal gyrus, left TPO junction, and the basal ganglia appears to be similarly affected by amyloid-burden across multiple cognitive tasks, and this potentially represents a task-general influence of amyloid burden on neural activity.
In addition to this quadratic relationship between amyloid SUVR and modulation, we observed a linear negative effect of SUVR in right lateral temporal (inferior, middle, and superior gyri), right hippocampus, and right anterior cingulate such that those with greater amyloid had more negative modulation (high demand < low demand) in these mostly task-negative regions. Although this sample was screened to be cognitively normal, this increased task-negativity and modulation in hippocampus for those with high amyloid appears consistent with commonly observed hippocampal hyperactivity in those with mild cognitive impairment (Dickerson et al. 2005; Celone et al. 2006). This relationship is also partly consistent with rodent research showing that amyloid-beta has a nonlinear causal relationship to hippocampal activity with low levels of amyloid promoting long-term potentiation and substantial amyloid leading to a collapse in potentiation (Puzzo et al. 2008; Abramov et al. 2009); here, a functional collapse at high amyloid levels was not observed. It would be informative to see whether those with Alzheimer’s disease show reduced hippocampal modulation analogous to their hypoactivation on episodic memory tasks (Dickerson et al. 2005; Celone et al. 2006) and potentiation impairments in rodents with high amyloid.
Findings here also supported our hypothesis that greater demand-related modulation would be predictive of higher fluid ability, as the ability to adaptively allocate neural resources to challenging tasks is likely an important factor in healthy cognitive aging (Mattay Venkata et al. 2006; Cappell et al. 2010; Bauer et al. 2015; Webb et al. 2020). The CRUNCH framework (Reuter-Lorenz and Cappell 2008) frames age-related cognitive decline as being partly due to age-related reductions in neural resources that limits efficient ramping up of neural activity as a task becomes more demanding. In line with this framework, this study observed that older adults with a more youthlike ability to modulate activity in response to task demands showed more youthlike cognitive performance. Critically, modulation interacted with amyloid and age such that the positive effect of modulation on fluid ability weakened with increased amyloid burden and age. It was only significant in those with practically no amyloid (SUVR < 1.01), well below a standard cutoff for amyloid positivity (Joshi et al. 2012; SUVR > 1.1). Somewhat paradoxically, the nonlinear relationship between SUVR and demand-related modulation indicated that several regions showed increased modulation at high levels of amyloid, despite this lack of a positive effect of modulation on cognition. Modulation in those with substantial amyloid may be inefficient, possibly due to amyloid-related changes to functional networks such as the frontoparietal and default mode network (Ben-Nejma et al. 2019; Hahn et al. 2019; Quevenco et al. 2020; Moffat et al. 2022). Additionally, those with very high amyloid SUVRs are more likely to have increased Alzheimer’s disease-related pathology, such as elevated tau or neurodegeneration (Jack et al. 2013), which could potentially in turn make modulation less effective. These findings add to the CRUNCH framework as they specify a new, yet common, situation where the benefits of modulation appear to break down.
The association between modulation and fluid ability also weakened as a function of age, with significant effects observed in late middle-age and those in their early 60s. It is likely that maintenance of youthlike strong demand-related modulation in the transition from middle-age to early older adulthood would allow for more effective allocation of neural resources across various tasks, thus supporting strong performance on the challenging tasks that comprised our fluid ability measure. In contrast, because BOLD activation for both high and low demand semantic judgments increases markedly with age (Wierenga et al. 2008; Hennessee et al. 2022) it seems likely that lower modulation in the oldest older adults often reflects a ceiling effect on activation such that ramping up activation further for high demand judgments would be both difficult due to resource limitations and unnecessary.
Conclusion
In conclusion, the current study demonstrated that amyloid-beta deposition shows a quadratic relationship to demand-related modulation in left middle temporal gyrus, right caudate, left putamen, and the left TPO junction with low levels of amyloid having a negative effect on modulation that reverses in those with substantially high amyloid burden. Furthermore, greater modulation was related to better cognitive function and this study specifies that this relationship is strongest in late middle age and early older age and in those with minimal amyloid deposition. Further examination of amyloid’s effect on demand-related modulation, especially with longitudinal research, will improve our understanding of why some individuals with amyloid burden nevertheless display strong cognitive function, and these changes in BOLD modulation may provide a useful indicator of who is most at risk for early cognitive decline.
