Abstract
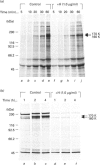
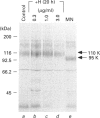
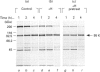
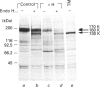
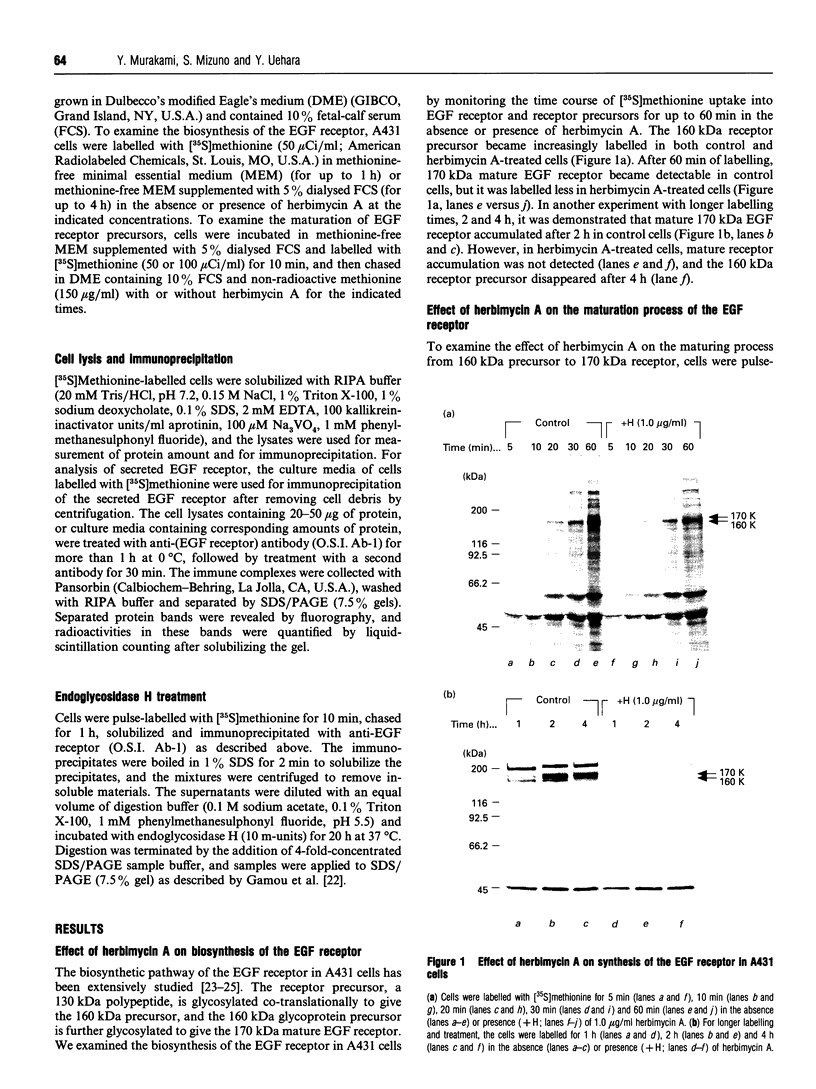
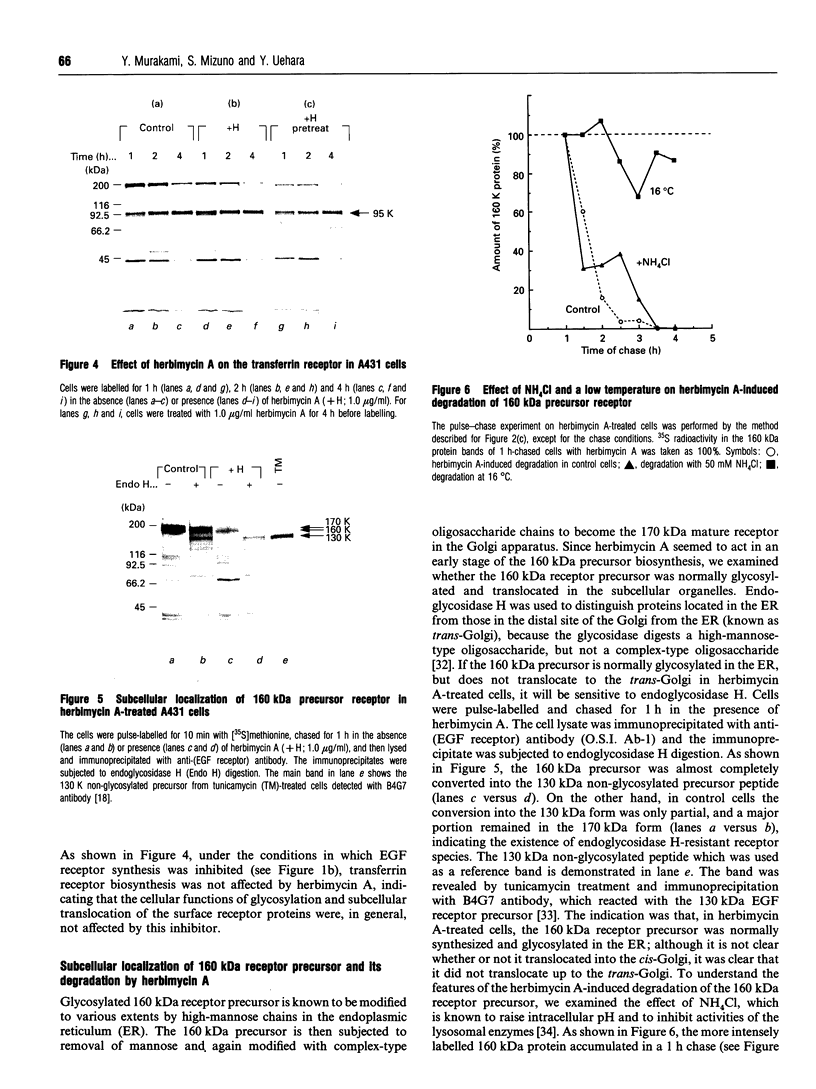
The effect of herbimycin A on the biosynthesis of epidermal growth factor (EGF) receptor was examined in human epidermoid carcinoma A431 cells. Cells were pulse-labelled with [35S]methionine, and EGF receptor biosynthesis was quantified by immunoprecipitation using a monoclonal anti-(EGF receptor) antibody. In the presence of herbimycin A, an immature 160 kDa EGF receptor precursor accumulated in 1 h and disappeared completely in 4 h. Pulse-labelled 160 kDa receptor precursor in the absence of herbimycin A, however, was converted normally into a 170 kDa one by chase with herbimycin A. Herbimycin A affected neither the synthesis of the secreted form of EGF receptor devoid of cytoplasmic domain, nor that of the transferrin receptor in A431 cells. The herbimycin A-induced degradation of 160 kDa EGF receptor precursor was not inhibited by an inhibitor of lysosomal enzymes, NH4Cl. Endoglycosidase H digestion of the 160 kDa precursor converted it into the deglycosylated 130 kDa precursor peptide. These results suggested that herbimycin A selectively acted on the EGF receptor precursor during the synthesis of the 160 kDa form, probably on the cytoplasmic domain, to form an aberrant molecule which was subjected to rapid degradation in the endoplasmic reticulum.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aboud-Pirak E., Hurwitz E., Pirak M. E., Bellot F., Schlessinger J., Sela M. Efficacy of antibodies to epidermal growth factor receptor against KB carcinoma in vitro and in nude mice. J Natl Cancer Inst. 1988 Dec 21;80(20):1605–1611. doi: 10.1093/jnci/80.20.1605. [DOI] [PubMed] [Google Scholar]
- Behzadian M. A., Shimizu N. Monoclonal antibody that immunoreacts with a subclass of human receptors for epidermal growth factor. Cell Struct Funct. 1985 Sep;10(3):219–232. doi: 10.1247/csf.10.219. [DOI] [PubMed] [Google Scholar]
- Brugge J. S. Interaction of the Rous sarcoma virus protein pp60src with the cellular proteins pp50 and pp90. Curr Top Microbiol Immunol. 1986;123:1–22. doi: 10.1007/978-3-642-70810-7_1. [DOI] [PubMed] [Google Scholar]
- Carlin C. R., Knowles B. B. Biosynthesis and glycosylation of the epidermal growth factor receptor in human tumor-derived cell lines A431 and Hep 3B. Mol Cell Biol. 1986 Jan;6(1):257–264. doi: 10.1128/mcb.6.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin C. R., Knowles B. B. Biosynthesis of the epidermal growth factor receptor in human epidermoid carcinoma-derived A431 cells. J Biol Chem. 1984 Jun 25;259(12):7902–7908. [PubMed] [Google Scholar]
- Carpenter G. Receptors for epidermal growth factor and other polypeptide mitogens. Annu Rev Biochem. 1987;56:881–914. doi: 10.1146/annurev.bi.56.070187.004313. [DOI] [PubMed] [Google Scholar]
