Abstract
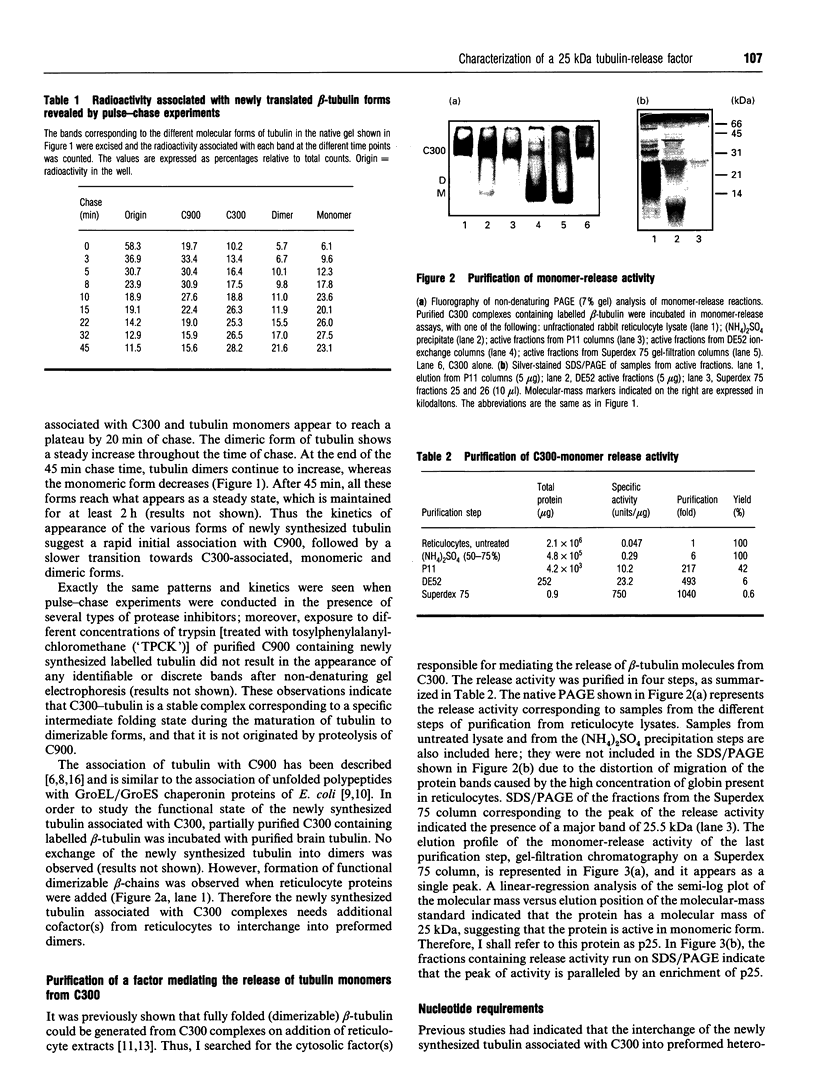
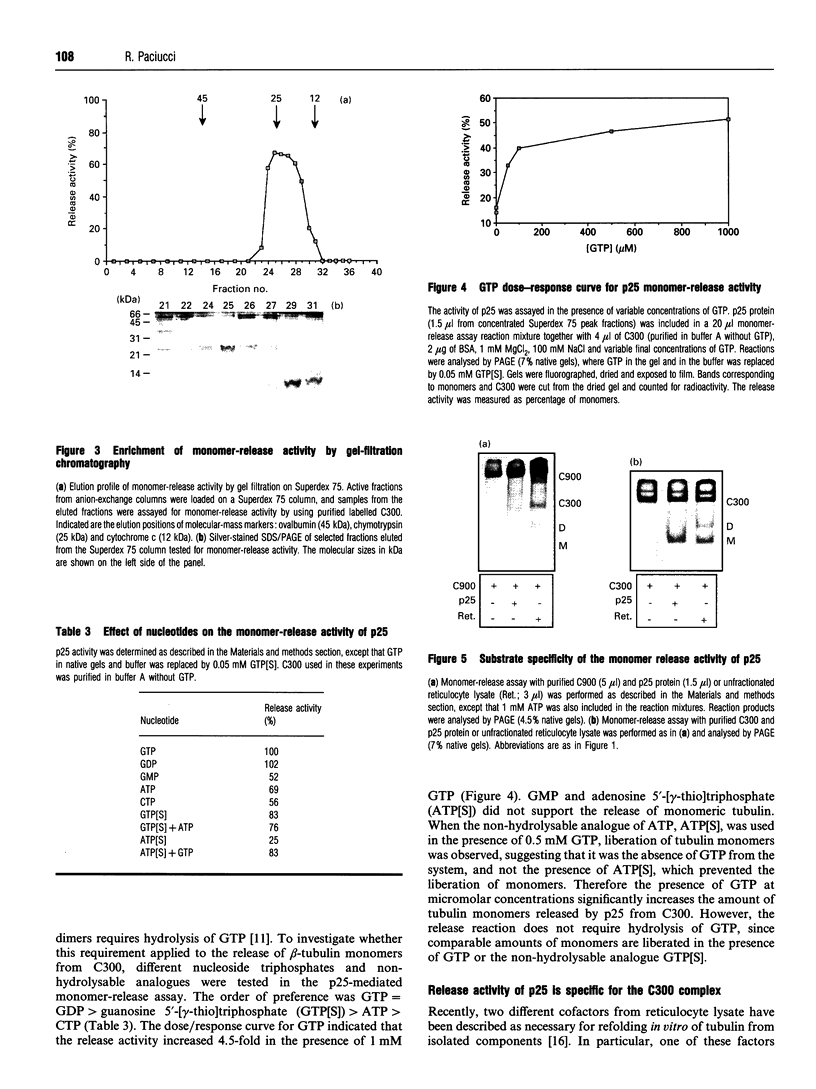
beta-Tubulin synthesized in vitro in rabbit reticulocyte lysate is found associated with 900 kDa complexes (C900) containing T Complex Polypeptide 1 (TCP1), heat-shock protein (hsp) 70 and other unidentified proteins, with smaller 300 kDa complexes (C300) of unknown nature, in dimeric association with reticulocyte alpha-tubulin and in monomeric forms. Pulse-chase experiments indicated that production of fully functional beta-tubulin was preceded by its association with C900 and C300 multimolecular complexes and by the appearance of beta-monomers. The high-molecular-mass forms appeared as intermediate products in the process leading to fully functional dimerizable beta-tubulin. C300-associated tubulin can be released as beta-monomer by addition of a cofactor present in reticulocyte lysate. Here a 25 kDa protein which releases tubulin monomers from C300 has been identified and characterized. The protein specifically released monomers from C300, but not from C900, in a process favoured by GTP.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bischoff F. R., Ponstingl H. Mitotic regulator protein RCC1 is complexed with a nuclear ras-related polypeptide. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10830–10834. doi: 10.1073/pnas.88.23.10830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Ellis R. J., van der Vies S. M. Molecular chaperones. Annu Rev Biochem. 1991;60:321–347. doi: 10.1146/annurev.bi.60.070191.001541. [DOI] [PubMed] [Google Scholar]
- Farr G. W., Yaffe M. B., Sternlicht H. Alpha-tubulin influences nucleotide binding to beta-tubulin: an assay using picomoles of unpurified protein. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5041–5045. doi: 10.1073/pnas.87.13.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontalba A., Paciucci R., Avila J., Zabala J. C. Incorporation of tubulin subunits into dimers requires GTP hydrolysis. J Cell Sci. 1993 Oct;106(Pt 2):627–632. doi: 10.1242/jcs.106.2.627. [DOI] [PubMed] [Google Scholar]
