Abstract
Background
Immune-related hepatitis (irH) is a serious immune-related adverse event (IRAE) that may result in morbidity, immune checkpoint inhibitor (ICI) therapy interruption and, rarely, mortality. The impact of underlying liver pathology, including liver metastasis, on the incidence of irH remains poorly understood.
Objectives
We hypothesized that the presence of underlying liver pathology increased the risk of irH in patients with cancer treated with ICI.
Patients and Methods
We conducted a retrospective case-control study of irH in patients with cancer receiving first ICI treatment from 2016–2020. Provider documented cases of ≥ grade 2 irH were identified and control matched in a 2:1 ratio based on age, sex, time of ICI initiation, and follow-up time. Conditional logistic regression was used to estimate the relationship between irH and liver metastasis at ICI initiation.
Results
Ninety-seven cases of irH were identified, 29% of which had liver metastases at time of ICI initiation. Thirty-eight percent of patients developed grade 2, 47% grade 3, and 14% grade 4 irH. When adjusted for covariates/confounders, the presence of liver metastasis was associated with increased odds of irH (aOR 2.79 95% CI 1.37–5.66, p = 0.005). The presence of liver metastases did not correlate with irH grade or rate of irH recurrence after ICI rechallenge.
Conclusions
Presence of liver metastases increased the odds of irH in patients with first-time ICI therapy. Limitations include the retrospective nature, moderate sample size, possible selection bias and confounding. Our findings are hypothesis-generating and warrant external validation as well as tissue and circulating biomarker exploration.
1. Introduction
Immune-related adverse events (IRAEs) are a serious but difficult to predict complication of immune checkpoint inhibitors (ICIs) that result in morbidity, ICI therapy interruption and, rarely, mortality [1–3]. This class of side effects is characterized by unchecked immune-system activation and damage to healthy tissue, but precise pathophysiology varies widely between tissue types. Immune-related adverse events can affect all organ systems, but most commonly affect the skin (rash, vitiligo), gastrointestinal (GI) tract (diarrhea, colitis), liver (hepatitis), and endocrine system (thyroiditis). Yet, many patients receiving ICIs never develop these complications. Why some patients develop IRAEs (with variable severity), remains a critical unanswered question in clinical immuno-oncology, highlighting the need for the development of predictive clinical and molecular biomarkers [3]. Given the remarkable increasing growth of ICI use, and the potential for large and sustained tumor response, understanding and subsequently minimizing the incidence and severity of IRAEs remains of utmost importance.
Immune-related hepatitis (irH) represents a significant proportion of reported IRAEs. Estimates differ, but one meta-analysis reported the all-grade incidence of irH as 6–30% [4]. Most studies related to irH are limited by lack of standardized nomenclature, with hepatotoxicity, immune-related hepatitis, and immune-mediated hepatitis often used interchangeably [5]. While there are no distinct diagnostic criteria for irH, it is usually suspected in patients with elevated liver biomarkers about 6–8 weeks following initiation of ICI therapy [4, 6]. However, irH remains a diagnosis of exclusion and other causes of liver biomarker elevations, including viral infection, other drug/toxin-mediated liver injury and metastatic disease must first be ruled out.
Risk factors for irH have not been robustly studied and are largely inferred from the broader IRAE literature. Combination ICI regimens, history of autoimmune disease, and history of prior IRAEs have been associated with IRAE incidence, but there are currently no robust, validated biomarkers that determine the risk of developing IRAEs [3, 4, 7–9]. To better understand the clinical correlates impacting the incidence of irH, we conducted a retrospective study of patients who developed irH. We hypothesized that presence of underlying liver pathology, in particular cirrhosis and liver metastasis, would increase the risk of irH in patients with cancer treated with ICI. Herein we describe the natural history of irH in our cohort and present initial findings on associated clinical risk factors.
2. Methods
2.1. Study Design and Approval
We conducted a retrospective case-control study in patients with cancer who received ICI at the University of Washington and Fred Hutchinson Cancer Center between 2016 and 2020 and who developed irH. Study protocols were approved by University of Washington’s Institutional Review Board (IRB ID: STUDY00008393). Due to the retrospective nature of the study, patient consent was waived by the IRB. This study was completed in compliance with ethical standards set forth by the Helsinki Declaration and its amendments. The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request and depending on institutional restrictions.
2.2. Case Identification
Our study population comprised patients with cancer who received first-dose ICI (PD-1: cemiplimab, nivolumab, pembrolizumab, PDL-1: atezolizumab, avelumab, durvalumab, CTLA-4: tremelimumab ipilimumab) between 2016–2020 (Fig. 1). From this initial population, cases of irH were identified via an initial screening and confirmed through manual chart review. Charts were selected for manual review if, within a year of ICI initiation, they had been assigned a liver-related ICD-10 diagnostic code and if they met or exceeded at least one biochemical criterion for grade 2 liver toxicity as defined by National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) V5 (Table 1) [10]. On manual chart review, irH was confirmed if patients had a documented diagnosis.
Fig. 1.
