Abstract
Density-dependent competition for food influences the foraging behaviour and demography of colonial animals, but how this influence varies across a species’ latitudinal range is poorly understood. Here we used satellite tracking from 21 Northern Gannet Morus bassanus colonies (39% of colonies worldwide, supporting 73% of the global population) during chick-rearing to test how foraging trip characteristics (distance and duration) covary with colony size (138–60 953 breeding pairs) and latitude across 89% of their latitudinal range (46.81–71.23° N). Tracking data for 1118 individuals showed that foraging trip duration and maximum distance both increased with square-root colony size. Foraging effort also varied between years for the same colony, consistent with a link to environmental variability. Trip duration and maximum distance also decreased with latitude, after controlling for colony size. Our results are consistent with density-dependent reduction in prey availability influencing colony size and reveal reduced competition at the poleward range margin. This provides a mechanism for rapid population growth at northern colonies and, therefore, a poleward shift in response to environmental change. Further work is required to understand when and how colonial animals deplete nearby prey, along with the positive and negative effects of social foraging behaviour.
Keywords: central place foraging, coloniality, species distributions, bio-logging, predator–prey, seabird
1. Background
Foraging is strongly affected by group living; there can be benefits via social information transfer [1,2] and costs via intraspecific competition for food [3–5]. Most studies of colonial animals suggest that foraging effort covaries with population size because large colonies deplete nearby prey and so individuals must travel further to forage [6–9]. This relationship may be altered by local environmental conditions that impact food availability and thus affect foraging behaviour [10,11], including at species’ range margins where competition may be lower [12]. Nevertheless, multi-population studies tend to be based on a small number of colonies and confined to a relatively small proportion of a species’ range [6,13] and rarely include populations at the extremes of their distribution. Understanding how foraging behaviour varies across the full extent of a species’ range could generate important insights into the causes and consequences of group living, as well as responses to global change.
Much research on foraging by colonial animals comes from seabirds. Over 95% of seabird species nest in colonies and they form some of the largest non-human vertebrate aggregations [14]. Density-dependent food competition arises in breeding seabirds where a zone of reduced prey accessibility occurs around colonies—Ashmole’s halo [3]. As a population grows, competition induces longer foraging trips, which reduces chick feeding rates and in turn reproductive success, as well as adult survival, eventually constraining colony size [3,15–18]. As well as direct measures of reduced prey availability around some colonies [5,19], support for this hypothesis comes primarily from inter-colony comparisons of foraging behaviour [6,8,9,13]. Density-dependent effects on seabird foraging interact with the effects of local environmental conditions [17]. This is important since reduced prey availability is thought to be the primary climate-related threat to seabirds [20], especially during breeding when birds are constrained to the colony [21,22]. Many seabird prey species are shifting poleward [23–26], and while some seabird populations mirror this [27–30], their ability to keep pace may be limited because individuals are generally faithful to their natal and breeding sites [31,32] and colony formation is rare [33].
Here, we study the foraging behaviour of Northern Gannets Morus bassanus (hereafter ‘gannet’) in relation to colony size and latitude. Gannet foraging trips are longer on average at larger colonies [6,17], and there is evidence for shorter foraging trips at more northerly colonies [34,35]. Gannets currently breed at 54 colonies across the North Atlantic, having experienced a rapid population increase over the last century [36,37]. This was probably influenced by reduced persecution [38] and increased food availability, potentially including fishery discards [39]. Since 1935, gannets have expanded their range northwards by 7.7°, with no southward change [27,40,41]. This may have been facilitated by ocean warming bringing influxes of prey in the north [42–45] but poor conditions in the south [46–49]. Increased availability of gannet prey species in the north, such as Atlantic Mackerel Scomber scombrus [42,44,45], may be linked to lower foraging effort [35]. More recently, population growth and range expansion may be impacted by thousands of gannets dying in an outbreak of highly pathogenic avian influenza (HPAI) H5N1 that began in 2021, but the scale of this is not yet fully known [50,51].
Here, we measured gannet foraging effort (foraging trip range and duration) from satellite tracking for 21 of the 54 known gannetries (varying in size from 138 to 60 953 nests and supporting approximately 73% of the global population [37]), spanning 89% of their latitudinal breeding extent. Birds were tracked for multiple years at some colonies, all before the recent HPAI outbreak. We tested how this covaries with colony size, latitude and year. We predict foraging effort to covary positively with colony size but also colonies at the expanding northern edge of the species’ range to have lower foraging effort than expected for their population size.
2. Methods
2.1. Foraging effort
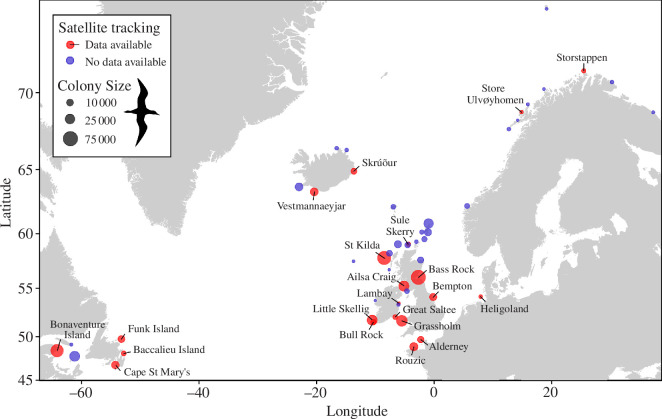
We collated information on foraging trip duration and maximum distance travelled from the colony for 21 colonies (electronic supplementary material, figure S1), calculated from more than 7100 trips for 1118 chick-rearing gannets, collected between 1998 and 2021. Ranging from 46.81° N to 71.23° N (2714 km at equivalent longitude), colonies span 89% of the latitudinal extent of the species’ breeding colonies (3045 km at equivalent longitude) from Cape St Mary’s (46.81° N) to Funk Island (49.75° N) in Newfoundland, and from Rouzic (48.90° N) to Bjørnøya, Svalbard (74.21° N) in the Northeast Atlantic ([27,52]; figure 1).
Figure 1.
All 54 Northern Gannet Morus bassanus colonies with circles proportional to colony size. Red circles indicate 21 colonies for which satellite tracking data were used in this study. No data available for colonies indicated by blue circles. Map adapted from tiles by Stamen Design, under Creative Commons (CC BY 3.0) using data by OpenStreetMap, under the Open Database Licence.
We combined seven published means and 14 direct estimates from tracking data located via the BirdLife International Seabird Tracking Database accessed at www.seabirdtracking.org (figure 1, table 1, electronic supplementary material, table S1). Multiple years of tracking data [2–11] were available for 13 colonies. Where multi-year data were available, we used the mean across all trips to produce colony-level values unless otherwise indicated. Sampling frequency varied from 1 s to 180 min. Only Great Saltee had this lowest frequency, and only for five of the 71 birds tracked. Most movement data came from precision global positioning system (GPS) devices (archival and remote download), except for St Kilda, UK, where birds were fitted with platform terminal transmitters (PTTs; which relayed locations via the ARGOS satellite systems with a median of 75 min between locations [12]). PTTs are less likely to record at regular intervals compared with GPS loggers [69], so we removed foraging trips with poor data quality (one trip with only two records and 12 with location intervals over 3 h).
Table 1.