- Frydman J., Nimmesgern E., Erdjument-Bromage H., Wall J. S., Tempst P., Hartl F. U. Function in protein folding of TRiC, a cytosolic ring complex containing TCP-1 and structurally related subunits. EMBO J. 1992 Dec;11(13):4767–4778. doi: 10.1002/j.1460-2075.1992.tb05582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Thomas J. O., Chow R. L., Lee G. H., Cowan N. J. A cytoplasmic chaperonin that catalyzes beta-actin folding. Cell. 1992 Jun 12;69(6):1043–1050. doi: 10.1016/0092-8674(92)90622-j. [DOI] [PubMed] [Google Scholar]
- Gao Y., Vainberg I. E., Chow R. L., Cowan N. J. Two cofactors and cytoplasmic chaperonin are required for the folding of alpha- and beta-tubulin. Mol Cell Biol. 1993 Apr;13(4):2478–2485. doi: 10.1128/mcb.13.4.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goloubinoff P., Christeller J. T., Gatenby A. A., Lorimer G. H. Reconstitution of active dimeric ribulose bisphosphate carboxylase from an unfoleded state depends on two chaperonin proteins and Mg-ATP. Nature. 1989 Dec 21;342(6252):884–889. doi: 10.1038/342884a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landry S. J., Gierasch L. M. Recognition of nascent polypeptides for targeting and folding. Trends Biochem Sci. 1991 Apr;16(4):159–163. doi: 10.1016/0968-0004(91)90060-9. [DOI] [PubMed] [Google Scholar]
- Langer T., Lu C., Echols H., Flanagan J., Hayer M. K., Hartl F. U. Successive action of DnaK, DnaJ and GroEL along the pathway of chaperone-mediated protein folding. Nature. 1992 Apr 23;356(6371):683–689. doi: 10.1038/356683a0. [DOI] [PubMed] [Google Scholar]
- Lewis V. A., Hynes G. M., Zheng D., Saibil H., Willison K. T-complex polypeptide-1 is a subunit of a heteromeric particle in the eukaryotic cytosol. Nature. 1992 Jul 16;358(6383):249–252. doi: 10.1038/358249a0. [DOI] [PubMed] [Google Scholar]
- Manning-Krieg U. C., Scherer P. E., Schatz G. Sequential action of mitochondrial chaperones in protein import into the matrix. EMBO J. 1991 Nov;10(11):3273–3280. doi: 10.1002/j.1460-2075.1991.tb04891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesi V. T., Ngo N. In vitro assembly of multiprotein complexes containing alpha, beta, and gamma tubulin, heat shock protein HSP70, and elongation factor 1 alpha. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):3028–3032. doi: 10.1073/pnas.90.7.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J., Langer T., Boteva R., Schramel A., Horwich A. L., Hartl F. U. Chaperonin-mediated protein folding at the surface of groEL through a 'molten globule'-like intermediate. Nature. 1991 Jul 4;352(6330):36–42. doi: 10.1038/352036a0. [DOI] [PubMed] [Google Scholar]
