Abstract
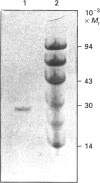
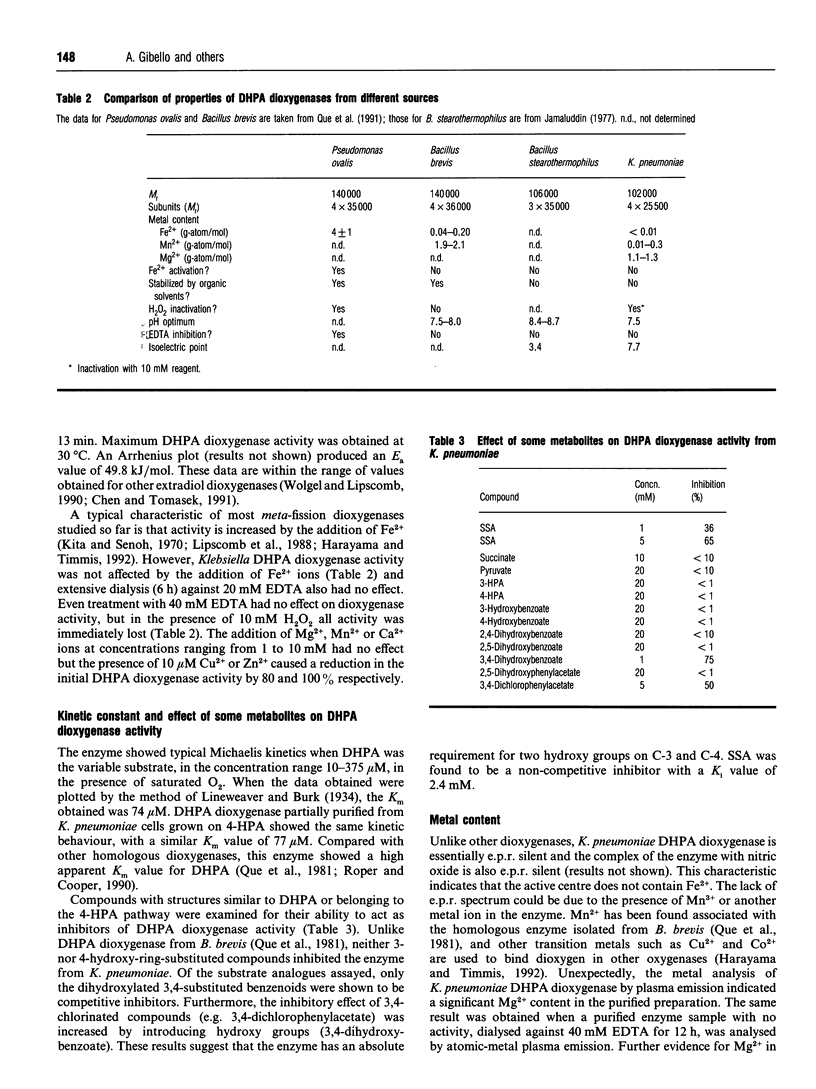
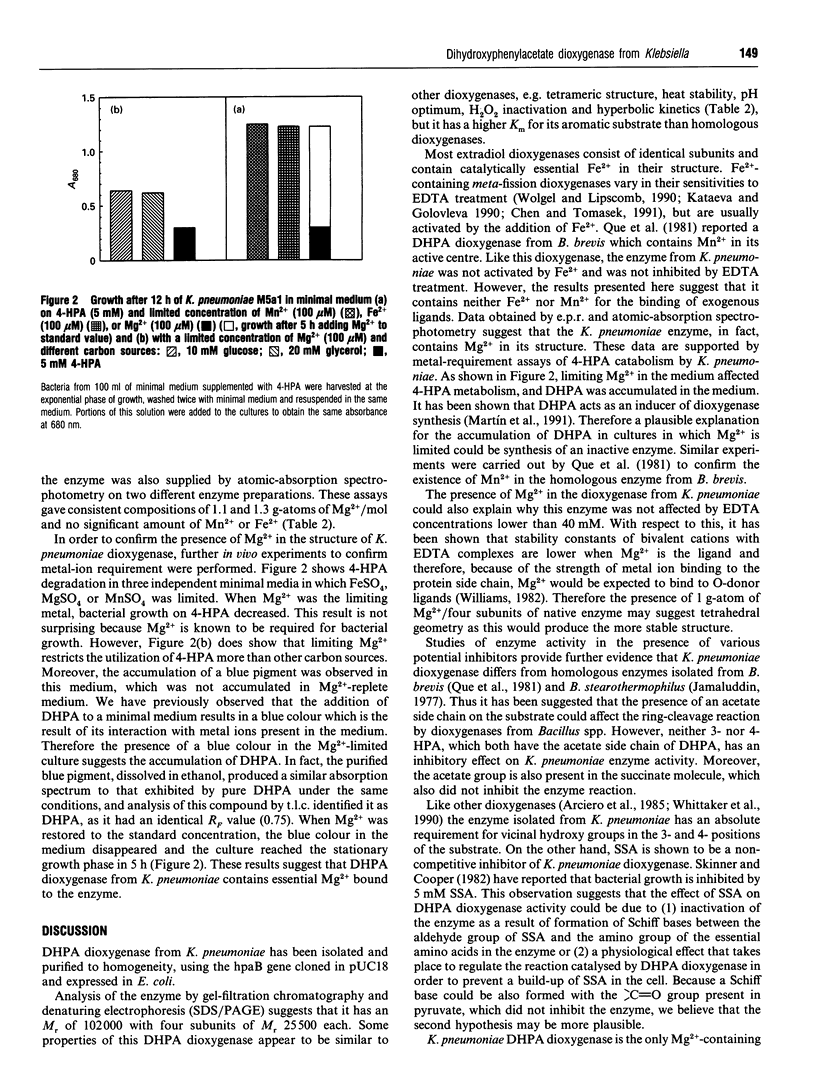
3,4-Dihydroxyphenylacetate 2,3-dioxygenase, an extradiol-ring-cleavage dioxygenase, has been purified from Klebsiella pneumoniae to homogeneity. The enzyme has an M(r) of 102,000 in its tetrameric form with an M(r) of 25,500 for each subunit. Unlike most other dioxygenases, the enzyme reported here contains Mg2+, as determined by atomic-absorption spectrophotometry and plasma emission metal analysis. The enzyme was shown to contain approx. 1 g-atom of Mg2+/mol of protein and we suggest an alpha 4 Mg2+ quaternary structure. This is the first report of a dioxygenase containing Mg2+ in its structure.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi T., Murooka Y., Harada T. Derepression of arylsulfatase synthesis in Aerobacter aerogenes by tyramine. J Bacteriol. 1973 Oct;116(1):19–24. doi: 10.1128/jb.116.1.19-24.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso J. M., Garrido-Pertierra A. Kinetic properties of 5-carboxymethyl-2-hydroxymuconate semialdehyde dehydrogenase from Escherichia coli. Biochimie. 1986 May;68(5):731–737. doi: 10.1016/s0300-9084(86)80167-5. [DOI] [PubMed] [Google Scholar]
- Arciero D. M., Orville A. M., Lipscomb J. D. [17O]Water and nitric oxide binding by protocatechuate 4,5-dioxygenase and catechol 2,3-dioxygenase. Evidence for binding of exogenous ligands to the active site Fe2+ of extradiol dioxygenases. J Biol Chem. 1985 Nov 15;260(26):14035–14044. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chen C. M., Tomasek P. H. 3,4-Dihydroxyxanthone dioxygenase from Arthrobacter sp. strain GFB100. Appl Environ Microbiol. 1991 Aug;57(8):2217–2222. doi: 10.1128/aem.57.8.2217-2222.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuskey S. M., Peccoraro V., Olsen R. H. Initial catabolism of aromatic biogenic amines by Pseudomonas aeruginosa PAO: pathway description, mapping of mutations, and cloning of essential genes. J Bacteriol. 1987 Jun;169(6):2398–2404. doi: 10.1128/jb.169.6.2398-2404.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Deschamps A. M., Richard C., Lebeault J. M. Bacteriology and nutrition of environmental strains of Klebsiella pneumoniae involved in wood and bark decay. Ann Microbiol (Paris) 1983 Mar-Apr;134A(2):189–196. doi: 10.1016/s0769-2609(83)80080-5. [DOI] [PubMed] [Google Scholar]