Foraging effort and population size for 21 Northern Gannet Morus bassanus colonies ordered by latitude [12,35,47,48,53–64]. Values are given ± s.d. where available. See electronic supplementary material, table S1 for longitude, years of counts and tracking data, sampling frequency, sample size and total distance travelled. *Colonies in the West Atlantic.
| colony | lat. | mean duration (h) | mean max. distance (km) | no. birds | source of tracking data | count (AOS/AON) | source of colony count |
|---|---|---|---|---|---|---|---|
| Cape St Mary’s* | 46.81 | 14.3 ± 0.5 | 72.4 ± 1.9 | 22 | [59] | 14 598 | [49] |
| Baccalieu Is* | 48.15 | 9.3 ± 7.3 | 39.9 ± 24.7 | 6 | [60]a | 2253 | [52] |
| Bonaventure* | 48.48 | 28 | 132 | 14 | [61] | 53 635 | |
| Rouzic | 48.90 | 25.1 ± 10.9 | 124.2 ± 50.0 | 169 | [48] | 20 400 | This study |
| Alderney | 49.71 | 23.0 | 122.0 | 60 | [47] | 7885 | [37] |
| Funk Is.* | 49.75 | 16.5 | 102.6 | 26 | [62] | 10 047 | [52] |
| Bull Rock | 51.58 | 11.9 ± 8.1 | 69.8 ± 34.1 | 14 | [12]a | 3694 | [65] |
| Grassholm | 51.73 | 21.8 ± 1.7 | 116.8 ± 7.4 | 304 | [63] | 36 011 | [66] |
| Little Skellig | 51.78 | 13.4 ± 11.8 | 96.5 ± 61.8 | 9 | [12]a | 29 683 | [65,67] |
| Great Saltee | 52.11 | 21.5 | 98.5 | 71 | [12]a; [64]; This study | 4722 | |
| Lambay | 53.50 | 11.6 ± 7.6 | 37.5 ± 19.6 | 3 | [12]a | 138 | JNCC 2010 |
| Bempton | 54.15 | 9.1 | 45.7 | 35 | [53]; This study | 11 061 | JNCC 2012 |
| Heligoland | 54.18 | 7.9 ± 8.0 | 42.0 ± 45.7 | 3 | [54] | 656 | [66] |
| Ailsa Craig | 55.25 | 26.2 ± 16.2 | 152.3 ± 70.5 | 16 | [12]a | 33 226 | |
| Bass Rock | 56.08 | 25.6 ± 12.6 | 200.1 ± 98.5 | 206 | [55–57]a | 60 953 | JNCC 2009 |
| St Kilda | 57.86 | 24.3 ± 14.8 | 164.2 ± 124.1 | 21 | [12]a | 60 290 | [66] |
| Sule Skerry | 59.08 | 14.4 ± 5.9 | 72.9 ± 19.8 | 2 | 1870 | ||
| Vestmann-Aeyjar | 63.36 | 10.2 ± 7.4 | 43.0 ± 27.0 | 9 | [35] | 15 044 | [68] |
| Skrúður | 64.90 | 4.8 ± 4.5 | 29.2 ± 24.2 | 27 | 6051 | ||
| Store Ulvøyhomen | 68.85 | 6.9 | 22.3 | 43 | [58] | 308 | [27] |
| Storstappen | 71.23 | 6.6 | 38.7 | 58 | 1244 |
Mean values were not available in the published sources, so tracking datasets were provided by the authors.
AON, apparently occupied nests; AOS, apparently occupied sites; JNCC, Joint Nature Conservation Committee Seabird Monitoring Program database (http://jncc.defra.gov.uk/smp/ accessed 14 January 2019).
2.2. Colony size
We collated gannet colony size based on occupied sites or nests (range: 138−60 953) for the year closest to tracking studies, with a mean difference of 1.75 years (range: 0−7; table 1; electronic supplementary material, table S1). Our study includes data from 21 colonies with a combined estimated population of 382 906 nests, approximately 73% of the global population (525 694 pairs from 54 colonies [66]; table 1). We treated the islands of the Vestmannaeyjar archipelago including Hellisey, and Les Etacs and Ortac in Alderney, as single colonies because of their proximity (approx. 5 km).
2.3. Statistical analysis
We tested the effects of colony size and latitude on foraging trip duration and maximum distance using linear models. We square-root transformed colony counts as relationships between mean trip duration and square-root colony size are approximately linear [6] and because foraging area (km2) increases with the square of distance (km) [70]. Traditional standard error calculations assume infinite populations. This is not normally problematic when sampling from large populations, but when the sample is a large proportion of the total population (greater than 5–10%), the overestimation of the standard error becomes important [71,72]. As we sampled 21 of the 54 known colonies (39%), we implemented a finite population correction in the ‘survey’ R package’s ‘svyglm’ function [73], using a conservative upper estimate of 60 colonies worldwide. We used the Rao–Scott working likelihood test for model selection, using the default linear combination of F distributions with 17 denominator degrees of freedom to generate the likelihood ratio [74]. We calculated adjusted pseudo r2 values in the ‘jtools’ R package [75], and delta (Δ) pseudo r2 values for each explanatory variable to compare their relative importance. We also ran these models excluding the six colonies for which nine or fewer individuals were tracked (electronic supplementary material, table S2). We did not have a large enough sample size to test for an interaction between latitude and colony size. We also tested how well trip duration predicts maximum distance (Results in electronic supplementary material, figure S2).
As 13 colonies had multiple years (range: 2−10) of tracking data (electronic supplementary material, table S3), we separately fitted linear mixed models explaining annual mean trip duration and maximum distance in relation to colony size, and either latitude or longitude using the ‘spaMM’ R package [76]. We fitted year as a linear numeric variable to check for and control for any potential long-term trends over the 24-year study period (1998−2021). We included colony ID by fitting it as a random intercept. To account for any spatial autocorrelation, we applied a spatial random effect using a Matérn correlation function to the great circle distances between colonies [76]. We checked the residuals using simulation with the ‘DHARMa’ R package [77]. We did not apply a finite population correction to the standard error because we were not aware of an applicable method for linear mixed models with mean sample sizes of fewer than 10 years within each colony and fewer than 30 colonies [72]. R scripts and data are available at https://github.com/bethclark36/ForagingEffortGannets [78], archived at https://doi.org/10.5281/zenodo.12796423 [79].
3. Results
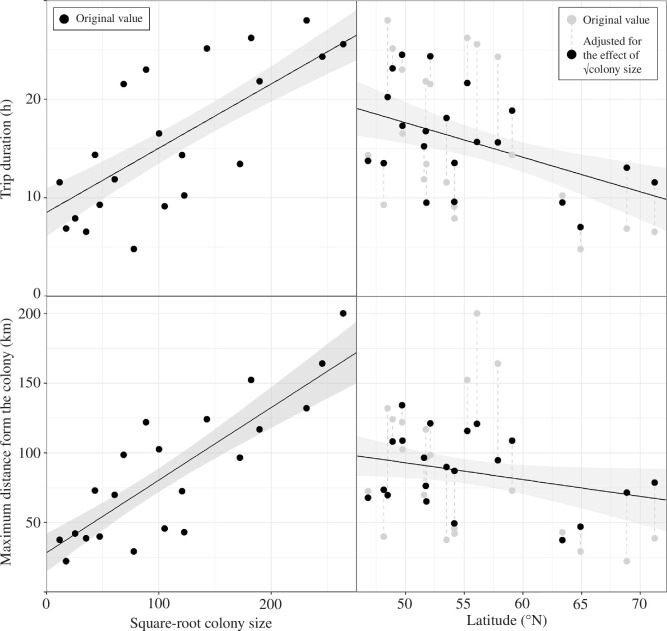
Foraging trip duration and maximum distance reached from the colony increased significantly linearly with square-root colony size, and trip duration significantly decreased with latitude—colony size had a much larger effect size than latitude (table 2; figure 2). Linear models including both square-root colony size and latitude had a pseudo-adjusted r2 of 0.62 for foraging trip duration and 0.72 for maximum distance. Results were similar when excluding six colonies with nine or fewer tracked individuals (electronic supplementary material, table S2).
Table 2.
Parameter estimates for linear models fitted with the finite population correction explaining Northern Gannet Morus bassanus colony means for foraging trip duration and maximum distance for 21 colonies. Delta (Δ) pseudo-adjusted r2 is the difference in pseudo-adjusted r2 between the models with and without the explanatory variable included and are thus a measure of the contribution of each variable to explaining the variation in foraging effort.
| foraging effort | explanatory variable | estimate ± s.e. | 2log LR | d.f. | p value | Δ pseudo r2 |
|---|---|---|---|---|---|---|
| trip duration (h) ~ | intercept | 27.808 ± 6.246 | — | — | — | — |
| √colony size | 0.065 ± 0.007 | 78.15 | 1,18 | <0.001 | 0.41 | |
| latitude | −0.350 ± 0.101 | 11.99 | 1,18 | 0.003 | 0.08 | |
| maximum distance (km) ~ | intercept | 94.623 ± 37.554 | — | — | — | — |
| √colony size | 0.521 ± 0.058 | 81.514 | 1,18 | <0.001 | 0.62 | |
| latitude | −1.202 ± 0.620 | 2.423 | 1,18 | 0.071 | 0.02 |
Figure 2.
