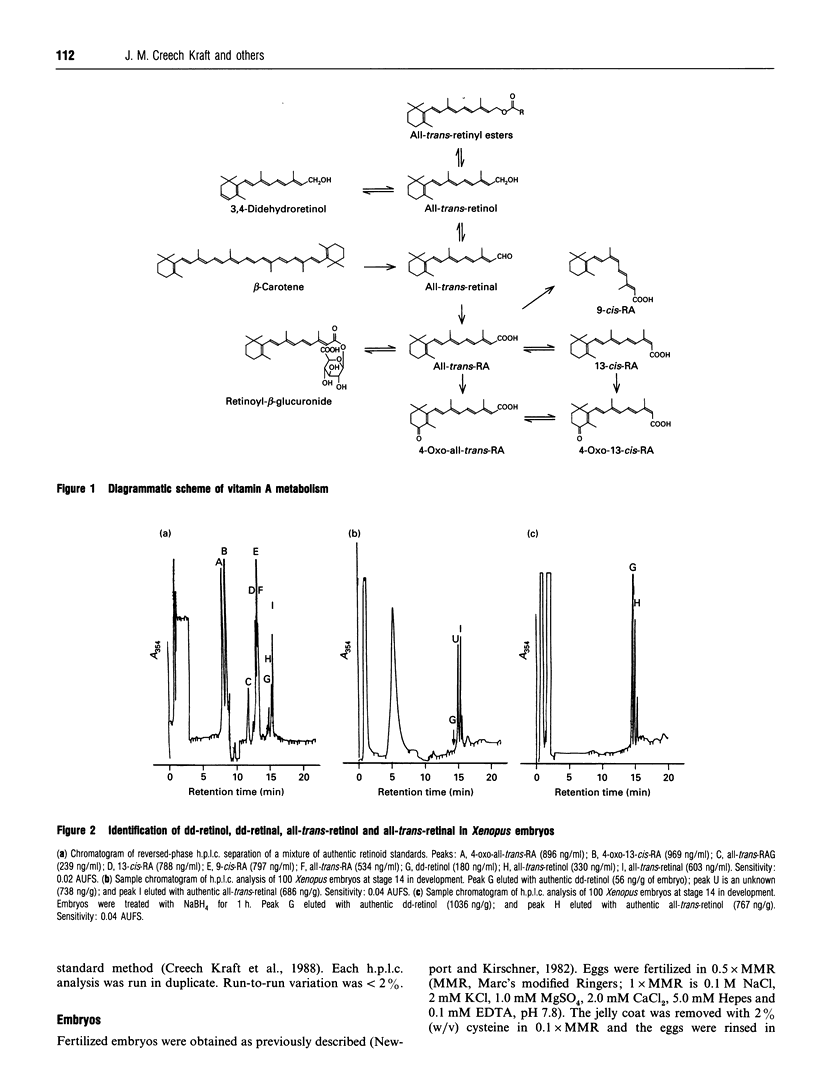
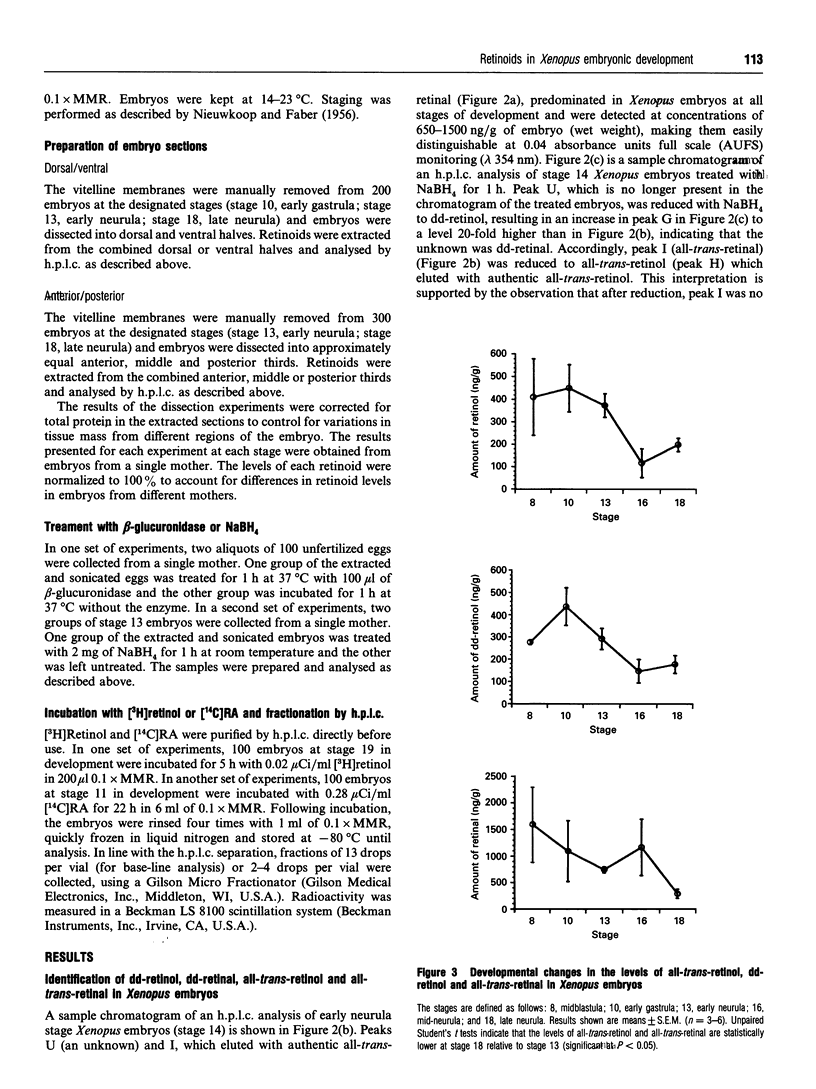
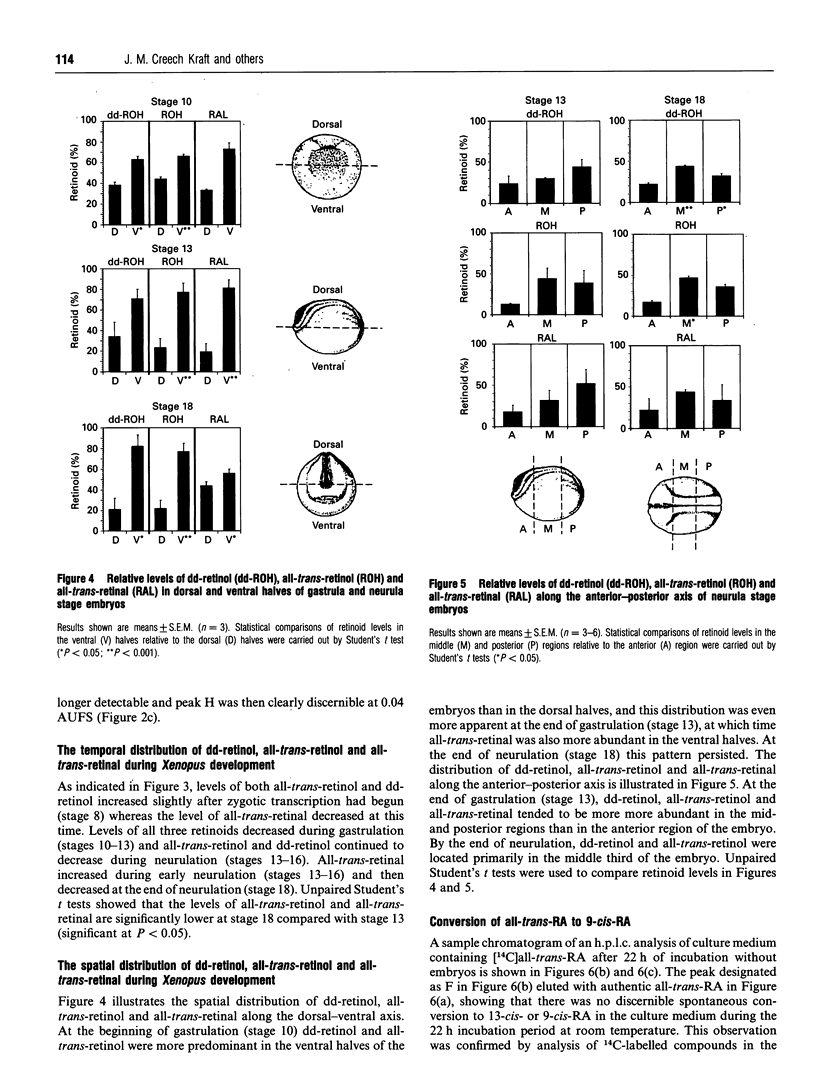
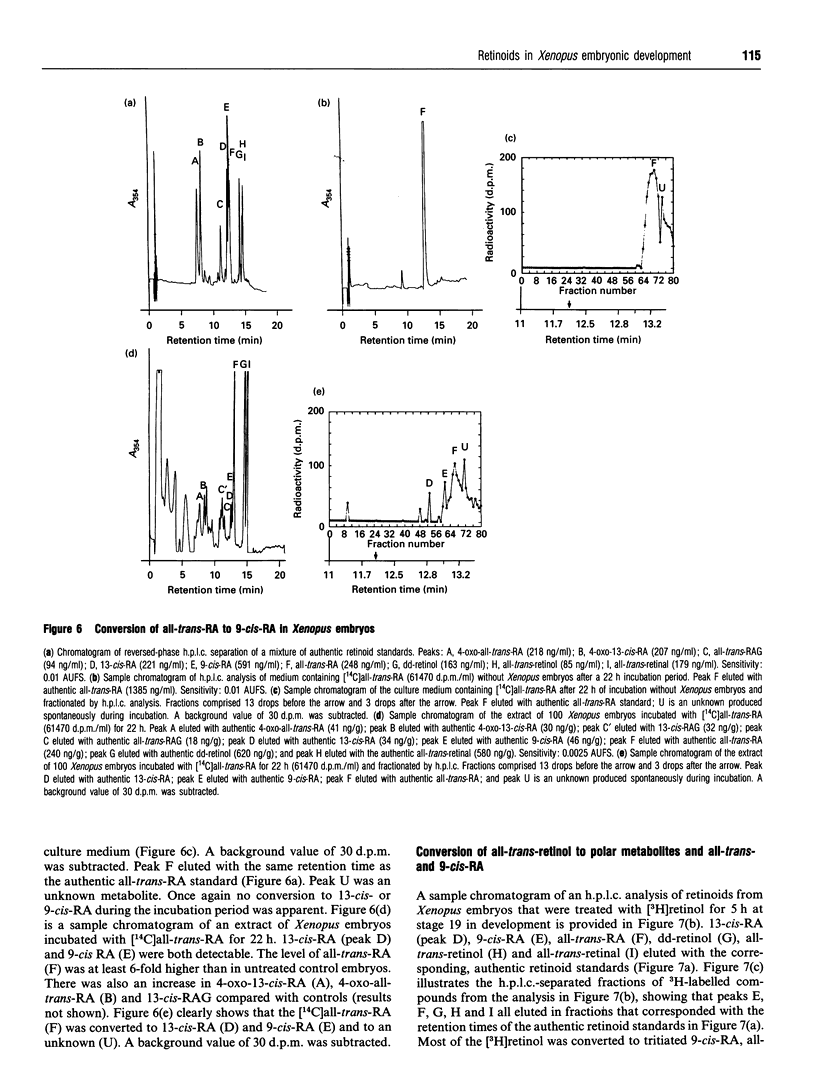
Abstract
Recently, the temporal and spatial distribution patterns of the retinoid receptor ligands 9-cis-retinoic acid and all-trans-retinoic acid were described in Xenopus embryos during early development [Creech Kraft, Schuh, Juchau and Kimelman (1994) Proc. Natl. Acad. Sci. U.S.A., in the press]. The present study demonstrates the presence and distribution of their likely precursors, all-trans-retinol, didehydroretinol, didehydroretinal and all-trans-retinal, as well as the occurrence of 4-oxo metabolites, in Xenopus embryos. The temporal and spatial distribution patterns of all-trans-retinol, didehydroretinol and all-trans-retinal did not coincide with that observed for 9-cis-retinoic acid but, in certain regards, were similar to the patterns delineated for all-trans-retinoic acid and all-trans-retinoyl beta-glucuronide. Evidence is presented that 9-cis-retinoic acid can be synthesized from both all-trans-retinoic acid and all-trans-retinol in Xenopus embryos, suggesting that the difference between the distributions of 9-cis-retinoic acid and the other retinoids may be caused by selective synthesis and/or protein binding of the 9-cis isomer.