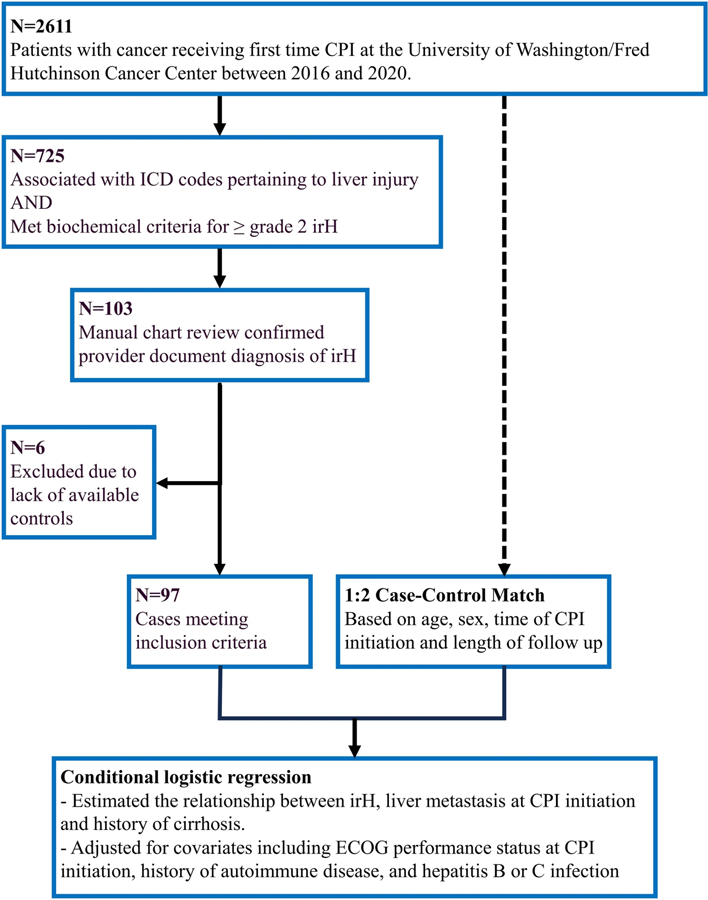
Cohort diagram; 103 patients receiving immune checkpoint inhibitor (ICI) therapy met laboratory thresholds for grade 2 immune related hepatitis (irH) and had an accompanying provider documented diagnosis of irH; 97 cases were included in the analysis and matched with controls based on age, sex, time of ICI initiation and length of follow-up. ECOG Eastern Cooperative Oncology Group
Table 1.
Immune-related hepatitis severity grading criteria
| Lab | ULN | Grade 2 threshold | Grade 2 value | Grade 3 threshold | Grade 3 value | Grade 4 threshold | Grade 4 value |
|---|---|---|---|---|---|---|---|
| AST | 33 | 3×ULN | 99 | 5×ULN | 165 | >20×ULN | 660 |
| ALT | 48 | 3×ULN | 144 | 5× ULN | 240 | >20×ULN | 960 |
| AP | 122 | 2.5×ULN | 305 | 5×ULN | 610 | >20×ULN | 2440 |
| Total bilirubin | 1.3 | 1.5×ULN | 1.95 | 3× ULN | 3.9 | >10×ULN | 13 |
| Albumina | 3.5 | 3.0 | 2.0 | N/A |
Thresholds for irH severity grade as defined by NCI Common Terminology Criteria for Adverse Events V5, and the corresponding laboratory value
ALT alanine transaminase, AP alkaline phosphatase, AST aspartate transaminase, irH immune-related hepatitis, NA not applicable, NCI National Cancer Institute, ULN upper limit of normal
Albumin thresholds reported as the lower limit of normal
2.3. Chart Review
Charts were abstracted using Redcap data collection software supported by the Institute of Translational Health Sciences (ITHS) [11, 12]. Baseline demographic and clinical characteristics were collected at the time of cancer diagnosis and ICI therapy onset. A patient was considered to have cirrhosis if there was a documented diagnosis; radiographic findings of liver disease without clinical correlation were not included in our study. For each course of ICI therapy, type of ICI(s), use of concomitant chemotherapy or targeted molecular therapies, concurrent radiotherapy, stage, sites of metastasis, and Eastern Cooperative Oncology Group (ECOG) performance status at ICI initiation were collected. Laboratory data, including aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (AP), total bilirubin, and albumin, were recorded at time of ICI initiation. Rechallenge was defined as resumption of the same ICI regimen, or monotherapy if a combination ICI as used. If a patient was treated with multiple lines of ICI therapy, each line of therapy was documented individually and IRAE outcomes collected.
For those lines of ICI therapy resulting in irH, additional laboratory and clinical data were collected at time of onset and throughout irH progression. Immune-related hepatitis onset was defined as the point at which a patient met or exceeded a least one biochemical threshold for grade 2 liver toxicity (Table 1). If a patient’s pre-ICI liver biomarker values exceeded these thresholds, irH was considered in patients whose AST and ALT exceeded 5× their pre-ICI baseline or AP exceeded 2× their pre-ICI baseline. Date and value of maximum AST, ALT, AP, total bilirubin, and minimum albumin value were recorded as well as date of normalization for each laboratory value. Date of irH resolution was defined as the date at which all laboratory values were within normal range or reached pre-ICI baseline. In cases where steroids or other immunosuppressive therapies were required, irH resolution was defined as the date when laboratory parameters maintained the pre-ICI baseline status after these therapies were discontinued. Overall irH grade was based on highest individual grades for AST, ALT, AP and total bilirubin per NCI CTCAE V5 criteria.