Mean foraging trip duration and maximum distance from the colony for 21 Northern Gannet Morus bassanus colonies in relation to colony size and latitude. Black lines show the prediction from a finite population corrected linear model ±95% confidence intervals (grey ribbon) using the mean value of latitude to predict the effect of square-root (√) colony size and vice versa. To visually correct for √colony size, we subtracted the √colony size effect (grey dashed line) from each data point (grey circle) and then added the √colony size effect for the mean √colony size (black circle). See electronic supplementary material, figure S3 for figure 2 with points labelled by colony name.
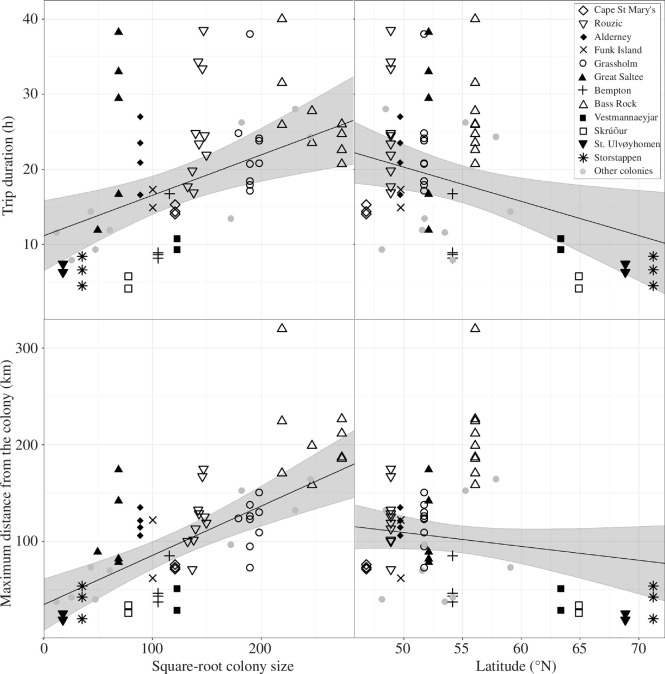
When modelling annual means, trip duration and maximum distance increase with colony size and decreased with latitude (figure 3). Square-root colony size appeared in the top models for trip duration and maximum distance, with a substantial drop in pseudo r2 if not included (table 3). Latitude also appeared in the top and second-best models for trip duration, with the third model falling below the heuristic 2 ΔAIC cut-off. Longitude and the linear effects of year were consistently indicated to be of lesser importance than both colony size and latitude in these models, with their inclusion being limited to either models substantially worse than the top model, or in models with both colony size and latitude. The top models for maximum distance did not show a pattern of preferentially including latitude, longitude or the linear effect of year, indicating little evidence that they were having a large effect. Trip duration and maximum distance varied substantially at some colonies among years within the same colony (figure 3).
Figure 3.
Annual mean foraging trip duration and maximum distance from the colony for 21 Northern Gannet Morus bassanus colonies in relation to colony size and latitude. Black symbols show the 13 colonies for which multiple years of tracking data were available (2−11 years). Grey circles indicate the eight colonies where only 1 year of data was available. Black lines show the prediction from a linear mixed model with colony fitted as a random intercept ±95% confidence intervals (grey ribbon) using the mean value of latitude to predict the effect of square-root colony size and vice versa.
Table 3.
Candidate models for linear mixed models for Northern Gannet Morus bassanus annual colony means for foraging trip duration and maximum distance from the colony, with colony and a distance matrix fitted as random effects. mAIC = marginal Akaike information criterion.
| response | fixed effects | mAIC | Δ mAIC | pseudo r2 | |||
|---|---|---|---|---|---|---|---|
| foraging trip duration ~ | √colony size | +latitude | 442.01 | 0 | 0.450 | ||
| √colony size | +latitude | +year | 443.98 | 1.97 | 0.451 | ||
| √colony size | 444.60 | 2.59 | 0.410 | ||||
| √colony size | +longitude | 446.47 | 4.46 | 0.411 | |||
| √colony size | +year | 446.49 | 4.48 | 0.411 | |||
| latitude | 447.87 | 5.86 | 0.379 | ||||
| √colony size | +longitude | +year | 448.35 | 6.34 | 0.412 | ||
| latitude | +year | 449.30 | 7.29 | 0.384 | |||
| longitude | 451.90 | 9.89 | 0.338 | ||||
| year | 452.47 | 10.46 | 0.332 | ||||
| longitude | +year | 452.97 | 10.96 | 0.348 | |||
| none | 454.01 | 12.00 | 0.272 | ||||
| maximum distance from the colony ~ | √colony size | 655.24 | 0 | 0.632 | |||
| √colony size | +latitude | 655.42 | 0.18 | 0.642 | |||
| √colony size | +longitude | 656.41 | 1.17 | 0.636 | |||
| √colony size | +year | 656.62 | 1.38 | 0.635 | |||
| √colony size | +latitude | +year | 656.71 | 1.47 | 0.646 | ||
| √colony size | +longitude | +year | 657.70 | 2.46 | 0.640 | ||
| latitude | 668.74 | 13.50 | 0.545 | ||||
| latitude | +year | 670.64 | 15.40 | 0.546 | |||
| longitude | 671.34 | 16.10 | 0.526 | ||||
| year | 671.78 | 16.54 | 0.523 | ||||
| none | 673.16 | 17.92 | 0.481 | ||||
| longitude | +year | 673.17 | 17.93 | 0.527 |
4. Discussion
Gannet foraging trip duration and maximum distance from the colony significantly increased with colony size, and after controlling for this, trip duration significantly decreased with latitude. We also recorded interannual variation within colonies but no long-term trend over the study period. We discuss these results in the context of density-dependent food competition, potential mechanisms of latitude effects, and how colonial animals might respond to global change.
4.1. Foraging effort and colony size
Our results provide a clear illustration that foraging effort increases with colony size in gannets, supporting previous studies of this species that used colony-based observations rather than biologging [6,17]. Our results are also consistent with multi-colony comparisons of other seabirds including shearwaters [80], tropical sulids [8] and guillemots [9]. Few studies have measured foraging behaviour across so many sites, but Patterson et al. [9] is a clear exception. They used tracking data from 29 murre colonies (7.6% of the 384 regional Common Murre Uria aalge and Thick-billed Murre Uria lomvia colonies) to estimate that foraging range scales with the cube root of colony size rather than the square-root used in our analysis.
Among gannets, relationships between colony size and foraging metrics were strong with delta pseudo r2 values of 0.41 for trip duration and 0.62 for maximum distance travelled. Differences in explanatory power between duration and distance may relate to individual foraging site fidelity. For example, adult gannets learn foraging locations [81], which are highly spatially repeatable both within and among years, while trip duration tends to be more variable presumably due to differences in routes, wind conditions and food availability among trips [56,82].
4.2. Interannual variation in foraging effort
Multi-year tracking revealed interannual variation in foraging effort within colonies, sometimes by tens of hours, but no detectable long-term trend over the study period. This is probably due to variable local environmental conditions since seabird foraging trips tend to be longer during years of poor environmental conditions [10,11,83,84]. ‘Good’ and ‘bad’ conditions for foraging gannets are unlikely to be synchronized across all colonies, especially for those that are far apart. Continued repeated tracking over a longer period could shed light on progressive changes in environmental conditions, particularly in a comparison between long-term trends in colonies in the far south and north.
