Abstract
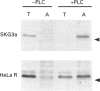
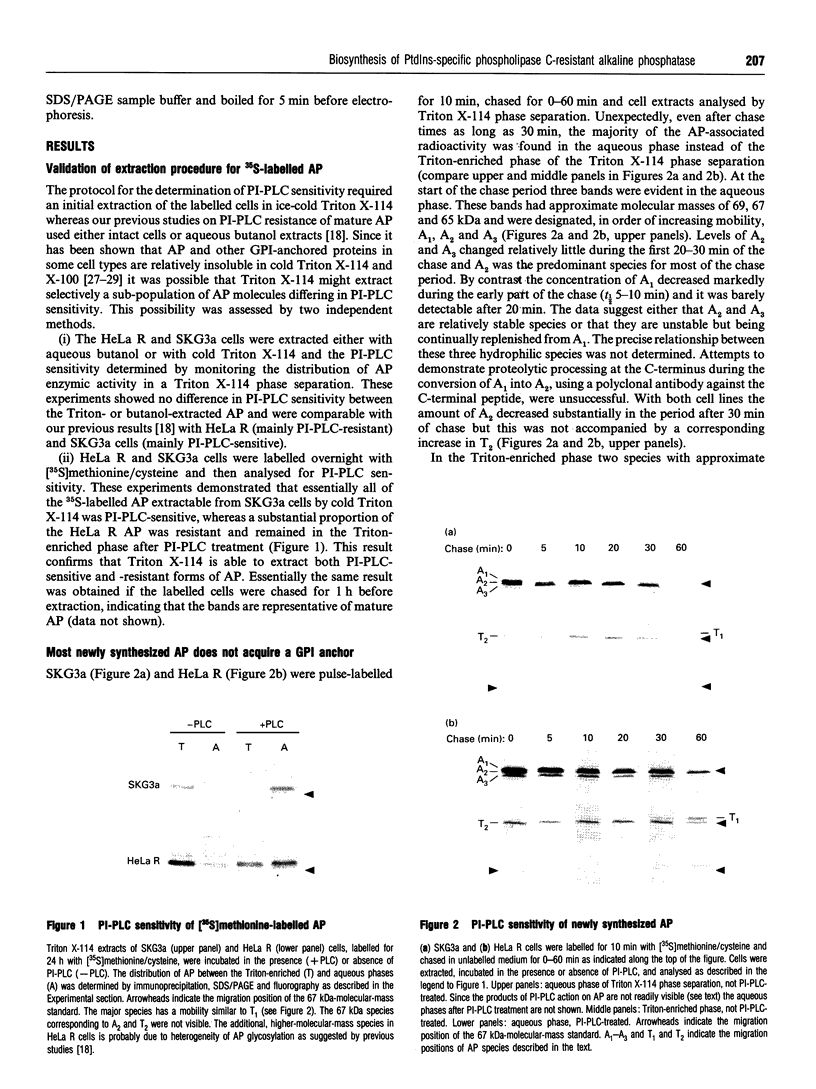
Previous studies have shown that some cells (e.g. SKG3a) express human placental alkaline phosphatase (AP) in a form which can be released from the membrane by bacterial PtdIns-specific phospholipase C (PI-PLC) while others (e.g. HeLa) are relatively resistant to this enzyme. Chemical and enzymic degradation studies have suggested that the PI-PLC resistance of AP is due to inositol acylation of its glycosylphosphatidylinositol (GPI) anchor. In order to identify the biosynthetic origin of PI-PLC resistance we determined the PI-PLC sensitivity of AP in 35S-labelled cells (10 min pulse; 0-60 min chase) by Triton X-114 phase separation. At the beginning of the chase period, the majority of the AP synthesized was hydrophilic, indicating that it had not acquired a GPI anchor. The concentration of hydrophilic AP species decreased with a t1/2 of 30-60 min but was not processed to an endoglycosidase H-resistant species or secreted into the medium. In both SKG3a and HeLa cells all of the hydrophobic, GPI-anchored AP detectable at the beginning of the chase was PI-PLC sensitive. PI-PLC-resistant species of AP were only observed in HeLa cells and these only appeared after about 30 min. The delayed appearance of PI-PLC resistance was unexpected as previous studies have suggested that candidate GPI-anchor precursors are PI-PLC-resistant as a result of inositol acylation. This work reveals unanticipated complexities in the biosynthesis of AP and its GPI anchor.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown D. A., Rose J. K. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992 Feb 7;68(3):533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- Cerneus D. P., Ueffing E., Posthuma G., Strous G. J., van der Ende A. Detergent insolubility of alkaline phosphatase during biosynthetic transport and endocytosis. Role of cholesterol. J Biol Chem. 1993 Feb 15;268(5):3150–3155. [PubMed] [Google Scholar]
- Conzelmann A., Spiazzi A., Bron C. Glycolipid anchors are attached to Thy-1 glycoprotein rapidly after translation. Biochem J. 1987 Sep 15;246(3):605–610. doi: 10.1042/bj2460605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross G. A. Glycolipid anchoring of plasma membrane proteins. Annu Rev Cell Biol. 1990;6:1–39. doi: 10.1146/annurev.cb.06.110190.000245. [DOI] [PubMed] [Google Scholar]
- Englund P. T. The structure and biosynthesis of glycosyl phosphatidylinositol protein anchors. Annu Rev Biochem. 1993;62:121–138. doi: 10.1146/annurev.bi.62.070193.001005. [DOI] [PubMed] [Google Scholar]
- Fasel N., Rousseaux M., Schaerer E., Medof M. E., Tykocinski M. L., Bron C. In vitro attachment of glycosyl-inositolphospholipid anchor structures to mouse Thy-1 antigen and human decay-accelerating factor. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6858–6862. doi: 10.1073/pnas.86.18.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson M. A. Site of palmitoylation of a phospholipase C-resistant glycosylphosphatidylinositol membrane anchor. Biochem J. 1992 Jun 1;284(Pt 2):297–300. doi: 10.1042/bj2840297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose S., Ravi L., Prince G. M., Rosenfeld M. G., Silber R., Andresen S. W., Hazra S. V., Medof M. E. Synthesis of mannosylglucosaminylinositol phospholipids in normal but not paroxysmal nocturnal hemoglobinuria cells. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6025–6029. doi: 10.1073/pnas.89.13.6025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper N. M., Turner A. J. Ectoenzymes of the kidney microvillar membrane. Differential solubilization by detergents can predict a glycosyl-phosphatidylinositol membrane anchor. Biochem J. 1988 Mar 15;250(3):865–869. doi: 10.1042/bj2500865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard A. D., Berger J., Gerber L., Familletti P., Udenfriend S. Characterization of the phosphatidylinositol-glycan membrane anchor of human placental alkaline phosphatase. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6055–6059. doi: 10.1073/pnas.84.17.6055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemmerson R., Low M. G. Phosphatidylinositol anchor of HeLa cell alkaline phosphatase. Biochemistry. 1987 Sep 8;26(18):5703–5709. doi: 10.1021/bi00392a019. [DOI] [PubMed] [Google Scholar]
- Klausner R. D., Sitia R. Protein degradation in the endoplasmic reticulum. Cell. 1990 Aug 24;62(4):611–614. doi: 10.1016/0092-8674(90)90104-m. [DOI] [PubMed] [Google Scholar]
- Kodukula K., Cines D., Amthauer R., Gerber L., Udenfriend S. Biosynthesis of phosphatidylinositol-glycan (PI-G)-anchored membrane proteins in cell-free systems: cleavage of the nascent protein and addition of the PI-G moiety depend on the size of the COOH-terminal signal peptide. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1350–1353. doi: 10.1073/pnas.89.4.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long K. E., Yomtovian R., Kida M., Knez J. J., Medof M. E. Time-dependent loss of surface complement regulatory activity during storage of donor blood. Transfusion. 1993 Apr;33(4):294–300. doi: 10.1046/j.1537-2995.1993.33493242635.x. [DOI] [PubMed] [Google Scholar]
- Low M. G., Finean J. B. Non-lytic release of acetylcholinesterase from erythrocytes by a phosphatidylinositol-specific phospholipase C. FEBS Lett. 1977 Oct 1;82(1):143–146. doi: 10.1016/0014-5793(77)80905-8. [DOI] [PubMed] [Google Scholar]
- Low M. G., Huang K. S. Factors affecting the ability of glycosylphosphatidylinositol-specific phospholipase D to degrade the membrane anchors of cell surface proteins. Biochem J. 1991 Oct 15;279(Pt 2):483–493. doi: 10.1042/bj2790483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low M. G., Stiernberg J., Waneck G. L., Flavell R. A., Kincade P. W. Cell-specific heterogeneity in sensitivity of phosphatidylinositol-anchored membrane antigens to release by phospholipase C. J Immunol Methods. 1988 Oct 4;113(1):101–111. doi: 10.1016/0022-1759(88)90386-9. [DOI] [PubMed] [Google Scholar]
- Low M. G. The glycosyl-phosphatidylinositol anchor of membrane proteins. Biochim Biophys Acta. 1989 Dec 6;988(3):427–454. doi: 10.1016/0304-4157(89)90014-2. [DOI] [PubMed] [Google Scholar]