2.4. Control Matching
Controls were matched to cases in a 2:1 ratio based on age (± 5 years), sex, time of ICI initiation (±1 year), and available follow-up time (control follow up time + 0.5 years ≥ case follow-up time). Controls were selected from our study population of patients with cancer receiving first-dose ICI but who did not have a documented diagnosis of irH (Fig. 1). Cases were excluded from further analysis if there were no available controls.
2.5. Analysis
Baseline characteristics of cases and controls were summarized with descriptive statistics and compared using chi-square tests and t-tests. The irH symptomatology, time to onset, grade, treatment course, and recurrence rate were analyzed with descriptive statistics. The R factor for liver injury was calculated for each case at time of irH onset and used to characterize cholestatic versus hepatocellular liver biomarker patterns [13].
Univariate logistic regression was used to estimate the relationship between irH, liver metastasis at ICI initiation, and history of cirrhosis, and adjusted for a priori identified covariates, including ECOG performance status at ICI initiation, combination (vs single agent) ICI, history of autoimmune disease, and hepatitis B or C infection. Subgroup analysis was performed using logistic regression to evaluate the relationship of irH severity/grade with history of cirrhosis and presence of liver metastasis.
All models and analysis were implemented in R version 4.1.2 (The R Foundation for Statistical Computing).
3. Results
3.1. Patient Selection and Baseline Characteristics
Overall, 2611 patients received their first dose of ICI between 2016 and 2020. Of these, 725 had ICD-10 code associations and biochemical criteria to qualify for manual chart review. Following chart review, 103 patients were found to have provider documented diagnoses of irH; six were excluded from analysis due to lack of available controls within ICI initiation window and insufficient follow-up time requirements (Fig. 1). The most common ICD-10 codes associated with irH in this cohort were autoimmune hepatitis (K75.4), other specified diseases of liver (K76.89), other specified abnormal findings of blood chemistry (R79.89), and unspecified inflammatory liver disease (K75.9) (Supplementary Table 1).
3.2. Immune‑Related Hepatitis Descriptive Analysis
Of the 97 cases of irH identified, 43% were women and median age was 62.1 years (interquartile range [IQR] 16.7). Tables 2 and 3 summarize baseline characteristics for cases and controls. The most common cancer types being treated were melanoma, renal cell carcinoma and non-small cell lung cancer; 94% of patients with irH had metastatic disease at time of ICI initiation, 29% of whom had liver metastasis. Five patients with irH had history of cirrhosis, with all having had underlying hepatitis C. Three of these patients with cirrhosis were receiving ICI therapy for treatment of hepatocellular carcinoma (HCC) and had previously received localized disease-directed therapy such as chemoablation or radiofrequency ablation.
Table 2.
Baseline demographic characteristics of the study population
| irH | No irH | p-Value | |
|---|---|---|---|
| N = 97 | N = 194 | ||
| Median age [IQR] | 62.1 [16.7] | 62.8 [16.3] | 0.895 |
| Sex | |||
| Male | 55 (56.7%) | 110 (56.7%) | 1 |
| Female | 42 (43.3%) | 84 (43.3%) | |
| Cancer diagnosis | |||
| Head and neck cancer | 10 (10.3%) | 15 (7.7%) | 0.460 |
| Lung cancer | 13 (13.4%) | 38 (19.6%) | 0.191 |
| Skin cancer | 42 (43.3%) | 34 (17.5%) | <0.001 |
| Breast cancer | 0 (0%) | 5 (2.6%) | 0.111 |
| Gastrointestinal cancer | 5 (5.2%) | 51 (26.3%) | <0.001 |
| Genitourinary cancer 22 (22.7%) | 37 (19.1%) | 0.470 | |
| Sarcoma | 4 (4.1%) | 10 (5.2%) | 0.698 |
| Hematologic malignancy | 0 (0%) | 2 (1.0%) | 0.316 |
| Other | 1 (1.0%) | 2 (1.0%) | 1 |
| History of cirrhosis | |||
| No | 92 (94.8%) | 172 (88.7%) | 0.086 |
| Yes | 5 (5.2%) | 22 (11.3%) | |
| History of autoimmune disease | |||
| No | 92 (94.8%) | 164 (84.5%) | 0.035 |
| Yes | 4 (4.1%) | 27 (13.9%) | |
| N/A | 1 (1.0%) | 3 (1.5%) | |
| Hepatitis B Infection | |||
| Uninfected/cleared | 95 (97.9%) | 187 (96.4%) | 0.473 |
| Infected/chronic/under treatment | 2 (2.1%) | 7 (3.6%) | |
| Hepatitis C infection | |||
| Uninfected/cleared | 90 (92.8%) | 178 (91.8%) | 0.759 |
| Infected/chronic/under treatment | 7 (7.2%) | 16 (8.2%) | |
| History of liver directed treatment (Y90, chemoablation, etc.) | |||
| No | 82 (84.5%) | 178 (91.8%) | 0.174 |
| Yes | 12 (12.4%) | 15 (7.7%) | |
| N/A | 3 (3.1%) | 1 (0.5%) | |
Data unavailable by chart review were recorded as N/A
Case and control subsets were compared using chi-square- and t-tests
IQR interquartile range, irH immune-related hepatitis, NA not applicable
Table 3.