4.3. Foraging effort and latitude
Controlling for colony size, we detected a latitudinal gradient in foraging trip duration and maximum distance. Such an effect was not detected in murres across a similar region [9]. There are at least three non-exclusive plausible explanations for why gannets have shorter foraging ranges in the north compared with similar-sized colonies further south. First, since gannets are primarily diurnal foragers, and generally rest on the water overnight [85], birds breeding in locations with long nights may have longer trips compared with high-latitude breeders experiencing shorter nights. Second, since many of the northerly colonies have fewer surrounding gannet populations, lower foraging effort may relate to reduced inter-colony competition as nearby colonies can increase intraspecific competition in seabirds [12,86–88]. Third, there may be an effect of climate change. At high latitudes, warming seas are associated with influxes of pelagic fish, potentially producing favourable gannet foraging conditions [27,42,44,89]. By contrast, warming at low latitudes is linked to reduced prey availability, high foraging effort and low breeding success on both sides of the Atlantic [46,47,59].
Understanding foraging ecology at high latitudes is timely because while gannet populations have increased throughout much of the twentieth and early twenty-first centuries [90], many seabirds on both sides of the North Atlantic have suffered declines in recent years, possibly linked to reduced food availability and quality driven by climate warming [65,91–93]. Most North Atlantic seabirds rely on forage fish, including sand eels Ammodytes spp. and Capelin Mallotus villosus [94], but warming reduces sand eel availability and quality [23]. For example, Atlantic Puffins Fratercula arctica breeding in declining populations had greater foraging ranges and lower chick provisioning rates [95]. Furthermore, Iceland and Norway have seen recent influxes of Atlantic Mackerel [42,44], which compete with and predate on sand eels and Capelin [96]. Similarly, increased Atlantic Herring Clupea harengus abundance has been linked to Capelin stock collapse and consequent decline in the vulnerable Black-legged Kittiwake Rissa tridactyla in Norway [89]. These changes heavily impact many seabird species, but mackerel and herring are excellent food for gannets due their large size and wide dietary breadth [39,45,97].
4.4. Mechanisms underpinning density dependence
The observed patterns are probably explained by density-dependent competition for food, leading to colony-specific halos of prey depletion or reduced prey accessibility [3]. Direct evidence for prey depletion by marine predators is scarce [5,19], but our results are consistent with previous evidence from comparing multiple colonies [6,8,9,13]. The strong positive relationship between population size and foraging trip length was apparent even though many of the sampled colonies are still growing [37] and therefore that food was not yet limiting colony size at some sites. Interannual variation in foraging effort indicates flexibility in response to fluctuating local environmental conditions, including marine heatwaves [49,98]. Breeding adult seabirds can somewhat buffer chick feeding against lower prey availability by increasing travel speed reducing resting time [98], but this may lead to carry-over effects and lower adult survival [18]. Our results are consistent with a reduced effect of competition at northern range margins for gannets [34,35]. This may allow for rapid population growth at small northern colonies and, therefore, a mechanism for a shift in the distribution of the species in response to climate change. However, we note that some northern colonies have decreased and some gone extinct, highlighting nuance in the patterns presented here [90]. We also suggest a more detailed analysis of the benefits as well as the costs of conspecific density since social interactions inform much about gannet foraging [99].
4.5. Monitoring and conservation
Monitoring foraging effort could allow us to identify struggling populations before demographic changes are detectable [18,95], particularly for long-lived, slow-breeding species [100]. Understanding the impact of environmental change on foraging behaviour may help mitigate climate change impacts by accounting for current and future foraging conditions in marine spatial planning [20], including for renewable energy development, and prioritizing restoration and area-based conservation measures [101,102]. Some species may be able to shift into more suitable regions, but some southward-shifting seabirds are already reaching the limits of available land for nesting [28,29]. Seabirds may also be restricted to foraging in suitable wave and wind regimes [103], and light availability near the poles [104,105]. Finally, the recent outbreak of HPAI (2021–2023; [51]) caused unprecedented seabird mortality across the North Atlantic and beyond, with thousands of gannets dying in 40 colonies on both sides of the Atlantic [50]. Our results provide baselines for gannet foraging trip metrics, which can be compared with data from during and after the outbreak to shed more light on density-dependence in colonial species. Five gannets tracked from the Bass Rock in 2022 made longer foraging trips during the outbreak and visited other colonies, which was not previously observed and could spread the disease [106]. After the 2022 outbreak, tracked gannets had shorter foraging trips than before, consistent with reduced intraspecific competition [106,107]. Reductions in colony size could greatly alter foraging seabird ranges with wide-ranging implications such as for marine spatial planning, further highlighting the importance of ongoing surveillance. Overall, understanding spatial variation in prey availability is key to future-proofing the conservation of seabirds and other colonial species.
5. Conclusion
Breeding gannets’ foraging effort increased with colony size across their latitudinal range, probably due to density-dependent competition for food. Moreover, we also found interannual variation, and an effect of latitude suggesting reduced intraspecific competition in the north of their distribution. These results, therefore, indicate how foraging flexibility may enable colonial animals to respond to environmental change but are ultimately limited by prey availability.
Acknowledgements
We thank Emma Wood for advice on analysis and presenting results, Alice Williams, Richard Phillips and Brendan Godley for comments on the text, and the Fundamental & Applied Biogeography group at the University of Exeter led by Regan Early for feedback. Fieldwork on Grassholm was made possible with the help of Greg Morgan and Lisa Morgan and with permission from RSPB. Many people assisted with fieldwork on Grassholm, including Richard Sherley, Anthony Bicknell, Ian R. Cleasby, James Grecian, Sam Patrick, Kelly Atkins, Kylie Scales, Tim Guilford, Claudia Stauss, Sylvie Vandanabeele, Nicola Childs, Pearl Costello, Rocio Moreno, Matthew Gummery, Lisa Sztukowski, Jana Jeglinski, Matthew Carter, Matthew Nicholson, Dimas Gianuca, Rhiannon Meier, Laura Zango, Kirsten Archibald, Jacob Gonzalez-Solis, Jen Tyler, Tommy Clay, Calum Laver, Melanie Wells, Zoe Deakin, Zoe Courchene, Richard Phillips, John Arnould, Emma Dwan, Jack Wright, Georgia Bardua, Paulo Catry, Sarah Parmor and Megan Francis. For St Kilda data collection and processing we thank Stuart Murray and Maria Bogdanova, and the UK Centre for Ecology & Hydrology for funding. For help with fieldwork on Rouzic, we thank François Siorat, Mélanie Le Nuz, Clara Péron, Tangi le Bot, Sam Patrick, Françoise Amélineau, Nory El Kasbi, Thierry Boulinier and Jérôme Fort. We acknowledge the support of Point Blue Conservation Science. Tracking data from Newfoundland was supported by NSERC grants to W.A.M.
Contributor Information
Bethany L. Clark, Email: Bethany.Clark@birdlife.org; bethany.louise.clark@gmail.com.
Freydís Vigfúsdóttir, Email: freydisv@gmail.com.
Sarah Wanless, Email: SWANL@ceh.ac.uk.
Keith C. Hamer, Email: k.c.hamer@leeds.ac.uk.
Thomas W. Bodey, Email: thomas.bodey@abdn.ac.uk.
Stuart Bearhop, Email: s.bearhop@exeter.ac.uk.
Ashley Bennison, Email: ashben@bas.ac.uk.
Jez Blackburn, Email: jezblackburn@sky.com.
Sam L. Cox, Email: samantha.cox@ucc.ie.
Kyle J. N. d’Entremont, Email: ky927448@dal.ca.
Stefan Garthe, Email: garthe@ftz-west.uni-kiel.de.
David Grémillet, Email: david.gremillet@cefe.cnrs.fr.
Mark Jessopp, Email: M.Jessopp@ucc.ie.
Jude Lane, Email: jude.lane@rspb.org.uk.
Amélie Lescroël, Email: alescroel@pointblue.org.
William A. Montevecchi, Email: mont@mun.ca.
David J. Pascall, Email: david.pascall@mrc-bsu.cam.ac.uk.
Pascal Provost, Email: pascal.provost@lpo.fr.
Ewan D. Wakefield, Email: ewan.wakefield@durham.ac.uk.
Victoria Warwick‐Evans, Email: vicrwi@bas.ac.uk.
Saskia Wischnewski, Email: saskia.wischnewski@rspb.org.uk.
Lucy J. Wright, Email: Lucy.Wright@rspb.org.uk.
Stephen C. Votier, Email: S.Votier@hw.ac.uk.