- Devi N. A., Kutty R. K., Vasantharajan V. N., Subba RAO P. V. Microbial metabolism of phenolic amines: degradation of dl-synephrine by an unidentified arthrobacter. J Bacteriol. 1975 Jun;122(3):866–873. doi: 10.1128/jb.122.3.866-873.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson D. T., Koch J. R., Schuld C. L., Kallio R. E. Oxidative degradation of aromatic hydrocarbons by microorganisms. II. Metabolism of halogenated aromatic hydrocarbons. Biochemistry. 1968 Nov;7(11):3795–3802. doi: 10.1021/bi00851a003. [DOI] [PubMed] [Google Scholar]
- Harayama S., Kok M., Neidle E. L. Functional and evolutionary relationships among diverse oxygenases. Annu Rev Microbiol. 1992;46:565–601. doi: 10.1146/annurev.mi.46.100192.003025. [DOI] [PubMed] [Google Scholar]
- Harayama S., Rekik M. Bacterial aromatic ring-cleavage enzymes are classified into two different gene families. J Biol Chem. 1989 Sep 15;264(26):15328–15333. [PubMed] [Google Scholar]
- Hareland W. A., Crawford R. L., Chapman P. J., Dagley S. Metabolic function and properties of 4-hydroxyphenylacetic acid 1-hydroxylase from Pseudomonas acidovorans. J Bacteriol. 1975 Jan;121(1):272–285. doi: 10.1128/jb.121.1.272-285.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpel M. R., Lipscomb J. D. Gentisate 1,2-dioxygenase from pseudomonas. Purification, characterization, and comparison of the enzymes from Pseudomonas testosteroni and Pseudomonas acidovorans. J Biol Chem. 1990 Apr 15;265(11):6301–6311. [PubMed] [Google Scholar]
- Jamaluddin M. P. Purification and properties of homoprotocatechuate 2,3-dioxygenase from Bacillus stearothermophilus. J Bacteriol. 1977 Feb;129(2):690–697. doi: 10.1128/jb.129.2.690-697.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. C., Cooper R. A. Catabolism of 3-hydroxybenzoate by the gentisate pathway in Klebsiella pneumoniae M5a1. Arch Microbiol. 1990;154(5):489–495. doi: 10.1007/BF00245233. [DOI] [PubMed] [Google Scholar]
- Kalb V. F., Jr, Bernlohr R. W. A new spectrophotometric assay for protein in cell extracts. Anal Biochem. 1977 Oct;82(2):362–371. doi: 10.1016/0003-2697(77)90173-7. [DOI] [PubMed] [Google Scholar]
- Kataeva I. A., Golovleva L. A. Catechol 2,3-dioxygenases from Pseudomonas aeruginosa 2x. Methods Enzymol. 1990;188:115–121. doi: 10.1016/0076-6879(90)88021-2. [DOI] [PubMed] [Google Scholar]