Supplementary Material
Acknowledgments
We thank Claudia Carreno and Micaela Chan, from Gagan Wig’s research lab, for their assistance in evaluating Freesurfer parcellation accuracy and providing image editing guidance. Avid Radiopharmaceuticals, Inc., a wholly owned subsidiary of Eli Lilly and Company, enabled use of the 18F-florbetapir tracer but did not provide direct funding and was not involved in data analysis or interpretation.
Contributor Information
Joseph P Hennessee, Center for Vital Longevity; Department of Psychology, School of Behavioral and Brain Sciences, University of Texas at Dallas, 1600 Viceroy Dr., Suite 800, Dallas, TX 75235, United States.
Tzu-Chen Lung, Center for Vital Longevity; Department of Psychology, School of Behavioral and Brain Sciences, University of Texas at Dallas, 1600 Viceroy Dr., Suite 800, Dallas, TX 75235, United States.
Denise C Park, Center for Vital Longevity; Department of Psychology, School of Behavioral and Brain Sciences, University of Texas at Dallas, 1600 Viceroy Dr., Suite 800, Dallas, TX 75235, United States; Department of Psychiatry, University of Texas Southwestern Medical Center, 5323 Harry Hines Blvd., Dallas, TX 75390, United States.
Kristen M Kennedy, Center for Vital Longevity; Department of Psychology, School of Behavioral and Brain Sciences, University of Texas at Dallas, 1600 Viceroy Dr., Suite 800, Dallas, TX 75235, United States.
Author contributions
Joseph Hennessee (Conceptualization, Methodology, Formal analysis, Visualization, Writing—original draft, Writing—review & editing), Tzu-Chen Lung (Methodology, Writing—review & editing), Denise C. Park (Conceptualization, Methodology, Supervision, Resources, Writing—review & editing, Project administration, Funding acquisition), Kristen Kennedy (Conceptualization, Methodology, Writing—review & editing).
Funding
This work was supported by National Institutes of Health: National Institute on Aging grants (5R37AG-006265-27, RC1AG036199) to DP.
Conflict of interest statement: None declared.
Data availability
Data and analysis code for this project are available at https://osf.io/7rsm3/? and unthresholded functional maps are available at https://neurovault.org/collections/ZWMWDJJV/.
References
- Abramov E, Dolev I, Fogel H, Ciccotosto GD, Ruff E, Slutsky I. Amyloid-β as a positive endogenous regulator of release probability at hippocampal synapses. Nat Neurosci. 2009:12(12):1567–1576. 10.1038/nn.2433. [DOI] [PubMed] [Google Scholar]
- Bauer E, Sammer G, Toepper M. Trying to put the puzzle together: age and performance level modulate the neural response to increasing task load within left rostral prefrontal cortex. Biomed Res Int. 2015:2015(1):1–11. 10.1155/2015/415458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Nejma IRH, Keliris AJ, Daans J, Ponsaerts P, Verhoye M, van der Linden A, Keliris GA. Increased soluble amyloid-beta causes early aberrant brain network hypersynchronisation in a mature-onset mouse model of amyloidosis. Acta Neuropathol Commun. 2019:7(1):180. 10.1186/s40478-019-0810-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex. 2009:19(12):2767–2796. 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991:82(4):239–259. 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Cappell KA, Gmeindl L, Reuter-Lorenz PA. Age differences in prefontal recruitment during verbal working memory maintenance depend on memory load. Cortex. 2010:46(4):462–473. 10.1016/j.cortex.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celone KA, Calhoun VD, Dickerson BC, Atri A, Chua EF, Miller SL, DePeau K, Rentz DM, Selkoe DJ, Blacker D, et al. Alterations in memory networks in mild cognitive impairment and Alzheimer’s disease: an independent component analysis. J Neurosci. 2006:26(40):10222–10231. 