Ethics
This work did not require ethical approval from a human subject or animal welfare committee.
Data accessibility
R scripts and data are available at [78], archived at [79]. Gannet tracking data used in this study are available to request from the BirdLife International Seabird Tracking Database (https://www.seabirdtracking.org/) with the following dataset IDs: 716–725, 728–734, 955–956, 1341–1342, 1472–1473, 1475–1478, 1543, 1636, 1638, 1653, 1660, 1793–1796, 2201. Electronic supplementary material, table S1 contains additional data for the 21 gannet colonies [108]. Figure S1 shows the relationship between trip duration and maximum distance. Electronic supplementary material, table S2 gives model estimates for colonies for which more than 10 individuals were tracked. Electronic supplementary material, table S3 gives the values derived from GPS data and colonies counts separated by year.
Declaration of AI use
We have not used AI-assisted technologies in creating this article.
Authors’ contributions
B.L.C.: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, visualization, writing—original draft, writing—review and editing; F.V.: conceptualization, investigation, writing—review and editing; S.W.: investigation, writing—review and editing; K.C.H.: investigation, writing—review and editing; T.W.B.: data curation, investigation, writing—review and editing; S.B.: investigation, writing—review and editing; A.B.: investigation, writing—review and editing; J.B.: investigation, writing—review and editing; S.L.C.: data curation, investigation, writing—review and editing; K.J.Nd’E.: data curation, investigation, writing—review and editing; S.G.: investigation, writing—review and editing; D.G.: investigation, writing—review and editing; M.J.: investigation, writing—review and editing; J.L.: data curation, investigation, writing—review and editing; A.L.: investigation, writing—review and editing; W.A.M.: investigation, writing—review and editing; D.J.P.: formal analysis, methodology, writing—review and editing; P.P.: investigation, writing—review and editing; E.D.W.: data curation, investigation, writing—review and editing; V.W.-E.: investigation, writing—review and editing; S.W.: data curation, investigation, writing—review and editing; L.J.W.: investigation, writing—review and editing; S.C.V.: funding acquisition, investigation, supervision, writing—original draft, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
This was supported by a NERC GW4+ Doctoral Training Partnership studentship to B.L.C. from the Natural Environment Research Council (NE/L002434/1), and a NERC Standard Grant to S.C.V. (NE/H007466/1).
References
- 1. Ward P, Zahavi A. 1973. The importance of certain assemblages of birds as “information‐centres” for food‐finding. Ibis 115, 517–534. ( 10.1111/j.1474-919X.1973.tb01990.x) [DOI] [Google Scholar]
- 2. Evans JC, Votier SC, Dall SRX. 2016. Information use in colonial living. Biol. Rev. Camb. Philos. Soc. 91, 658–672. ( 10.1111/brv.12188) [DOI] [PubMed] [Google Scholar]
- 3. Ashmole NP. 1963. The regulation of numbers of tropical oceanic birds. Ibis 103b, 458–473. ( 10.1111/j.1474-919X.1963.tb06766.x) [DOI] [Google Scholar]
- 4. Breed GA, Don Bowen W, Leonard ML. 2013. Behavioral signature of intraspecific competition and density dependence in colony-breeding marine predators. Ecol. Evol. 3, 3838–3854. ( 10.1002/ece3.754) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weber SB, et al. 2021. Direct evidence of a prey depletion “halo” surrounding a pelagic predator colony. Proc. Natl Acad. Sci. USA 118, e2101325118. ( 10.1073/pnas.2101325118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lewis S, Sherratt TN, Hamer KC, Wanless S. 2001. Evidence of intra-specific competition for food in a pelagic seabird. Nature New Biol. 412, 816–819. ( 10.1038/35090566) [DOI] [PubMed] [Google Scholar]
- 7. Ballance LT, Ainley DG, Ballard G, Barton K. 2009. An energetic correlate between colony size and foraging effort in seabirds, an example of the Adélie penguin Pygoscelis adeliae. J. Avian Biol. 40, 279–288. ( 10.1111/j.1600-048X.2008.04538.x) [DOI] [Google Scholar]
- 8. Oppel S, et al. 2015. Foraging distribution of a tropical seabird supports Ashmole’s hypothesis of population regulation. Behav. Ecol. Sociobiol. 69, 915–926. ( 10.1007/s00265-015-1903-3) [DOI] [Google Scholar]
- 9. Patterson A, et al. 2022. Foraging range scales with colony size in high-latitude seabirds. Curr. Biol. 32, 3800–3807.( 10.1016/j.cub.2022.06.084) [DOI] [PubMed] [Google Scholar]
- 10. Lewis S, Grémillet D, Daunt F, Ryan PG, Crawford RJM, Wanless S. 2006. Using behavioural and state variables to identify proximate causes of population change in a seabird. Oecologia 147, 606–614. ( 10.1007/s00442-005-0321-z) [DOI] [PubMed] [Google Scholar]
- 11. Piatt J, Harding A, Shultz M, Speckman S, van Pelt T, Drew G, Kettle A. 2007. Seabirds as indicators of marine food supplies: Cairns revisited. Mar. Ecol. Prog. Ser. 352, 221–234. ( 10.3354/meps07078) [DOI] [Google Scholar]
- 12. Wakefield ED, et al. 2013. Space partitioning without territoriality in gannets. Science 341, 68–70. ( 10.1126/science.1236077) [DOI] [PubMed] [Google Scholar]
- 13. Corman AM, Mendel B, Voigt CC, Garthe S. 2016. Varying foraging patterns in response to competition? A multicolony approach in a generalist seabird. Ecol. Evol. 6, 974–986. ( 10.1002/ece3.1884) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Coulson JC. 2002. Colonial breeding in seabirds. In Biology of marine birds (eds Schreiber EA, Burger J), pp. 87–114. Boca Raton, FL: CRC Press. ( 10.1201/9781420036305) [DOI] [Google Scholar]
- 15. Hunt GL, Eppley ZA, Schneider DC. 1986. Reproductive performance of seabirds: the importance of population and colony size. Auk. 103, 306–317. ( 10.1093/auk/103.2.306) [DOI] [Google Scholar]
- 16. Barbraud C, et al. 2018. Density dependence, prey accessibility and prey depletion by fisheries drive peruvian seabird population dynamics. Ecography 41, 1092–1102. ( 10.1111/ecog.02485) [DOI] [Google Scholar]
- 17. Davies R, Wanless S, Lewis S, Hamer K. 2013. Density-dependent foraging and colony growth in a pelagic seabird species under varying environmental conditions. Mar. Ecol. Prog. Ser. 485, 287–294. ( 10.3354/meps10348) [DOI] [Google Scholar]
- 18. Horswill C, Warwick-Evans V, Esmonde NPG, Reid N, Kirk H, Siddiqi-Davies KR, Josey SA, Wood MJ. 2023. Interpopulation differences and temporal synchrony in rates of adult survival between two seabird colonies that differ in population size and distance to foraging grounds. Ecol. Evol. 13, e10455. ( 10.1002/ece3.10455) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Birt V, Birt T, Goulet D, Cairns D, Montevecchi W. 1987. Ashmole’s halo: direct evidence for prey depletion by a seabird. Mar. Ecol. Prog. Ser. 40, 205–208. ( 10.3354/meps040205) [DOI] [Google Scholar]
- 20. Sydeman WJ, Thompson SA, Kitaysky A. 2012. Seabirds and climate change: roadmap for the future. Mar. Ecol. Prog. Ser. 454, 107–117. ( 10.3354/meps09806) [DOI] [Google Scholar]
- 21. Bertram DF, Harfenist A, Hedd A. 2009. Seabird nestling diets reflect latitudinal temperature-dependent variation in availability of key zooplankton prey populations. Mar. Ecol. Prog. Ser. 393, 199–210. ( 10.3354/meps08223) [DOI] [Google Scholar]