- Decker S. J. Aspects of the metabolism of the epidermal growth factor receptor in A431 human epidermoid carcinoma cells. Mol Cell Biol. 1984 Apr;4(4):571–575. doi: 10.1128/mcb.4.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorner A. J., Bole D. G., Kaufman R. J. The relationship of N-linked glycosylation and heavy chain-binding protein association with the secretion of glycoproteins. J Cell Biol. 1987 Dec;105(6 Pt 1):2665–2674. doi: 10.1083/jcb.105.6.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feig L. A. The many roads that lead to Ras. Science. 1993 May 7;260(5109):767–768. doi: 10.1126/science.8484117. [DOI] [PubMed] [Google Scholar]
- Fukazawa H., Mizuno S., Uehara Y. Effects of herbimycin A and various SH-reagents on p60v-src kinase activity in vitro. Biochem Biophys Res Commun. 1990 Nov 30;173(1):276–282. doi: 10.1016/s0006-291x(05)81053-8. [DOI] [PubMed] [Google Scholar]
- Gamou S., Hirai M., Rikimaru K., Enomoto S., Shimizu N. Biosynthesis of the epidermal growth factor receptor in human squamous cell carcinoma lines: secretion of the truncated receptor is not common to epidermal growth factor receptor-hyperproducing cells. Cell Struct Funct. 1988 Feb;13(1):25–38. doi: 10.1247/csf.13.25. [DOI] [PubMed] [Google Scholar]
- Gamou S., Shimizu N. Change in metabolic turnover is an alternate mechanism increasing cell surface epidermal growth factor receptor levels in tumor cells. J Biol Chem. 1987 May 15;262(14):6708–6713. [PubMed] [Google Scholar]
- Hendershot L., Bole D., Köhler G., Kearney J. F. Assembly and secretion of heavy chains that do not associate posttranslationally with immunoglobulin heavy chain-binding protein. J Cell Biol. 1987 Mar;104(3):761–767. doi: 10.1083/jcb.104.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins C. R., Trowbridge I. S. Internalization and processing of transferrin and the transferrin receptor in human carcinoma A431 cells. J Cell Biol. 1983 Aug;97(2):508–521. doi: 10.1083/jcb.97.2.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunts J., Gamou S., Hirai M., Shimizu N. Molecular mechanisms involved in increasing epidermal growth factor receptor levels on the cell surface. Jpn J Cancer Res. 1986 May;77(5):423–427. [PubMed] [Google Scholar]
- Kawamoto T., Mendelsohn J., Le A., Sato G. H., Lazar C. S., Gill G. N. Relation of epidermal growth factor receptor concentration to growth of human epidermoid carcinoma A431 cells. J Biol Chem. 1984 Jun 25;259(12):7761–7766. [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Levitzki A. Tyrphostins: tyrosine kinase blockers as novel antiproliferative agents and dissectors of signal transduction. FASEB J. 1992 Nov;6(14):3275–3282. doi: 10.1096/fasebj.6.14.1426765. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Bonifacino J. S., Yuan L. C., Klausner R. D. Degradation from the endoplasmic reticulum: disposing of newly synthesized proteins. Cell. 1988 Jul 15;54(2):209–220. doi: 10.1016/0092-8674(88)90553-3. [DOI] [PubMed] [Google Scholar]