10.1523/JNEUROSCI.2250-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Benedictis A, Duffau H, Paradiso B, Grandi E, Balbi S, Granieri E, Colarusso E, Chioffi F, Marras CE, Sarubbo S. Anatomo-functional study of the temporo-parieto-occipital region: dissection, tractographic and brain mapping evidence from a neurosurgical perspective. J Anat. 2014:225(2):132–151. 10.1111/joa.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire R, Hyman BT, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006:31(3):968–980. 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Salat DH, Greve DN, Chua EF, Rand-Giovannetti E, Rentz M, Bertram L, Mullin K, Tanzi RE, Blacker D, et al. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology. 2005:65(3):404–411. 10.1212/01.wnl.0000171450.97464.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman K, Tudorascu D, Agudelo C, Snitz B, Karim H, Cohen A, Mathis C, Price J, Weissfeld L, et al. Amyloid-beta deposition is associated with increased medial temporal lobe activation during memory encoding in the cognitively normal elderly. Am J Geriatr Psychiatry. 2017:25(5):551–560. 10.1016/j.jagp.2016.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom R, French J, Harman H, Dermen D. Manual for kit of factor referenced cognitive tests. Princeton (NJ): Educational Testing Service; 1976. [Google Scholar]
- Elman JA, Oh H, Madison CM, Baker SL, Vogel JW, Marks SM, Crowley S, O'Neil JP, Jagust WJ. Neural compensation in older people with brain amyloid-β deposition. Nat Neurosci. 2014:17(10):1316–1318. 10.1038/nn.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell ME, Chen X, Rundle MM, Chan MY, Wig GS, Park DC. Regional amyloid accumulation and cognitive decline in initially amyloid-negative adults. Neurology. 2018:2018(91):e1809–e1821. 10.1212/WNL.0000000000006469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster CM, Kennedy KM, Horn MM, Hoagey DA, Rodrigue KM. Both hyper- and hypo-activation to cognitive challenge are associated with increased beta-amyloid deposition in healthy aging: a nonlinear effect. NeuroImage. 2018:166:285–292. 10.1016/j.neuroimage.2017.10.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn A, Strandberg TO, Stomrud E, Nilsson M, van Westen D, Palmqvist S, Ossenkoppele R, Hansson O. Association between earliest amyloid uptake and functional connectivity in cognitively unimpaired elderly. Cereb Cortex. 2019:29(5):2173–2182. 10.1093/cercor/bhz020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy JA, Higgins GA. Alzheimer's disease: the amyloid cascade hypothesis. Science 1992:256(5054):184–185. 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- Hedden T, Park DC, Nisbett R, Ji LJ, Jing Q, Jiao S. Cultural variation in verbal versus spatial neuropsychological function across the life span. Neuropsychol. 2002:16(1):65–73. 10.1037/0894-4105.16.1.65. [DOI] [PubMed] [Google Scholar]
- Hennessee JP, Webb CE, Chen X, Kennedy KM, Wig GS, Park DC. Relationship of prefrontal brain lateralization to optimal cognitive function differs with age. NeuroImage. 2022:264(119736):1–10. 10.1016/j.neuroimage.2022.119736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman P, Morcom AM. Age-related changes in the neural networks supporting semantic cognition: a meta-analysis of 47 functional neuroimaging studies. Neurosci Biobehav Rev. 2018:84:134–150. 10.1016/j.neubiorev.2017.11.010. [DOI] [PubMed] [Google Scholar]