- 22. Le Bohec C, Durant JM, Gauthier-Clerc M, Stenseth NC, Park YH, Pradel R, Grémillet D, Gendner JP, Le Maho Y. 2008. King penguin population threatened by Southern Ocean warming. Proc. Natl Acad. Sci. USA 105, 2493–2497. ( 10.1073/pnas.0712031105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Arnott SA, Ruxton GD. 2002. Sandeel recruitment in the North Sea: demographic, climatic and trophic effects. Mar. Ecol. Prog. Ser. 238, 199–210. ( 10.3354/meps238199) [DOI] [Google Scholar]
- 24. Atkinson A, et al. 2019. Krill (Euphausia superba) distribution contracts southward during rapid regional warming. Nat. Clim. Chang. 9, 142–147. ( 10.1038/s41558-018-0370-z) [DOI] [Google Scholar]
- 25. Perry AL, Low PJ, Ellis JR, Reynolds JD. 2005. Climate change and distribution shifts in marine fishes. Science 308, 1912–1915. ( 10.1126/science.1111322) [DOI] [PubMed] [Google Scholar]
- 26. Olafsdottir AH, Utne KR, Jacobsen JA, Jansen T, Óskarsson GJ, Nøttestad L, Elvarsson BÞ, Broms C, Slotte A. 2019. Geographical expansion of Northeast Atlantic mackerel (Scomber scombrus) in the Nordic Seas from 2007 to 2016 was primarily driven by stock size and constrained by low temperatures. Deep Sea Res. Part II: Topical Stud. in Oceanogr. 159, 152–168. ( 10.1016/j.dsr2.2018.05.023) [DOI] [Google Scholar]
- 27. Barrett RT, Strøm H, Melnikov M. 2017. On the polar edge: the status of the northern gannet (Morus bassanus) in the Barents Sea in 2015-16. Polar Res. 36, 1390384. ( 10.1080/17518369.2017.1390384) [DOI] [Google Scholar]
- 28. Crawford RJM, Makhado AB, Whittington PA, Randall RM, Oosthuizen WH, Waller LJ. 2015. A changing distribution of seabirds in South Africa — the possible impact of climate and its consequences. Front. Ecol. Evol. 3, 10. ( 10.3389/fevo.2015.00010) [DOI] [Google Scholar]
- 29. Dunlop JN. 2009. The population dynamics of tropical seabird establishing frontier colonies on islands off south-western Australia. Mar. Ornithol. 37, 99–105. [Google Scholar]
- 30. Munilla I, Genovart M, Paiva VH, Velando A. 2016. Colony foundation in an oceanic seabird. PLoS One 11, e0147222. ( 10.1371/journal.pone.0147222) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bried J, Jouventin P. 2002. Site and mate choice in seabirds: an evolutionary approach. In Biology of marine birds (eds Schreiber EA, Burger J), pp. 263–305. Boca Raton, FL: CRC Press. ( 10.1201/9781420036305) [DOI] [Google Scholar]
- 32. Matthiopoulos J, Harwood J, Thomas L. 2005. Metapopulation consequences of site fidelity for colonially breeding mammals and birds. J. Anim. Ecol. 74, 716–727. ( 10.1111/j.1365-2656.2005.00970.x) [DOI] [Google Scholar]
- 33. Kildaw SD, Irons DB, Nysewander DR, Buck CL. 2005. Formation and growth of new seabird colonies: the significance of habitat quality. Mar. Ornithol. 33, 49–58. [Google Scholar]
- 34. Pettex E, Barrett RT, Lorentsen SH, Bonadonna F, Pichegru L, Pons JB, Grémillet D. 2015. Contrasting population trends at seabirds colonies: is food limitation a factor in Norway? J. Ornithol. 156, 397–406. ( 10.1007/s10336-014-1137-6) [DOI] [Google Scholar]
- 35. Clark BL, Vigfúsdóttir F, Jessopp MJ, Burgos JM, Bodey TW, Votier SC. 2020. Gannets are not attracted to fishing vessels in Iceland—potential influence of a discard ban and food availability. ICES J. Mar. Sci. 77, 692–700. ( 10.1093/icesjms/fsz233) [DOI] [Google Scholar]
- 36. Montevecchi W, Myers R. 1997. Centurial and decadal oceanographic influences on changes in northern gannet populations and diets in the north-west Atlantic: implications for climate change. ICES J. Mar. Sci. 54, 608–614. ( 10.1006/jmsc.1997.0265) [DOI] [Google Scholar]
- 37. Murray S, Harris MP, Wanless S. 2015. The status of the gannet in Scotland in 2013-14. Scot. Birds 35, 3–18. [Google Scholar]
- 38. Serjeantson D. 2001. The great auk and the gannet: a prehistoric perspective on the extinction of the great auk. Intl. J. Osteoarchaeol. 11, 43–55. ( 10.1002/oa.545) [DOI] [Google Scholar]
- 39. Votier SC, Bicknell A, Cox SL, Scales KL, Patrick SC. 2013. A bird’s eye view of discard reforms: bird-borne cameras reveal seabird/fishery interactions. PLoS One 8, e57376. ( 10.1371/journal.pone.0057376) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wynne Edwards V, Lockley R, Morrey Salmon H. 1936. The distribution and numbers of breeding gannets (Sula bassana L). Br. Birds. 29, 262–276. [Google Scholar]
- 41. Grémillet D, Pichegru L, Siorat F, Georges J. 2006. Conservation implications of the apparent mismatch between population dynamics and foraging effort in French northern gannets from the English Channel. Mar. Ecol. Prog. Ser. 319, 15–25. ( 10.3354/meps319015) [DOI] [Google Scholar]
- 42. Berge J, Heggland K, Lønne OJ, Cottier F, Hop H, Gabrielsen GW, Nøttestad L, Misund OA. 2015. First records of Atlantic mackerel (Scomber scombrus) from the Svalbard archipelago, Norway, with possible explanations for the extension of its distribution. ARCTIC 68, 54. ( 10.14430/arctic4455) [DOI] [Google Scholar]
- 43. Dalpadado P, Ingvaldsen RB, Stige LC, Bogstad B, Knutsen T, Ottersen G, Ellertsen B. 2012. Climate effects on Barents Sea ecosystem dynamics. ICES J. Mar. Sci. 69, 1303–1316. ( 10.1093/icesjms/fss063) [DOI] [Google Scholar]
- 44. Astthorsson OS, Valdimarsson H, Gudmundsdottir A, Óskarsson GJ. 2012. Climate-related variations in the occurrence and distribution of mackerel (Scomber scombrus) in Icelandic waters. ICES J. Mar. Sci. 69, 1289–1297. ( 10.1093/icesjms/fss084) [DOI] [Google Scholar]
- 45. Vigfúsdóttir F, Lilliendahl K, Garðarsson A. 2009. Fæða súlu við ísland: diets of Northern Gannet in Iceland (Icelandic with English summary). Blki. 30, 55–60. ( 10.13140/RG.2.2.31812.78727) [DOI] [Google Scholar]
- 46. Montevecchi WA, et al. 2021. Ocean heat wave induces breeding failure at the southern breeding limit of the northern gannet Morus bassanus. Mar. Ornithol. 49, 71–78. [Google Scholar]
- 47. Warwick-Evans V, Atkinson PW, Arnould JPY, Gauvain R, Soanes L, Robinson LA, Green JA. 2016. Changes in behaviour drive inter-annual variability in the at-sea distribution of northern gannets. Mar. Biol. 163, 156. ( 10.1007/s00227-016-2922-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Le Bot T, Lescroël A, Fort J, Péron C, Gimenez O, Provost P, Grémillet D. 2019. Fishery discards do not compensate natural prey shortage in Northern Gannets from the English Channel. Biol. Conserv. 236, 375–384. ( 10.1016/j.biocon.2019.05.040) [DOI] [Google Scholar]
- 49. d’Entremont KJN, Guzzwell LM, Wilhelm SI, Friesen VL, Davoren GK, Walsh CJ, Montevecchi WA. 2022. Northern gannets (Morus bassanus) breeding at their southern limit struggle with prey shortages as a result of warming waters. ICES J. Mar. Sci. 79, 50–60. ( 10.1093/icesjms/fsab240) [DOI] [Google Scholar]
- 50. Lane JV, et al. 2024. High pathogenicity avian influenza (H5N1) in northern gannets (Morus bassanus): global spread, clinical signs and demographic consequences. Ibis 166, 633–650. ( 10.1111/ibi.13275) [DOI] [Google Scholar]
- 51. Xie R, et al. 2023. The episodic resurgence of highly pathogenic avian influenza H5 virus. Nature 622, 810–817. ( 10.1038/s41586-023-06631-2) [DOI] [PubMed] [Google Scholar]
- 52. Chardine JW, Rail JF, Wilhelm S. 2013. Population dynamics of Northern Gannets in North America, 1984-2009. J. Field Ornithol. 84, 187–192. ( 10.1111/jofo.12017) [DOI] [Google Scholar]
- 53. Langston RHW, Teuten E, Butler A. 2013. Foraging ranges of northern gannets Morus bassanus in relation to proposed offshore wind farms in the UK: 2010-2012. RSPB report to UK Department of Energy and Climate Change. See https://assets.publishing.service.gov.uk/media/5a75086ce5274a3cb28691f3/OESEA2_North_Sea_Gannet_Tracking_Year3_Report.pdf.