Clinical characteristics of the study population at ICI initiation
| irH | No irH | p-Value | |
|---|---|---|---|
| N = 97 | N = 194 | ||
| Single-agent ICI therapy | |||
| Atezolizumab | 4 (4.1%) | 4 (1.8%) | 0.070 |
| Avelumab | 0 (0%) | 1 (0.5%) | 0.580 |
| Cemiplimab | 2 (1.0%) | 0 (0%) | 0.010 |
| Durvalumab | 0 (0%) | 0 (0%) | N/A |
| Ipilimumab | 5 (5.1%) | 9 (6.0%) | 0.260 |
| Nivolumab | 13 (13.4%) | 85 (42.8%) | 0.002 |
| Pembrolizumab | 30 (30.9%) | 78 (40.2%) | 0.279 |
| Tremelimumab | 0 (0%) | 0 (0%) | N/A |
| Combination ICI Therapy | |||
| No | 54 (55.7%) | 177 (91.2%) | <0.001 |
| Yes | 43 (44.3%) | 17 (8.8%) | |
| ECOG Performance Score at ICI initiation | |||
| 0 and 1 | 71 (73.2%) | 129 (66.5%) | 0.054 |
| 2+ | 12 (12.4%) | 46 (23.7%) | |
| N/A | 14 (14.4%) | 19 (9.8%) | |
| Presence of metastases at ICI initiation | |||
| Any | 91 (93.8%) | 172 (88.7%) | 0.160 |
| Adrenal | 6 (6.2%) | 17 (8.8%) | 0.442 |
| Bladder/ureter/kidney | 6 (6.2%) | 12 (6.2%) | 1.00 |
| Bone | 26 (26.8%) | 53 (27.3%) | 0.926 |
| Bowel | 1 (1.0%) | 0 (0.0%) | 0.156 |
| Brain/CNS | 10 (10.3%) | 13 (6.7%) | 0.282 |
| Liver | 28 (28.9%) | 33 (17.0%) | 0.019 |
| Lung | 45 (46.4%) | 68 (35.1%) | 0.061 |
| Lymph node | 55 (56.7%) | 116 (59.8%) | 0.613 |
| Other | 5 (5.2%) | 19 (9.8%) | 0.175 |
| Peritoneum | 4 (4.1%) | 25 (12.9%) | 0.019 |
| Soft tissue | 19 (19.6%) | 28 (14.4%) | 0.260 |
| Vascular | 1 (1.0%) | 11 (5.7%) | 0.061 |
| Baseline liver tests | |||
| ALT | 18.0 [13.0–31.5] | 19.0 [11.0–34.5] | 0.641 |
| AST | 20.0 [17.0–26.5] | 19.0 [14.0–38.0] | 0.118 |
| AP | 69.0 [56.0–82.5] | 79.0 [61.0–133] | 0.009 |
| Total bilirubin | 0.50 [0.4–0.7] | 0.50 [0.4–0.6] | 0.325 |
| Albumin | 4.00 [3.7–4.3] | 3.80 [3.3–4.1] | 0.709 |
Descriptive categorical measures are expressed in as number (n) and percentage (%), quantitative metrics are expressed as median and IQR
Data unavailable by chart review were recorded as N/A. Case and control subsets were compared using chi-square- and t-tests
ALT alanine transaminase, AP alkaline phosphatase, AST aspartate transaminase, CNS central nervous system, ECOG Eastern Cooperative Oncology Group, ICI immune checkpoint inhibitor, IQR interquartile range, irH immune-related hepatitis, NA not applicable
In our cohort of irH patients, 26 had mildly abnormal liver biomarkers prior to ICI administration and none met grade 2 thresholds prior to ICI initiation requiring higher thresholds. Cirrhosis (2) and liver metastasis (5) represented a minority of irH cases with abnormal baseline biomarkers. One hundred patients in the control group had abnormal baseline liver biomarkers, although patients with cirrhosis (22) or liver metastases (24) accounted for a larger proportion. Abnormal baseline liver biomarkers were driven largely by AST elevations in irH cases (15), while controls most commonly had derangements in albumin (59) (Supplementary Table 2). The etiology of baseline liver biomarker abnormalities and AP isoenzyme fractionation were not consistently reported by providers.
Median number of ICI doses prior to irH onset was 2 (IQR 3), equating to 49 days (IQR 71). Most cases of irH presented with a variety of non-specific symptoms including fatigue (35%), nausea/vomiting (24%), and fevers (15%). Notably, 29% of irH cases were asymptomatic and were identified during routine laboratory monitoring. Only seven patients underwent liver biopsy for diagnostic confirmation. Liver injury presented in a mixed cholestatic-hepatocellular pattern with median R factor of 3.30 (IQR 3.82) at irH onset. Peak laboratory values included a median AST of 164 (IQR 264), median ALT 222 (IQR 206), and median AP 148 (IQR 251) (Table 4). These liver biomarker values translated to 38% grade 2, 47% grade 3 and 14% grade 4 irH.
Table 4.