- Malik A. S., Low M. G. Conversion of human placental alkaline phosphatase from a high Mr form to a low Mr form during butanol extraction. An investigation of the role of endogenous phosphoinositide-specific phospholipases. Biochem J. 1986 Dec 1;240(2):519–527. doi: 10.1042/bj2400519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puoti A., Conzelmann A. Structural characterization of free glycolipids which are potential precursors for glycophosphatidylinositol anchors in mouse thymoma cell lines. J Biol Chem. 1992 Nov 5;267(31):22673–22680. [PubMed] [Google Scholar]
- Roberts W. L., Myher J. J., Kuksis A., Low M. G., Rosenberry T. L. Lipid analysis of the glycoinositol phospholipid membrane anchor of human erythrocyte acetylcholinesterase. Palmitoylation of inositol results in resistance to phosphatidylinositol-specific phospholipase C. J Biol Chem. 1988 Dec 15;263(35):18766–18775. [PubMed] [Google Scholar]
- Seetharam B., Tiruppathi C., Alpers D. H. Membrane interactions of rat intestinal alkaline phosphatase: role of polar head groups. Biochemistry. 1985 Nov 5;24(23):6603–6608. doi: 10.1021/bi00344a045. [DOI] [PubMed] [Google Scholar]
- Singh N., Singleton D., Tartakoff A. M. Anchoring and degradation of glycolipid-anchored membrane proteins by L929 versus by LM-TK- mouse fibroblasts: implications for anchor biosynthesis. Mol Cell Biol. 1991 May;11(5):2362–2374. doi: 10.1128/mcb.11.5.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takami N., Oda K., Ikehara Y. Aberrant processing of alkaline phosphatase precursor caused by blocking the synthesis of glycosylphosphatidylinositol. J Biol Chem. 1992 Jan 15;267(2):1042–1047. [PubMed] [Google Scholar]
- Takami N., Ogata S., Oda K., Misumi Y., Ikehara Y. Biosynthesis of placental alkaline phosphatase and its post-translational modification by glycophospholipid for membrane-anchoring. J Biol Chem. 1988 Feb 25;263(6):3016–3021. [PubMed] [Google Scholar]
- Tartakoff A. M., Singh N. How to make a glycoinositol phospholipid anchor. Trends Biochem Sci. 1992 Nov;17(11):470–473. doi: 10.1016/0968-0004(92)90491-q. [DOI] [PubMed] [Google Scholar]
- Toutant J. P., Roberts W. L., Murray N. R., Rosenberry T. L. Conversion of human erythrocyte acetylcholinesterase from an amphiphilic to a hydrophilic form by phosphatidylinositol-specific phospholipase C and serum phospholipase D. Eur J Biochem. 1989 Apr 1;180(3):503–508. doi: 10.1111/j.1432-1033.1989.tb14674.x. [DOI] [PubMed] [Google Scholar]
- Urakaze M., Kamitani T., DeGasperi R., Sugiyama E., Chang H. M., Warren C. D., Yeh E. T. Identification of a missing link in glycosylphosphatidylinositol anchor biosynthesis in mammalian cells. J Biol Chem. 1992 Apr 5;267(10):6459–6462. [PubMed] [Google Scholar]
- Walter E. I., Ratnoff W. D., Long K. E., Kazura J. W., Medof M. E. Effect of glycoinositolphospholipid anchor lipid groups on functional properties of decay-accelerating factor protein in cells. J Biol Chem. 1992 Jan 15;267(2):1245–1252. [PubMed] [Google Scholar]
- Walter E. I., Roberts W. L., Rosenberry T. L., Ratnoff W. D., Medof M. E. Structural basis for variations in the sensitivity of human decay accelerating factor to phosphatidylinositol-specific phospholipase C cleavage. J Immunol. 1990 Feb 1;144(3):1030–1036. [PubMed] [Google Scholar]
- Wong Y. W., Low M. G. Phospholipase resistance of the glycosyl-phosphatidylinositol membrane anchor on human alkaline phosphatase. Clin Chem. 1992 Dec;38(12):2517–2525. [PubMed] [Google Scholar]