- May W. S., Jr, Cuatrecasas P. Transferrin receptor: its biological significance. J Membr Biol. 1985;88(3):205–215. doi: 10.1007/BF01871086. [DOI] [PubMed] [Google Scholar]
- Mayes E. L., Waterfield M. D. Biosynthesis of the epidermal growth factor receptor in A431 cells. EMBO J. 1984 Mar;3(3):531–537. doi: 10.1002/j.1460-2075.1984.tb01842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick F. Signal transduction. How receptors turn Ras on. Nature. 1993 May 6;363(6424):15–16. doi: 10.1038/363015a0. [DOI] [PubMed] [Google Scholar]
- Merlino G. T., Xu Y. H., Ishii S., Clark A. J., Semba K., Toyoshima K., Yamamoto T., Pastan I. Amplification and enhanced expression of the epidermal growth factor receptor gene in A431 human carcinoma cells. Science. 1984 Apr 27;224(4647):417–419. doi: 10.1126/science.6200934. [DOI] [PubMed] [Google Scholar]
- Moroni M. C., Willingham M. C., Beguinot L. EGF-R antisense RNA blocks expression of the epidermal growth factor receptor and suppresses the transforming phenotype of a human carcinoma cell line. J Biol Chem. 1992 Feb 5;267(4):2714–2722. [PubMed] [Google Scholar]
- Murakami Y., Fukazawa H., Mizuno S., Uehara Y. Conversion of epidermal growth factor (EGF) into a stimulatory ligand for A431-cell growth by herbimycin A by decreasing the level of expression of EGF receptor. Biochem J. 1994 Jul 1;301(Pt 1):57–62. doi: 10.1042/bj3010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami Y., Uehara Y., Yamamoto C., Fukazawa H., Mizuno S. Induction of hsp 72/73 by herbimycin A, an inhibitor of transformation by tyrosine kinase oncogenes. Exp Cell Res. 1991 Aug;195(2):338–344. doi: 10.1016/0014-4827(91)90382-5. [DOI] [PubMed] [Google Scholar]
- Ozawa S., Ueda M., Ando N., Abe O., Hirai M., Shimizu N. Stimulation by EGF of the growth of EGF receptor-hyperproducing tumor cells in athymic mice. Int J Cancer. 1987 Nov 15;40(5):706–710. doi: 10.1002/ijc.2910400523. [DOI] [PubMed] [Google Scholar]
- Ozawa S., Ueda M., Ando N., Abe O., Shimizu N. High incidence of EGF receptor hyperproduction in esophageal squamous-cell carcinomas. Int J Cancer. 1987 Mar 15;39(3):333–337. doi: 10.1002/ijc.2910390311. [DOI] [PubMed] [Google Scholar]
- Poole B., Ohkuma S. Effect of weak bases on the intralysosomal pH in mouse peritoneal macrophages. J Cell Biol. 1981 Sep;90(3):665–669. doi: 10.1083/jcb.90.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainsbury J. R., Farndon J. R., Needham G. K., Malcolm A. J., Harris A. L. Epidermal-growth-factor receptor status as predictor of early recurrence of and death from breast cancer. Lancet. 1987 Jun 20;1(8547):1398–1402. doi: 10.1016/s0140-6736(87)90593-9. [DOI] [PubMed] [Google Scholar]