- 54. Garthe S, Markones N, Corman AM. 2017. Possible impacts of offshore wind farms on seabirds: a pilot study in Northern Gannets in the southern North Sea. J. Ornithol. 158, 345–349. ( 10.1007/s10336-016-1402-y) [DOI] [Google Scholar]
- 55. Hamer K, Humphreys E, Garthe S, Hennicke J, Peters G, Grémillet D, Phillips R, Harris M, Wanless S. 2007. Annual variation in diets, feeding locations and foraging behaviour of gannets in the North Sea: flexibility, consistency and constraint. Mar. Ecol. Prog. Ser. 338, 295–305. ( 10.3354/meps338295) [DOI] [Google Scholar]
- 56. Wakefield ED, Cleasby IR, Bearhop S, Bodey TW, Davies RD, Miller PI, Newton J, Votier SC, Hamer KC. 2015. Long-term individual foraging site fidelity—why some gannets don’t change their spots. Ecology 96, 3058–3074. ( 10.1890/14-1300.1) [DOI] [PubMed] [Google Scholar]
- 57. Lane J, Spracklen D, Hamer K. 2019. Effects of windscape on three-dimensional foraging behaviour in a wide-ranging marine predator, the Northern Gannet. Mar. Ecol. Prog. Ser. 628, 183–193. ( 10.3354/meps13089) [DOI] [Google Scholar]
- 58. Pettex E, Lorentsen SH, Grémillet D, Gimenez O, Barrett RT, Pons JB, Bohec C, Bonadonna F. 2012. Multi-scale foraging variability in northern gannet (Morus bassanus) fuels potential foraging plasticity. Mar. Biol. 159, 2743–2756. ( 10.1007/s00227-012-2035-1) [DOI] [Google Scholar]
- 59. d’Entremont K, Davoren G, Walsh C, Wilhelm S, Montevecchi W. 2022. Intra- and inter-annual shifts in foraging tactics by parental northern gannets Morus bassanus indicate changing prey fields. Mar. Ecol. Prog. Ser. 698, 155–170. ( 10.3354/meps14164) [DOI] [Google Scholar]
- 60. Montevecchi WA, et al. 2012. Tracking seabirds to identify ecologically important and high risk marine areas in the western North Atlantic. Biol. Conserv. 156, 62–71. ( 10.1016/j.biocon.2011.12.001) [DOI] [Google Scholar]
- 61. Garthe S, Montevecchi WA, Chapdelaine G, Rail JF, Hedd A. 2007. Contrasting foraging tactics by northern gannets (Sula bassana) breeding in different oceanographic domains with different prey fields. Mar. Biol. 151, 687–694. ( 10.1007/s00227-006-0523-x) [DOI] [Google Scholar]
- 62. Garthe S, Montevecchi WA, Davoren GK. 2011. Inter‐annual changes in prey fields trigger different foraging tactics in a large marine predator. Limnol. Oceanogr. 56, 802–812. ( 10.4319/lo.2011.56.3.0802) [DOI] [Google Scholar]
- 63. Clark B, et al. 2021. Sexual segregation of gannet foraging over 11 years: movements vary but isotopic differences remain stable. Mar. Ecol. Prog. Ser. 661, 1–16. ( 10.3354/meps13636) [DOI] [Google Scholar]
- 64. Hamer KC, Phillips RA, Hill JK, Wanless S, Wood AG. 2001. Contrasting foraging strategies of gannets Morus bassanus at two North Atlantic colonies: foraging trip duration and foraging area fidelity. Mar. Ecol. Prog. Ser. 224, 283–290. ( 10.3354/meps224283) [DOI] [Google Scholar]
- 65. Wanless S, Harris M, Redman P, Speakman J. 2005. Low energy values of fish as a probable cause of a major seabird breeding failure in the North Sea. Mar. Ecol. Prog. Ser. 294, 1–8. ( 10.3354/meps294001) [DOI] [Google Scholar]
- 66. Murray S, Morgan G, Harris MP. 2015. A count of northern gannets on grassholm in 2015. Br. Birds. 108, 691–692. [Google Scholar]
- 67. Newton SF, Harris MP, Murray S. 2015. Census of gannet Morus bassanus in Ireland in 2013–14. Ir. Birds 10, 215–220. [Google Scholar]
- 68. Garðarsson A. 2019. Íslenskar súlubyggðir 2013-2014: Icelandic colonies of the northern gannet in 2013-2014 (Icelandic, English summary). Bliki. 33, 69–71. [Google Scholar]
- 69. Wilson R, et al. 2002. Remote-sensing systems and seabirds: their use, abuse and potential for measuring marine environmental variables. Mar. Ecol. Prog. Ser. 228, 241–261. ( 10.3354/meps228241) [DOI] [Google Scholar]
- 70. Gaston AJ, Ydenberg RC, Smith GEJ. 2007. Ashmole’s halo and population regulation in seabirds. Mar. Ornithol. 35, 119–126. [Google Scholar]
- 71. Cochran WG. 1977. Sampling techniques, 3rd edn. New York, NY: John Wiley & Sons. [Google Scholar]
- 72. Lai MHC, Kwok OM, Hsiao YY, Cao Q. 2018. Finite population correction for two-level hierarchical linear models. Psychol. Methods 23, 94–112. ( 10.1037/met0000137) [DOI] [PubMed] [Google Scholar]
- 73. Lumley T. 2004. Analysis of complex survey samples. J. Stat. Softw. 9, 1–19. ( 10.18637/jss.v009.i08) [DOI] [Google Scholar]
- 74. Lumley T, Scott A. 2014. Tests for regression models fitted to survey data. Aust. N. Z. J. Stat. 56, 1–14. ( 10.1111/anzs.12065) [DOI] [Google Scholar]
- 75. Long JA. 2019. Jtools: analysis and presentation of social scientific data. R package version 2.0.0. See https://cran.r-project.org/package=jtools.
- 76. Rousset F, Ferdy JB. 2014. Testing environmental and genetic effects in the presence of spatial autocorrelation. Ecography 37, 781–790. ( 10.1111/ecog.00566) [DOI] [Google Scholar]
- 77. Hartig F. 2020. DHARMa: residual diagnostics for hierarchical (multi-level/mixed) regression models. See https://CRAN.R-project.org/package=DHARMa.
- 78. Clark BL . 2024. ForagingEffortGannets. GitHub. See https://github.com/bethclark36/ForagingEffortGannets.