- Huijbers W, Mormino EC, Schultz AP, Wigman S, Ward AM, Larvie M, Amariglio RE, Marshall GA, Rentz DM, Johnson KA, et al. Amyloid-b deposition in mild cognitive impairment is associated with increased hippocampal activity, atrophy and clinical progression. Brain. 2015:138(4):1023–1035. 10.1093/brain/awv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingala S, Tomassen J, Collij LE, Prent N, van ‘t Ent D, ten Kate M, Konijnenberg E, Yaqub M, Scheltens P, EJC DG, et al. Amyloid-driven disruption of default mode network connectivity in cognitively healthy individuals. Brain Commun. 2021:3(4):fcab201. 10.1093/braincomms/fcab201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CRJ, Wiste HJ, Weigand SD, Knopman DS, Lowe V, Vemuri P, Mielke MM, Jones DT, Senjem ML, Gunter JL, et al. Amyloid-first and neurodegeneration-first profiles characterize incident amyloid PET positivity. Neurology. 2013:81(20):1732–1740. 10.1212/01.wnl.0000435556.21319.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M, Shepardson N, Yang T, Chen G, Walsh D, Selkoe DJ. Soluble amyloid β-protein dimers isolated from Alzheimer cortex directly induce tau hyperphosphorylation and neuritic degeneration. Proc Natl Acad Sci USA. 2011:108(14):5819–5824. 10.1073/pnas.1017033108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi AD, Pontecorvo MJ, Clark CM, Carpenter AP, Jennings DL, Sadowsky CH, Adler LP, Kovnat KD, Seibyl JP, Arora A, et al. the Florbetapir F 18 Study Investigators performance characteristics of amyloid PET with Florbetapir F 18 in patients with Alzheimer’s disease and cognitively normal subjects. J Nucl Med. 2012:53(3):378–384. 10.2967/jnumed.111.090340. [DOI] [PubMed] [Google Scholar]
- Kennedy KM, Rodrigue KM, Bischof GN, Hebrank AC, Reuter-Lorenz PA, Park DC. Age trajectories of functional activation under conditions of low and high processing demands: an adult lifespan fMRI study of the aging brain. NeuroImage. 2015:104:21–34. 10.1016/j.neuroimage.2014.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy KM, Foster CM, Rodrigue KM. Increasing beta-amyloid deposition in cognitively healthy aging predicts nonlinear change in BOLD modulation to difficulty. NeuroImage. 2018:183:142–149. 10.1016/j.neuroimage.2018.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks SM, Lockhart SN, Baker, Jagust WJ. Tau and β-amyloid are associated with medial temporal lobe structure, function, and memory encoding in normal aging. J Neurosci. 2017:37(12):3192–3201. 10.1523/JNEUROSCI.3769-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattay VS, Fera F, Tessitore A, Hariri AR, Berman KF, Das S, Meyer-Lindenberg A, Goldberg TE, Callicott JH, et al. Neurophysiological correlates of age-related changes in working memory capacity. Neurosci Lett. 2006:392(1–2):32–37. 10.1016/j.neulet.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Moffat G, Zhukovsky P, Coughlan G, Voineskos AN. Unravelling the relationship between amyloid accumulation and brain network function in normal aging and very mild cognitive decline: a longitudinal analysis. Brain Commun. 2022:4(6):fcac282. 10.1093/braincomms/fcac282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan KA, Jefferies E, Visser M, Lambon Ralph MA. Going beyond inferior prefrontal involvement in semantic control: evidence for the additional contribution of dorsal angular gyrus and posterior middle temporal cortex. J Cogn Neurosci. 2013:25(11):1824–1850. 10.1162/jocn_a_00442. [DOI] [PubMed] [Google Scholar]
- Oh H, Steffener J, Razlighi QR, Habeck C, Stern Y. β-Amyloid deposition is associated with decreased right prefrontal activation during task switching among cognitively normal elderly. J Neurosci. 2016:36(6):1962–1970. 10.1523/JNEUROSCI.3266-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop JJ, Mucke L. Amyloid-β–induced neuronal dysfunction in Alzheimer’s disease: from synapses toward neural networks. Nat Neurosci. 2010:13(7):812–818. 10.1038/nn.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK. Models of visuospatial and verbal memory across the adult life span. Psychol Aging. 2002:17(2):299–320. 10.1037/0882-7974.17.2.299. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interactions in multiple linear regression, multilevel modeling, and latent curve analysis. J Educ Behav Stat. 2006:31(4):437–448. 10.3102/10769986031004437. [DOI] [Google Scholar]