Median maximum and minimum laboratory values during irH course and associated IQ values
| Value | Median | IQ1 | IQ3 |
|---|---|---|---|
| ALT | 222 | 149 | 355 |
| AST | 164 | 106 | 370 |
| AP | 148 | 97 | 348 |
| Total bilirubin | 0.9 | 0.5 | 1.8 |
| Albumin | 3.4 | 3.1 | 3.7 |
Maximum values are reported for all laboratory tests except albumin, for which the minimum value was recorded during irH course
ALT alanine transaminase, AP alkaline phosphatase, AST aspartate transaminase, IQ interquartile, irH immune-related hepatitis, NA not applicable
Most common ICIs used were pembrolizumab monotherapy (30), nivolumab monotherapy (13) and ipilimumab/nivolumab combination (43) (Table 3). Following irH diagnosis, 83 patients had ICI treatment held; 32 patients were rechallenged with the same ICI, 13 (41%) of whom had recurrence of irH. Twenty-seven patients received multiple lines of ICI therapy, but only one patient developed recurrent irH on a subsequent ICI regimen. Steroids were required in 78 cases, 10 required additional non-steroidal immunosuppressant. The most common non-steroidal immunosuppressant used was mycophenolate (90%), indications for use varied but were predominantly due to steroid-refractory cases.
3.3. Immune‑Related Hepatitis and Risk Factor Analysis
We performed univariate and multivariable conditional logistic regression models to evaluate the relationship between history of prior liver pathology (either cirrhosis or liver metastases) and subsequent development of irH. We found that history of cirrhosis was associated with a lower risk of irH, an effect that attained significance after adjusting for covariates (adjusted [a] OR 0.14, 95% CI 0.02–0.75). Conversely, the presence of liver metastasis was associated with increased odds of irH (aOR 2.79 95% CI 1.37–5.66) (Table 5). Rate of recurrence after ICI rechallenge did not have a statistically significant relationship with presence of liver metastases (OR 0.73, 95% CI 0.11–4.69) or history of cirrhosis (OR 0.75, 95% CI 0.06–9.22). Cirrhosis and presence of liver metastases did not correlate with irH grade.
Table 5.
Univariable and multivariable conditional logistic regression analysis of irH risk factors
| Model type | OR (95% CI) | p-Value |
|---|---|---|
| Unadjusted model | ||
| Liver metastasis at ICI initiation | 2.14 (1.15 to 3.96) | 0.015 |
| History of cirrhosis | 0.40 (0.14 to 1.11) | 0.080 |
| Adjusted model 1—adjusted for ECOG Performance Score, history of autoimmune disease, Hepatitis B and Hepatitis C | ||
| Liver metastasis at ICI initiation | 2.46 (1.25 to 4.85) | 0.009 |
| History of cirrhosis | 0.14 (0.02 to 0.74) | 0.020 |
| Adjusted model 2—adjusted for dual ICI, ECOG Performance Score, history of autoimmune disease, Hepatitis B and Hepatitis C | ||
| Liver metastasis at ICI initiation | 2.79 (1.37 to 5.66) | 0.005 |
| History of cirrhosis | 0.14 (0.02 to 0.75) | 0.022 |
CI confidence interval, ECOG Eastern Cooperative Oncology Group, ICI immune checkpoint inhibitor, irH immune-related hepatitis, OR odds ratio
4. Discussion
This retrospective case-control study identified nearly 100 cases of irH and evaluated the relationship between underlying liver pathology and incidence of this IRAE. Herein, we described the clinical features and time course of irH. The presence of hepatic metastases at ICI initiation was associated with a significantly higher risk of irH. Immune-related hepatitis severity and rate of irH recurrence after ICI rechallenge was independent of both history of cirrhosis and the presence of liver metastasis.
Our description of the natural history of irH aligns with previously described clinical courses and prompts several questions for future analysis. Immune-related hepatitis onset after two doses of ICI therapy, or approximately 6–8 weeks, is well established in previous literature, as is the frequent asymptomatic presentation [7, 14]. Interestingly, patients often did not redevelop irH after rechallenging with the same agent. Even fewer patients who developed irH with first ICI exposure had a recurrence of irH when trialed with a different ICI agent. These findings align with previous observations of irH recurrence in a melanoma-specific cohort [15]. We found no significant associations between recurrence rate and liver metastases or cirrhosis, although our findings are limited by low case numbers. Understanding more about why and how the initial exposure to ICI agents provokes irH and identifying features of patients with persistent or recurrent irH following rechallenge remain very important topics for future study. If validated through larger studies, these data may inform adjusted guidelines with respect to rechallenge in mild-moderate cases of irH.
The liver’s position as an immune protected space poses particularly interesting questions regarding the risk of developing irH. The high levels of antigen exposure from gut pathogens requires the liver to have exquisitely tuned immune tolerance to avoid a state of chronic inflammation [16, 17]. Immune dysfunction in cirrhosis presents an additional layer of complexity as the balance of immunodeficiency and systemic inflammation vary depending on disease stage. While systemic inflammation dominates early-stage cirrhosis, immunodeficiency from impaired pathogen defense and T-cell depletion is hallmark of advanced disease [16]. Several mechanisms of immune overactivation have been proposed for IRAEs; however, immunostaining of irH biopsies appear rich in T-lymphocytes with a CD8+ pre-dominance suggesting immune-cell infiltration as the underlying immune mechanism over autoantibody deposition or cytokine release [7, 8, 18].