Full text
PDF
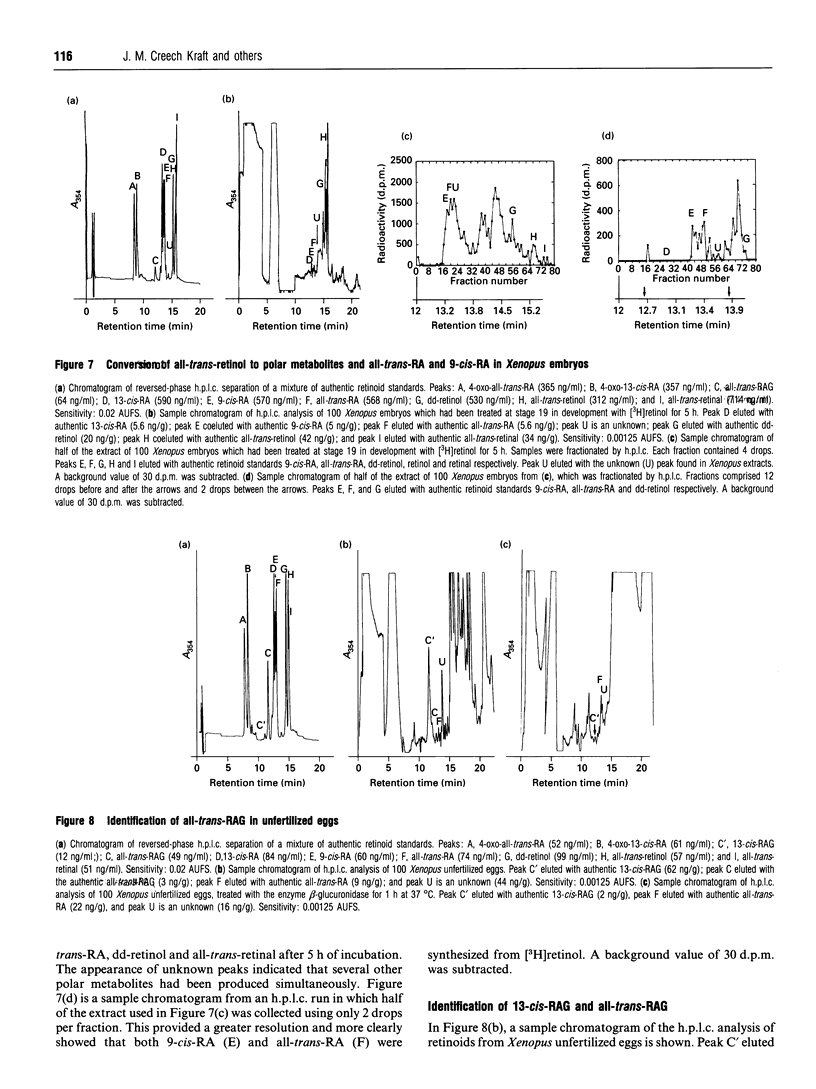
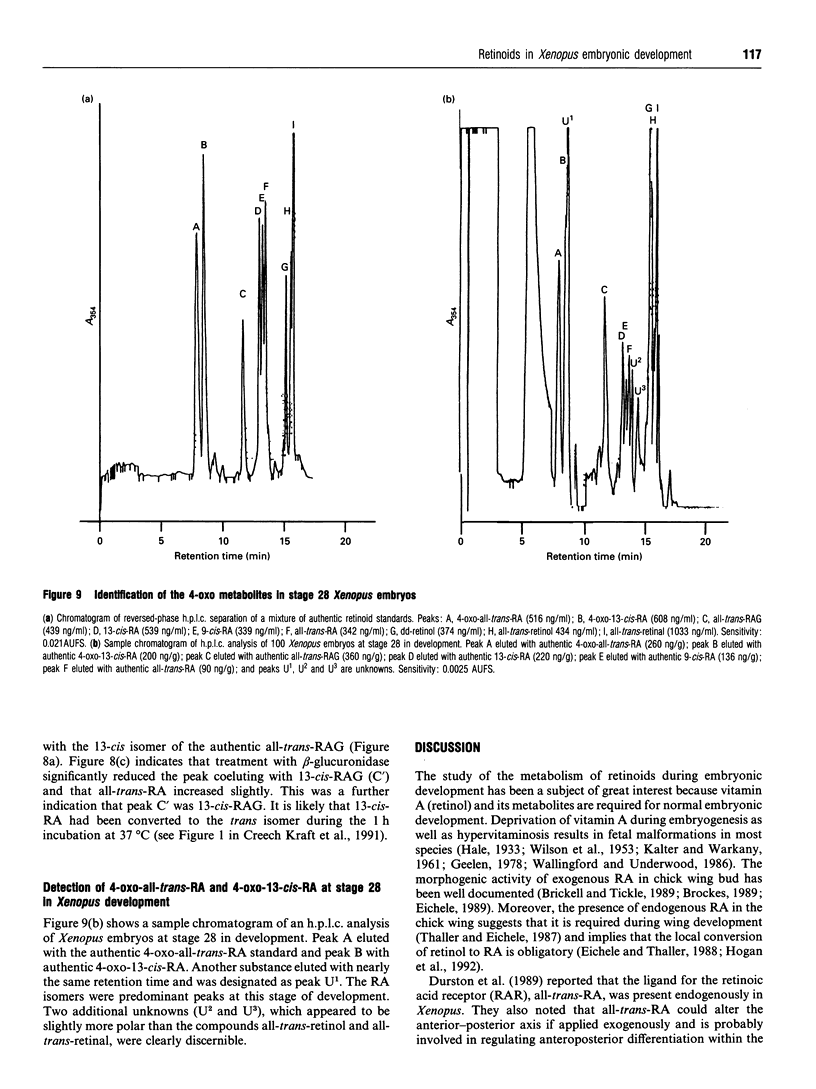


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azuma M., Seki T., Fujishita S. Changes of egg retinoids during the development of Xenopus laevis. Vision Res. 1990;30(10):1395–1400. doi: 10.1016/0042-6989(90)90020-l. [DOI] [PubMed] [Google Scholar]
- Barua A. B., Olson J. A. Chemical synthesis and growth-promoting activity of all-trans-retinyl beta-D-glucuronide. Biochem J. 1987 May 15;244(1):231–234. doi: 10.1042/bj2440231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg B., Mangelsdorf D. J., Dyck J. A., Bittner D. A., Evans R. M., De Robertis E. M. Multiple retinoid-responsive receptors in a single cell: families of retinoid "X" receptors and retinoic acid receptors in the Xenopus egg. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2321–2325. doi: 10.1073/pnas.89.6.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickell P. M., Tickle C. Morphogens in chick limb development. Bioessays. 1989 Nov;11(5):145–149. doi: 10.1002/bies.950110508. [DOI] [PubMed] [Google Scholar]
- Brockes J. P. Retinoids, homeobox genes, and limb morphogenesis. Neuron. 1989 Apr;2(4):1285–1294. doi: 10.1016/0896-6273(89)90066-4. [DOI] [PubMed] [Google Scholar]
- Creech Kraft J., Eckhoff C., Kochhar D. M., Bochert G., Chahoud I., Nau H. Isotretinoin (13-cis-retinoic acid) metabolism, cis-trans isomerization, glucuronidation, and transfer to the mouse embryo: consequences for teratogenicity. Teratog Carcinog Mutagen. 1991;11(1):21–30. doi: 10.1002/tcm.1770110104. [DOI] [PubMed] [Google Scholar]
- Creech Kraft J., Löfberg B., Chahoud I., Bochert G., Nau H. Teratogenicity and placental transfer of all-trans-, 13-cis-, 4-oxo-all-trans-, and 4-oxo-13-cis-retinoic acid after administration of a low oral dose during organogenesis in mice. Toxicol Appl Pharmacol. 1989 Aug;100(1):162–176. doi: 10.1016/0041-008x(89)90099-9. [DOI] [PubMed] [Google Scholar]