- Lee Y. L., Dagley S. Comparison of two dioxygenases from Pseudomonas putida. J Bacteriol. 1977 Sep;131(3):1016–1017. doi: 10.1128/jb.131.3.1016-1017.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín M., Gibello A., Fernández J., Ferrer E., Garrido-Pertierra A. Catabolism of 3- and 4-hydroxyphenylacetic acid by Klebsiella pneumoniae. J Gen Microbiol. 1991 Mar;137(3):621–628. doi: 10.1099/00221287-137-3-621. [DOI] [PubMed] [Google Scholar]
- Nielsen B. L., Brown L. R. The basis for colored silver-protein complex formation in stained polyacrylamide gels. Anal Biochem. 1984 Sep;141(2):311–315. doi: 10.1016/0003-2697(84)90047-2. [DOI] [PubMed] [Google Scholar]
- Que L., Jr, Widom J., Crawford R. L. 3,4-Dihydroxyphenylacetate 2,3-dioxygenase. A manganese(II) dioxygenase from Bacillus brevis. J Biol Chem. 1981 Nov 10;256(21):10941–10944. [PubMed] [Google Scholar]
- Roper D. I., Cooper R. A. Subcloning and nucleotide sequence of the 3,4-dihydroxyphenylacetate (homoprotocatechuate) 2,3-dioxygenase gene from Escherichia coli C. FEBS Lett. 1990 Nov 26;275(1-2):53–57. doi: 10.1016/0014-5793(90)81437-s. [DOI] [PubMed] [Google Scholar]
- Skinner M. A., Cooper R. A. An Escherichia coli mutant defective in the NAD-dependent succinate semialdehyde dehydrogenase. Arch Microbiol. 1982 Sep;132(3):270–275. doi: 10.1007/BF00407964. [DOI] [PubMed] [Google Scholar]
- Sparnins V. L., Chapman P. J. Catabolism of L-tyrosine by the homoprotocatechuate pathway in gram-positive bacteria. J Bacteriol. 1976 Jul;127(1):362–366. doi: 10.1128/jb.127.1.362-366.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoelstra S. F. Degradation of tyrosine in anaerobically stored piggery wastes and in pig feces. Appl Environ Microbiol. 1978 Nov;36(5):631–638. doi: 10.1128/aem.36.5.631-638.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Whittaker J. W., Orville A. M., Lipscomb J. D. Protocatechuate 3,4-dioxygenase from Brevibacterium fuscum. Methods Enzymol. 1990;188:82–88. doi: 10.1016/0076-6879(90)88016-4. [DOI] [PubMed] [Google Scholar]
- Williams R. J. Free manganese (II) and iron (II) cations can act as intracellular cell controls. FEBS Lett. 1982 Apr 5;140(1):3–10. doi: 10.1016/0014-5793(82)80508-5. [DOI] [PubMed] [Google Scholar]
- Wolgel S. A., Lipscomb J. D. Protocatechuate 2,3-dioxygenase from Bacillus macerans. Methods Enzymol. 1990;188:95–101. doi: 10.1016/0076-6879(90)88018-6. [DOI] [PubMed] [Google Scholar]