Most data reported on the rates of irH in ICI therapy in patients with cirrhosis are linked to their use in treatment of HCC. Pembrolizumab and nivolumab are currently approved as second-line agents for advanced HCC [19]. Initial studies evaluating the safety of nivolumab (Checkmate 040 trial) reported treatment-related aminotransferase elevations between 6–15%, comparable to the incidence of irH in other cancer types [9–20]. However, the corresponding pembrolizumab trial reported a notably lower irH incidence at 3% [21]. Subsequent meta-analysis of single-agent ICI therapy in primary liver cancers showed that the incidence of both all-grade and grade ≥3 AST, ALT, bilirubin and hepatobiliary disorders were significantly higher in primary liver cancers than other cancer types [5, 22]. However, these studies were limited by the significant variation in terminology regarding elevated aminotransferase values across the clinical trials queried.
The negative association between cirrhosis and irH found in our study suggests that the underlying immunodeficiency of cirrhosis may outweigh baseline inflammation from chronic liver disease. This was contrary to our hypothesized effect and may still represent a misattribution due to low case numbers. This negative association could also be due to missed cases of irH given underlying hepatocyte exhaustion leading to limited rise in aminotransferase values. Several articles have noted a discordance between the degree of liver test elevation and histopathologic severity of irH [7, 23]. Our findings might therefore suggest the need for lower diagnostic threshold for irH in patients with cirrhosis. A larger cohort of patients with cirrhosis undergoing ICI therapy will be needed to confirm this effect and to parse out the individual effects of HCC and underlying cirrhosis on irH incidence.
Like primary liver tumors, the impact of liver metastasis on rates of irH has limited data. Liver metastases can cause hypoperfusion of surrounding healthy liver parenchyma, damage which can be exacerbated by ICI therapy [5]. The interplay between metastatic disease and the liver parenchyma has unique implications for irH. In the setting of the liver’s immune tolerant environment, liver metastases have been suggested to induce peripheral tolerance to distant metastasis sites, accounting for the poor response to pembrolizumab therapy compared to patients with melanoma and non-liver metastases [17, 24]. Similar lower response rates to ICIs were also noted among patients with advanced urothelial carcinoma with liver metastases [25]. It has been suggested that hepatic metastases may induce a peripheral immune tolerance mechanism through sequestration and apoptosis of antigen-specific CD8+ T-cells in the liver [26]. Despite the literature on the association between hepatic metastases and reduced ICI efficacy, data have not been extensive regarding the mechanism and role of such metastases on hepatic IRAEs. Two small cohort studies, including 8 and 43 patients with irH, respectively, could not draw statistically significant conclusions regarding liver metastasis [27, 28]. The small sample size and related low power may limit the robustness of such analysis.
Our study represents one of the largest known cohorts to examine the relationship between liver metastasis and irH. As hypothesized, the presence of liver metastasis was associated with an increased risk of irH. In the absence of routine liver biopsy, it is difficult to definitively separate the role of irH from direct hepatocellular injury due to metastatic progression. However, by requiring a provider-adjudicated diagnosis of irH, we aimed to limit this confounding variable [29]. Our findings suggest that the putative immunologic effects of liver tumor microenvironment may possibly play a role in irH, but perhaps not as one might expect. Given the role of liver metastases on peripheral ICI efficacy and the reported association between ICI efficacy and IRAE incidence, one might infer that the immune tolerance of hepatic metastases would possibly reduce the rate of irH [1, 26]. Confirming and reconciling our findings with this model remains a plan for future studies, although it highlights the complexity and unique position of the hepatic immune environment compared to the periphery. The role of the underlying tumor type in augmenting an immunogenic role of liver metastases also remains an important question for future subgroup analysis.
This study is one of the few to include grade 2 irH. Most case reports, metanalyses, and retrospective studies are limited to severe manifestations (grade 3–4) of irH, which can result in acute liver failure and, sometimes death [6]. The milder grade 2 irH, which can require treatment interruption and systemic steroids, has rarely been considered in outcomes research. Our chart review found that mild versus moderate/severe irH represented a separation in clinical management between primary oncologist and specialist hepatologist. This can introduce heterogeneity in management and hinders study of the disease as a spectrum [29]. We found this milder form of the disease represented nearly one-third of our cohort, suggesting a large gap in our knowledge about disease course and outcomes.
As the classification of grade 2 versus grade 3 irH can represent the difference between treatment hold and permanent discontinuation, understanding which patients are likely to progress to severe irH could improve therapeutic outcomes [4, 30, 31]. To investigate this question, we performed subgroup analysis of irH risk factors according to irH grade. In our cohort of patients with irH, neither the presence of cirrhosis nor presence of liver metastasis varied according to irH grade. A larger study will be necessary to query how the degree of liver disease varies with irH grade. This is particularly important with cirrhosis, as advanced disease and HCC covary and their individual effects will need to be assessed separately. This may present a challenge as patients with truly advanced liver disease are not considered candidates for ICI. For now, our study represents a step in appreciating the spectrum of disease and bridging this clinical gap in irH management.
Our study has several limitations inherent to its design. The relatively small sample size may have limited the statistical significance of the findings. Additional limitations include its retrospective nature, lack of randomization, and a heterogenous population (e.g., across tumor types, ICI used, sites of metastasis, performance status, prior therapies) leading to potential confounding. The incidence of early, subclinical liver disease may likely be unrepresented in our cohort leading to potential misclassification bias. Due to the inherent variability of reporting non-oncologic radiographic findings in staging studies, incidental evidence of liver disease was not included. Similarly, the retrospective nature of our study precludes the study of relative liver tumor burden/volume on the risk of irH.