- Durston A. J., Timmermans J. P., Hage W. J., Hendriks H. F., de Vries N. J., Heideveld M., Nieuwkoop P. D. Retinoic acid causes an anteroposterior transformation in the developing central nervous system. Nature. 1989 Jul 13;340(6229):140–144. doi: 10.1038/340140a0. [DOI] [PubMed] [Google Scholar]
- Eichele G. Retinoids and vertebrate limb pattern formation. Trends Genet. 1989 Aug;5(8):246–251. doi: 10.1016/0168-9525(89)90096-6. [DOI] [PubMed] [Google Scholar]
- Ellinger-Ziegelbauer H., Dreyer C. A retinoic acid receptor expressed in the early development of Xenopus laevis. Genes Dev. 1991 Jan;5(1):94–104. doi: 10.1101/gad.5.1.94. [DOI] [PubMed] [Google Scholar]
- Geelen J. A. Hypervitaminosis A induced teratogenesis. CRC Crit Rev Toxicol. 1979 Nov;6(4):351–375. doi: 10.3109/10408447909043651. [DOI] [PubMed] [Google Scholar]
- Heyman R. A., Mangelsdorf D. J., Dyck J. A., Stein R. B., Eichele G., Evans R. M., Thaller C. 9-cis retinoic acid is a high affinity ligand for the retinoid X receptor. Cell. 1992 Jan 24;68(2):397–406. doi: 10.1016/0092-8674(92)90479-v. [DOI] [PubMed] [Google Scholar]
- Hogan B. L., Thaller C., Eichele G. Evidence that Hensen's node is a site of retinoic acid synthesis. Nature. 1992 Sep 17;359(6392):237–241. doi: 10.1038/359237a0. [DOI] [PubMed] [Google Scholar]
- KALTER H., WARKANY J. Experimental production of congenital malformations in strains of inbred mice by maternal treatment with hypervitaminosis A. Am J Pathol. 1961 Jan;38:1–21. [PMC free article] [PubMed] [Google Scholar]
- Kraft J. C., Juchau M. R. 9-cis-retinoic acid: a direct-acting dysmorphogen. Biochem Pharmacol. 1993 Aug 17;46(4):709–716. doi: 10.1016/0006-2952(93)90558-e. [DOI] [PubMed] [Google Scholar]
- Levin A. A., Sturzenbecker L. J., Kazmer S., Bosakowski T., Huselton C., Allenby G., Speck J., Kratzeisen C., Rosenberger M., Lovey A. 9-cis retinoic acid stereoisomer binds and activates the nuclear receptor RXR alpha. Nature. 1992 Jan 23;355(6358):359–361. doi: 10.1038/355359a0. [DOI] [PubMed] [Google Scholar]