- Ruddon R. W., Krzesicki R. F., Norton S. E., Beebe J. S., Peters B. P., Perini F. Detection of a glycosylated, incompletely folded form of chorionic gonadotropin beta subunit that is a precursor of hormone assembly in trophoblastic cells. J Biol Chem. 1987 Sep 15;262(26):12533–12540. [PubMed] [Google Scholar]
- Sternlicht H., Farr G. W., Sternlicht M. L., Driscoll J. K., Willison K., Yaffe M. B. The t-complex polypeptide 1 complex is a chaperonin for tubulin and actin in vivo. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9422–9426. doi: 10.1073/pnas.90.20.9422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland T. W., Pierce J. G. The beta subunits of glycoprotein hormones. Formation of three-dimensional structure during cell-free biosynthesis of lutropin-beta. J Biol Chem. 1985 May 10;260(9):5816–5819. [PubMed] [Google Scholar]
- Strickland T. W., Puett D. The kinetic and equilibrium parameters of subunit association and gonadotropin dissociation. J Biol Chem. 1982 Mar 25;257(6):2954–2960. [PubMed] [Google Scholar]
- Yaffe M. B., Farr G. W., Miklos D., Horwich A. L., Sternlicht M. L., Sternlicht H. TCP1 complex is a molecular chaperone in tubulin biogenesis. Nature. 1992 Jul 16;358(6383):245–248. doi: 10.1038/358245a0. [DOI] [PubMed] [Google Scholar]
- Yaffe M. B., Farr G. W., Sternlicht H. Kinetics of beta-tubulin exchange following translation. Evidence for a slow conformational change in beta-tubulin necessary for incorporation into heterodimers. J Biol Chem. 1989 Nov 15;264(32):19045–19051. [PubMed] [Google Scholar]
- Yaffe M. B., Farr G. W., Sternlicht H. Translation of beta-tubulin mRNA in vitro generates multiple molecular forms. J Biol Chem. 1988 Nov 5;263(31):16023–16031. [PubMed] [Google Scholar]
- Yen T. J., Machlin P. S., Cleveland D. W. Autoregulated instability of beta-tubulin mRNAs by recognition of the nascent amino terminus of beta-tubulin. Nature. 1988 Aug 18;334(6183):580–585. doi: 10.1038/334580a0. [DOI] [PubMed] [Google Scholar]
- Zabala J. C., Cowan N. J. Tubulin dimer formation via the release of alpha- and beta-tubulin monomers from multimolecular complexes. Cell Motil Cytoskeleton. 1992;23(3):222–230. doi: 10.1002/cm.970230306. [DOI] [PubMed] [Google Scholar]