- Puzzo D, Privitera L, Leznik E, Fà M, Staniszewski A, Palmeri A, Arancio O. Picomolar amyloid-β positively modulates synaptic plasticity and memory in hippocampus. J Neurosci. 2008:28(53):14537–14545. 10.1523/JNEUROSCI.2692-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quevenco FC, van Bergen JM, Treyer V, Studer ST, Kagerer SM, Meyer R, Gietl AF, Kaufmann PA, Nitsch RM, Hock C, et al. Functional brain network connectivity patterns associated with normal cognition at old-age, local β-amyloid, tau, and APOE4. Front Aging Neurosci. 2020:12:46. 10.3389/fnagi.2020.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven JC. Progressive matrices: a perceptual test of intelligence, sets a, B, C, D, and E. London (UK): HK Lewis; 1938. [Google Scholar]
- Reuter-Lorenz PA, Cappell KA. Neurocognitive aging and the compensation hypothesis. Curr Dir Psychol Sci. 2008:17(3):177–182. 10.1111/j.1467-8721.2008.00570.x. [DOI] [Google Scholar]
- Rieck JR, Rodrigue KM, Boylan MA, Kennedy KM. Age-related reduction of BOLD modulation to cognitive difficulty predicts poorer task accuracy and poorer fluid reasoning ability. NeuroImage. 2017:147:262–271. 10.1016/j.neuroimage.2016.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigue KM, Kennedy KM, Devous MD Sr, Rieck JR, Hebrank AC, Diaz-Arrastia R, Mathews D, Park DC. β-Amyloid burden in healthy aging: regional distribution and cognitive consequences. Neurology. 2012:2012(6):387–395. 10.1212/WNL.0b013e318245d295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA, Babcock RL. Decomposing adult age differences in working memory. Dev Psychol. 1991:27(5):763–776. 10.1037/0012-1649.27.5.763. [DOI] [Google Scholar]
- Salthouse TA, Pink JE, Tucker-Drob EM. Contextual analysis of fluid intelligence. Intelligence. 2008:36(5):464–486. 10.1016/j.intell.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz AP, Chhatwal JP, Hedden T, Mormino EC, Hanseeuw BJ, Sepulcre J, Huijbers W, LaPoint M, Buckley RF, Johnson KA, et al. Phases of hyperconnectivity and hypoconnectivity in the defaunlt mode and salience networks track with amyloid and tau in clinically normal individuals. J Neurosci. 2017:37(16):4323–4331. 10.1523/JNEUROSCI.3263-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med. 2016:8(6):595–608. 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Dickerson BC, Pihlajamaki M, Vannini P, LaViolette PS, Vitolo OV, Hedden T, Becker JA, Rentz DM, Selkoe DJ, et al. Functional alterations in memory networks in early Alzheimer’s disease. NeuroMolecular Med. 2010:12(1):27–43. 10.1007/s12017-009-8109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner ML, Engle RW. Is working memory capacity task dependent? J Mem Lang. 1989:28(2):127–154. 10.1016/0749-596X(89)90040-5. [DOI] [Google Scholar]
- Webb CE, Rodrigue KM, Hoagey DA, Foster CM, Kennedy KM. Contributions of white matter connectivity and BOLD modulation to cognitive aging: a lifespan structure-function association study. Cereb Cortex. 2020:30(3):1649–1661. 10.1093/cercor/bhz193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler adult intelligence scale-III (WAIS-III). New York (NY): Psychological Corporation; 1997. [Google Scholar]
- Wierenga CE, Benjamin M, Gopinath K, Perlstein WM, Leonard CM, Rothi LJG, Conway T, Cato MA, Briggs R, et al. Age-related changes in word retrieval: role of bilateral frontal and subcortical networks. Neurobiol Aging. 2008:29(3):436–451. 10.1016/j.neurobiolaging.2006.10.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and analysis code for this project are available at https://osf.io/7rsm3/? and unthresholded functional maps are available at https://neurovault.org/collections/ZWMWDJJV/.