Despite its relatively small sample size, our study still represents one of the largest cohorts of irH in the published literature and generates relevant hypotheses to be tested in larger studies. Our stringent inclusion criteria aimed to avoid common confounding causes of increasing aminotransferase values during ICI therapy. Additional strengths include the inclusion of irH grade 2 and the analysis of clinical variables by irH grade. We hope our study can raise awareness of this important and clinically relevant IRAE, provide diagnostic insight as to its presentation and progression, and prompt further research into its clinical correlates, risk factors and clinical outcomes. Moreover, future studies may include biomarker exploration for the diagnosis, risk stratification, prognosis and management of irH and other IRAEs [32].
5. Conclusion
We demonstrated an independent positive association between liver metastases and irH after adjustment for relevant covariates that may act as confounders. Our findings are hypothesis-generating and warrant external validation.
Supplementary Material
Key Points.
The liver’s position as a presumed immune protected space poses particularly interesting questions regarding the risk of developing immune-related adverse events such as immune-related hepatitis.
We describe the presentation, disease course and risk factors in 97 patients with cancer treated with immune checkpoint therapy who developed grade 2–4 immune-related hepatitis.
Patients with cancer who had liver metastases at the time of immune checkpoint inhibitor therapy had increased odds of developing immune-related hepatitis.
Acknowledgements
R. Talukder was supported by the National Cancer Institute under training grant T32CA009515. D. Makrakis and D.R. Bakaloudi acknowledge support from KureIt Cancer Research. P. Grivas acknowledges the Seattle Translational Tumor Research program.
Funding
No external funding was used in the preparation of this manuscript.
Footnotes
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s11523-023-00980-8.
Declarations
Conflicts of interest David Hockenbery has been consultant for VectivBio AG. Petros Grivas has done consulting for 4D Pharma, Aadi Bioscience, Asieris Pharmaceuticals, Astellas Pharma, AstraZeneca, BostonGene, Bristol Myers Squibb, CG Oncology, Dyania Health, Exelixis, Fresenius Kabi, G1 Therapeutics, Genentech, Gilead Sciences, Guardant Health, ImmunityBio, Infinity Pharmaceuticals, Janssen, Lucence, Mirati Therapeutics, MSD, Pfizer, PureTech, Regeneron, Roche, Seattle Genetics, Silverback Therapeutics, Strata Oncology, QED Therapeutics, Merck KGaA, UroGen Pharma. Dr. Grivas also wishes to declare institutional research grants from Bavarian Nordic, Bristol Myers Squibb, Clovis Oncology, Debiopharm Group, G1 Therapeutics, Gilead Sciences, GlaxoSmithKline, Mirati Therapeutics, MSD, Pfizer, QED Therapeutics, and Merck KGaA. Ali R. Khaki has done consulting for Janssen, participates in an advisory board with Seagen/Astellas and has research collaborations with Tempus Labs. The other authors have no disclosures.
Ethics approval Study protocol was approved by University of Washington’s Institutional Review Board (IRB ID: STUDY00008393). This study was completed in compliance with ethical standards set forth by the Helsinki Declaration and its amendments.
Consent to participate Due to the retrospective nature of the study, patient consent was waived by the IRB.
Consent for publication Not applicable.
Code availability Not applicable.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request and depending on institutional restrictions.
References
- 1.Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 2018;378:158–68. [DOI] [PubMed] [Google Scholar]
- 2.Kennedy LC, Bhatia S, Thompson JA, Grivas P. Preexisting autoimmune disease: implications for immune checkpoint inhibitor therapy in solid tumors. J Natl Compr Canc Netw 2019;17:750–7. [DOI] [PubMed] [Google Scholar]
- 3.Kennedy LC, Wong KM, Kamat NV, Khaki AR, Bhatia S, Thompson JA, et al. Untangling the multidisciplinary care web: streamlining care through an immune-related adverse events (IRAE) tumor board. Target Oncol 2020;15:541–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grover S, Rahma OE, Hashemi N, Lim RM. Gastrointestinal and hepatic toxicities of checkpoint inhibitors: algorithms for management. Am Soc Clin Oncol Educ Book 2018;38:13–9. [DOI] [PubMed] [Google Scholar]
- 5.Fu J, Li WZ, McGrath NA, Lai CW, Brar G, Xiang YQ, et al. Immune checkpoint inhibitor associated hepatotoxicity in primary liver cancer versus other cancers: a systematic review and meta-analysis. Front Oncol 2021. 10.3389/fonc.2021.650292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suzman DL, Pelosof L, Rosenberg A, Avigan MI. Hepatotoxicity of immune checkpoint inhibitors: an evolving picture of risk associated with a vital class of immunotherapy agents. Liver Int 2018;38:976–87. [DOI] [PubMed] [Google Scholar]
- 7.Kleiner DE, Berman D. Pathologic changes in ipilimumab-related hepatitis in patients with metastatic melanoma. Dig Dis Sci 2012;57:2233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zen Y, Yeh MM. Checkpoint inhibitor-induced liver injury: a novel form of liver disease emerging in the era of cancer immunotherapy. Semin Diagn Pathol 2019;36:434–40. [DOI] [PubMed] [Google Scholar]
- 9.De Martin E, Michot JM, Rosmorduc O, Guettier C, Samuel D. Liver toxicity as a limiting factor to the increasing use of immune checkpoint inhibitors. JHEP Rep 2020. 10.1016/j.jhepr.2020.100170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) v5.0. National Cancer Institute. 2017. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_60. Accessed 5 Feb 2023.