- Santon J. B., Cronin M. T., MacLeod C. L., Mendelsohn J., Masui H., Gill G. N. Effects of epidermal growth factor receptor concentration on tumorigenicity of A431 cells in nude mice. Cancer Res. 1986 Sep;46(9):4701–4705. [PubMed] [Google Scholar]
- Schneider C., Sutherland R., Newman R., Greaves M. Structural features of the cell surface receptor for transferrin that is recognized by the monoclonal antibody OKT9. J Biol Chem. 1982 Jul 25;257(14):8516–8522. [PubMed] [Google Scholar]
- Sharma S., Rodgers L., Brandsma J., Gething M. J., Sambrook J. SV40 T antigen and the exocytotic pathway. EMBO J. 1985 Jun;4(6):1479–1489. doi: 10.1002/j.1460-2075.1985.tb03806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderquist A. M., Carpenter G. Glycosylation of the epidermal growth factor receptor in A-431 cells. The contribution of carbohydrate to receptor function. J Biol Chem. 1984 Oct 25;259(20):12586–12594. [PubMed] [Google Scholar]
- Tarentino A. L., Maley F. Purification and properties of an endo-beta-N-acetylglucosaminidase from Streptomyces griseus. J Biol Chem. 1974 Feb 10;249(3):811–817. [PubMed] [Google Scholar]
- Uehara Y., Fukazawa H., Murakami Y., Mizuno S. Irreversible inhibition of v-src tyrosine kinase activity by herbimycin A and its abrogation by sulfhydryl compounds. Biochem Biophys Res Commun. 1989 Sep 15;163(2):803–809. doi: 10.1016/0006-291x(89)92293-6. [DOI] [PubMed] [Google Scholar]
- Uehara Y., Murakami Y., Mizuno S., Kawai S. Inhibition of transforming activity of tyrosine kinase oncogenes by herbimycin A. Virology. 1988 May;164(1):294–298. doi: 10.1016/0042-6822(88)90649-6. [DOI] [PubMed] [Google Scholar]
- Uehara Y., Murakami Y., Sugimoto Y., Mizuno S. Mechanism of reversion of Rous sarcoma virus transformation by herbimycin A: reduction of total phosphotyrosine levels due to reduced kinase activity and increased turnover of p60v-src1. Cancer Res. 1989 Feb 15;49(4):780–785. [PubMed] [Google Scholar]
- Ullrich A., Coussens L., Hayflick J. S., Dull T. J., Gray A., Tam A. W., Lee J., Yarden Y., Libermann T. A., Schlessinger J. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. 1984 May 31-Jun 6Nature. 309(5967):418–425. doi: 10.1038/309418a0. [DOI] [PubMed] [Google Scholar]
- Velu T. J., Beguinot L., Vass W. C., Willingham M. C., Merlino G. T., Pastan I., Lowy D. R. Epidermal-growth-factor-dependent transformation by a human EGF receptor proto-oncogene. Science. 1987 Dec 4;238(4832):1408–1410. doi: 10.1126/science.3500513. [DOI] [PubMed] [Google Scholar]
- Wong A. J., Bigner S. H., Bigner D. D., Kinzler K. W., Hamilton S. R., Vogelstein B. Increased expression of the epidermal growth factor receptor gene in malignant gliomas is invariably associated with gene amplification. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6899–6903. doi: 10.1073/pnas.84.19.6899. [DOI] [PMC free article] [PubMed] [Google Scholar]