- 79. Clark BL. 2024. ForagingEffortGannets: code and data. Zenodo. 10.5281/zenodo.12796423 [DOI]
- 80. Paiva VH, Ramos JA, Nava C, Neves V, Bried J, Magalhães M. 2018. Inter-sexual habitat and isotopic niche segregation of the endangered monteiro’s storm-petrel during breeding. Zoology (Jena). 126, 29–35. ( 10.1016/j.zool.2017.12.006) [DOI] [PubMed] [Google Scholar]
- 81. Votier SC, et al. 2017. Effects of age and reproductive status on individual foraging site fidelity in a long-lived marine predator. Proc. Biol. Sci. 284, 20171068. ( 10.1098/rspb.2017.1068) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Patrick SC, et al. 2014. Individual differences in searching behaviour and spatial foraging consistency in a central place marine predator. Oikos 123, 33–40. ( 10.1111/j.1600-0706.2013.00406.x) [DOI] [Google Scholar]
- 83. Paiva VH, Geraldes P, Ramirez I, Werner AC, Garthe S, Ramos JA. 2013. Overcoming difficult times: the behavioural resilience of a marine predator when facing environmental stochasticity. Mar. Ecol. Prog. Ser. 486, 277–288. ( 10.3354/meps10332) [DOI] [Google Scholar]
- 84. Thorne LH, et al. 2015. Foraging behavior links climate variability and reproduction in North Pacific albatrosses. Mov. Ecol. 3, 27. ( 10.1186/s40462-015-0050-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Furness RW, Garthe S, Trinder M, Matthiopoulos J, Wanless S, Jeglinski J. 2018. Nocturnal flight activity of Northern Gannets Morus bassanus and implications for modelling collision risk at offshore wind farms. Environ. Impact Assess. Rev. 73, 1–6. ( 10.1016/j.eiar.2018.06.006) [DOI] [Google Scholar]
- 86. Ainley DG, Ford RG, Brown ED, Suryan RM, Irons DB. 2003. PREY resources, competition, and geographic structure of kittiwake colonies in Prince William Sound. Ecology 84, 709–723. ( 10.1890/0012-9658(2003)084[0709:PRCAGS]2.0.CO;2) [DOI] [Google Scholar]
- 87. Cairns DK. 1989. The regulation of seabird colony size: a hinterland model. Am. Nat. 134, 141–146. ( 10.1086/284970) [DOI] [Google Scholar]
- 88. Furness RW, Birkhead TR. 1984. Seabird colony distributions suggest competition for food supplies during the breeding season. Nature 311, 655–656. ( 10.1038/311655a0) [DOI] [Google Scholar]
- 89. Barrett RT. 2007. Food web interactions in the southwestern Barents Sea: black-legged kittiwakes Rissa tridactyla respond negatively to an increase in herring Clupea harengus. Mar. Ecol. Prog. Ser. 349, 269–276. ( 10.3354/meps07116) [DOI] [Google Scholar]
- 90. Jeglinski JWE, et al. 2023. Metapopulation regulation acts at multiple spatial scales: insights from a century of seabird colony census data. Ecol. Monogr. 93, 1–29. ( 10.1002/ecm.1569) [DOI] [Google Scholar]
- 91. Carroll MJ, et al. 2015. Effects of sea temperature and stratification changes on seabird breeding success. Clim. Res. 66, 75–89. ( 10.3354/cr01332) [DOI] [Google Scholar]
- 92. Frederiksen M, Anker-Nilssen T, Beaugrand G, Wanless S. 2013. Climate, copepods and seabirds in the boreal northeast Atlantic – current state and future outlook. Glob. Chang. Biol. 19, 364–372. ( 10.1111/gcb.12072) [DOI] [PubMed] [Google Scholar]
- 93. Sandvik H, Erikstad KE, Barrett RT, Yoccoz NG. 2005. The effect of climate on adult survival in five species of North Atlantic seabirds. J. Anim. Ecol. 74, 817–831. ( 10.1111/j.1365-2656.2005.00981.x) [DOI] [Google Scholar]
- 94. Lilliendahl K, Solmundsson J. 1997. An estimate of summer food consumption of six seabird species in Iceland. ICES J. Mar. Sci. 54, 624–630. ( 10.1006/jmsc.1997.0240) [DOI] [Google Scholar]
- 95. Fayet AL, Clucas GV, Anker-Nilssen T, Syposz M, Hansen ES. 2021. Local prey shortages drive foraging costs and breeding success in a declining seabird, the Atlantic puffin. J. Anim. Ecol. 90, 1152–1164. ( 10.1111/1365-2656.13442) [DOI] [PubMed] [Google Scholar]
- 96. Óskarsson GJ, Gudmundsdottir A, Sveinbjörnsson S, Sigurðsson Þ. 2016. Feeding ecology of mackerel and dietary overlap with herring in Icelandic waters. Mar. Biol. Res. 12, 16–29. ( 10.1080/17451000.2015.1073327) [DOI] [Google Scholar]
- 97. Lewis S, Sherratt TN, Hamer KC, Harris MP, Wanless S. 2003. Contrasting diet quality of Northern Gannets Morus bassanus at two colonies. Ardea. 91, 167–176. [Google Scholar]
- 98. Osborne OE, O’Hara P, Whelan S, Zandbergen P, Hatch SA, Elliott KH. 2020. Breeding seabirds increase foraging range in response to an extreme marine heatwave. Mar. Ecol. Prog. Ser. 646, 161–173. ( 10.3354/meps13392) [DOI] [Google Scholar]
- 99. Jones TB, Patrick SC, Arnould JPY, Rodríguez-Malagón MA, Wells MR, Green JA. 2018. Evidence of sociality in the timing and location of foraging in a colonial seabird. Biol. Lett. 14, 20180214. ( 10.1098/rsbl.2018.0214) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Bakker VJ, Finkelstein ME, Doak DF, VanderWerf EA, Young LC, Arata JA, Sievert PR, Vanderlip C. 2018. The albatross of assessing and managing risk for long-lived pelagic seabirds. Biol. Conserv. 217, 83–95. ( 10.1016/j.biocon.2017.08.022) [DOI] [Google Scholar]
- 101. Grémillet D, Boulinier T. 2009. Spatial ecology and conservation of seabirds facing global climate change: a review. Mar. Ecol. Prog. Ser. 391, 121–137. ( 10.3354/meps08212) [DOI] [Google Scholar]
- 102. Arafeh-Dalmau N, et al. 2023. Integrating climate adaptation and transboundary management: guidelines for designing climate-smart marine protected areas. One Earth 6, 1523–1541. ( 10.1016/j.oneear.2023.10.002) [DOI] [Google Scholar]
- 103. Suryan RM, et al. 2008. Wind, waves, and wing loading: morphological specialization may limit range expansion of endangered albatrosses. PLoS One 3, e4016. ( 10.1371/journal.pone.0004016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. White CR, Green JA, Martin GR, Butler PJ, Grémillet D. 2013. Energetic constraints may limit the capacity of visually guided predators to respond to Arctic warming. J. Zool. (Lond.) 289, 119–126. ( 10.1111/j.1469-7998.2012.00968.x) [DOI] [Google Scholar]
- 105. Ballard G, Toniolo V, Ainley DG, Parkinson CL, Arrigo KR, Trathan PN. 2010. Responding to climate change: Adélie penguins confront astronomical and ocean boundaries. Ecology 91, 2056–2069. ( 10.1890/09-0688.1) [DOI] [PubMed] [Google Scholar]
- 106. Jeglinski JWE, et al. 2024. HPAIV outbreak triggers short-term colony connectivity in a seabird metapopulation. Sci. Rep. 14, 3126. ( 10.1038/s41598-024-53550-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Grémillet D, et al. 2023. Strong breeding colony fidelity in Northern Gannets following high pathogenicity avian influenza virus (HPAIV) outbreak. Biol. Conserv. 286, 110269. ( 10.1016/j.biocon.2023.110269) [DOI] [Google Scholar]
- 108. Clark BL, Vigfúsdóttir F, Wanless S, Hamer K, Bodey TW, Bearhop Set al. 2024. . Supplementary material from: Northern Gannet foraging trip length increases with colony size and decreases with latitude. FigShare ( 10.6084/m9.figshare.c.7410582) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
R scripts and data are available at [78], archived at [79]. Gannet tracking data used in this study are available to request from the BirdLife International Seabird Tracking Database (https://www.seabirdtracking.org/) with the following dataset IDs: 716–725, 728–734, 955–956, 1341–1342, 1472–1473, 1475–1478, 1543, 1636, 1638, 1653, 1660, 1793–1796, 2201. Electronic supplementary material, table S1 contains additional data for the 21 gannet colonies [108]. Figure S1 shows the relationship between trip duration and maximum distance. Electronic supplementary material, table S2 gives model estimates for colonies for which more than 10 individuals were tracked. Electronic supplementary material, table S3 gives the values derived from GPS data and colonies counts separated by year.