- Newport J., Kirschner M. A major developmental transition in early Xenopus embryos: I. characterization and timing of cellular changes at the midblastula stage. Cell. 1982 Oct;30(3):675–686. doi: 10.1016/0092-8674(82)90272-0. [DOI] [PubMed] [Google Scholar]
- Pijnappel W. W., Hendriks H. F., Folkers G. E., van den Brink C. E., Dekker E. J., Edelenbosch C., van der Saag P. T., Durston A. J. The retinoid ligand 4-oxo-retinoic acid is a highly active modulator of positional specification. Nature. 1993 Nov 25;366(6453):340–344. doi: 10.1038/366340a0. [DOI] [PubMed] [Google Scholar]
- Sharpe C. R. Two isoforms of retinoic acid receptor alpha expressed during Xenopus development respond to retinoic acid. Mech Dev. 1992 Nov;39(1-2):81–93. doi: 10.1016/0925-4773(92)90028-i. [DOI] [PubMed] [Google Scholar]
- Sive H. L., Draper B. W., Harland R. M., Weintraub H. Identification of a retinoic acid-sensitive period during primary axis formation in Xenopus laevis. Genes Dev. 1990 Jun;4(6):932–942. doi: 10.1101/gad.4.6.932. [DOI] [PubMed] [Google Scholar]
- Thaller C., Eichele G. Characterization of retinoid metabolism in the developing chick limb bud. Development. 1988 Jul;103(3):473–483. doi: 10.1242/dev.103.3.473. [DOI] [PubMed] [Google Scholar]
- Thaller C., Eichele G. Identification and spatial distribution of retinoids in the developing chick limb bud. Nature. 1987 Jun 18;327(6123):625–628. doi: 10.1038/327625a0. [DOI] [PubMed] [Google Scholar]
- Thaller C., Hofmann C., Eichele G. 9-cis-retinoic acid, a potent inducer of digit pattern duplications in the chick wing bud. Development. 1993 Jul;118(3):957–965. doi: 10.1242/dev.118.3.957. [DOI] [PubMed] [Google Scholar]
- WILSON J. G., ROTH C. B., WARKANY J. An analysis of the syndrome of malformations induced by maternal vitamin A deficiency. Effects of restoration of vitamin A at various times during gestation. Am J Anat. 1953 Mar;92(2):189–217. doi: 10.1002/aja.1000920202. [DOI] [PubMed] [Google Scholar]