- 11.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG, et al. Research electronic data capture (REDCap)—A meta-data-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: building an international community of software partners. J Biomed Inform 2019. 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chalasani NP, Hayashi PH, Bonkovsky HL, Navarro VJ, Lee WM, Fontana RJ, et al. Practice Parameters Committee of the American College of Gastroenterology ACG Clinical Guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol 2014;109:950–66. [DOI] [PubMed] [Google Scholar]
- 14.Patrinely JR Jr, McGuigan B, Chandra S, Fenton SE, Chowdhary A, Kennedy LB, et al. A multicenter characterization of hepatitis associated with immune checkpoint inhibitors. Oncoimmunology 2021. 10.1080/2162402X.2021.1875639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li M, Sack JS, Rahma OE, Hodi FS, Zucker SD, Grover S, et al. Outcomes after resumption of immune checkpoint inhibitor therapy after high-grade immune-mediated hepatitis. Cancer. 2020;126:5088–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Albillos A, Martin-Mateos R, Van der Merwe S, Wiest R, Jalan R, Álvarez-Mon M, et al. Cirrhosis-associated immune dysfunction. Nat Rev Gastroenterol Hepatol 2022;19:112–34. [DOI] [PubMed] [Google Scholar]
- 17.Lee JC, Green MD, Huppert LA, Chow C, Pierce RH, Daud AI, et al. The liver-immunity nexus and cancer immunotherapy. Clin Cancer Res 2022;28:5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Martin E, Michot JM, Papouin B, Champiat S, Mateus C, Lambotte O, et al. Characterization of liver injury induced by cancer immunotherapy using immune checkpoint inhibitors. J Hepatol 2018;68:1181–90. [DOI] [PubMed] [Google Scholar]
- 19.Keenan BP, Fong L, Kelley RK. Immunotherapy in hepatocellular carcinoma: the complex interface between inflammation, fibrosis, and the immune response. J Immunother Cancer. 2019. 10.1186/s40425-019-0749-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol 2018;19:940–52. [DOI] [PubMed] [Google Scholar]
- 22.Brown ZJ, Heinrich B, Steinberg SM, Yu SJ, Greten TF. Safety in treatment of hepatocellular carcinoma with immune checkpoint inhibitors as compared to melanoma and non-small cell lung cancer. J Immunother Cancer. 2017. 10.1186/s40425-017-0298-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reynolds K, Thomas M, Dougan M. Diagnosis and management of hepatitis in patients on checkpoint blockade. Oncologist 2018;23:991–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tumeh PC, Hellmann MD, Hamid O, Tsai KK, Loo KL, Gubens MA, et al. Liver metastasis and treatment outcome with anti-PD-1 monoclonal antibody in patients with melanoma and NSCLC. Cancer Immunol Res 2017;5:417–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Makrakis D, Talukder R, Lin GI, Diamantopoulos LN, Dawsey S, Gupta S, et al. Association between sites of metastasis and outcomes with immune checkpoint inhibitors in advanced urothelial carcinoma. Clin Genitourin Cancer. 2022;20:e440–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu J, Green MD, Li S, Sun Y, Choi JE, et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat Med 2021;27:152–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Romanski NA, Holmstroem RB, Ellebaek E, Svane IM. Characterization of risk factors and efficacy of medical management of immune-related hepatotoxicity in real-world patients with metastatic melanoma treated with immune checkpoint inhibitors. Eur J Cancer. 2020;130:211–8. [DOI] [PubMed] [Google Scholar]
- 28.Sawada K, Hayashi H, Nakajima S, Hasebe T, Fujiya M, Okumura T. Non-alcoholic fatty liver disease is a potential risk factor for liver injury caused by immune checkpoint inhibitor. J Gastroenterol Hepatol 2020;35:1042–8. [DOI] [PubMed] [Google Scholar]
- 29.Tsung I, Dolan R, Lao CD, Fecher L, Riggenbach K, Yeboah-Korang A, et al. Liver injury is most commonly due to hepatic metastases rather than drug hepatotoxicity during pembrolizumab immunotherapy. Aliment Pharmacol Ther 2019;50:800–8. [DOI] [PubMed] [Google Scholar]
- 30.Jennings JJ, Mandaliya R, Nakshabandi A, Lewis JH. Hepatotoxicity induced by immune checkpoint inhibitors: a comprehensive review including current and alternative management strategies. Expert Opin Drug Metab Toxicol 2019;15:231–44. [DOI] [PubMed] [Google Scholar]
- 31.Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J Clin Oncol 2018;36:1714–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Michailidou D, Khaki AR, Morelli MP, Diamantopoulos L, Singh N, Grivas P. Association of blood biomarkers and autoimmunity with immune related adverse events in patients with cancer treated with immune checkpoint inhibitors. Sci Rep 2021. 10.1038/s41598-021-88307-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request and depending on institutional restrictions.


