Abstract
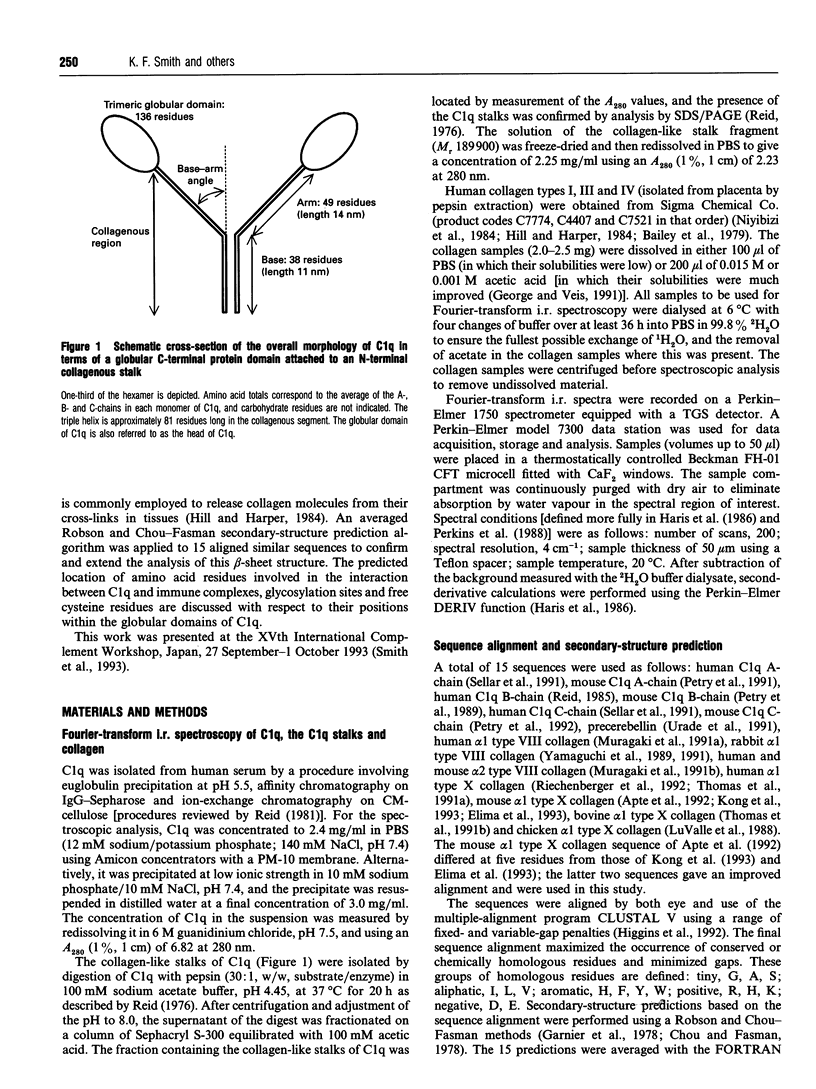
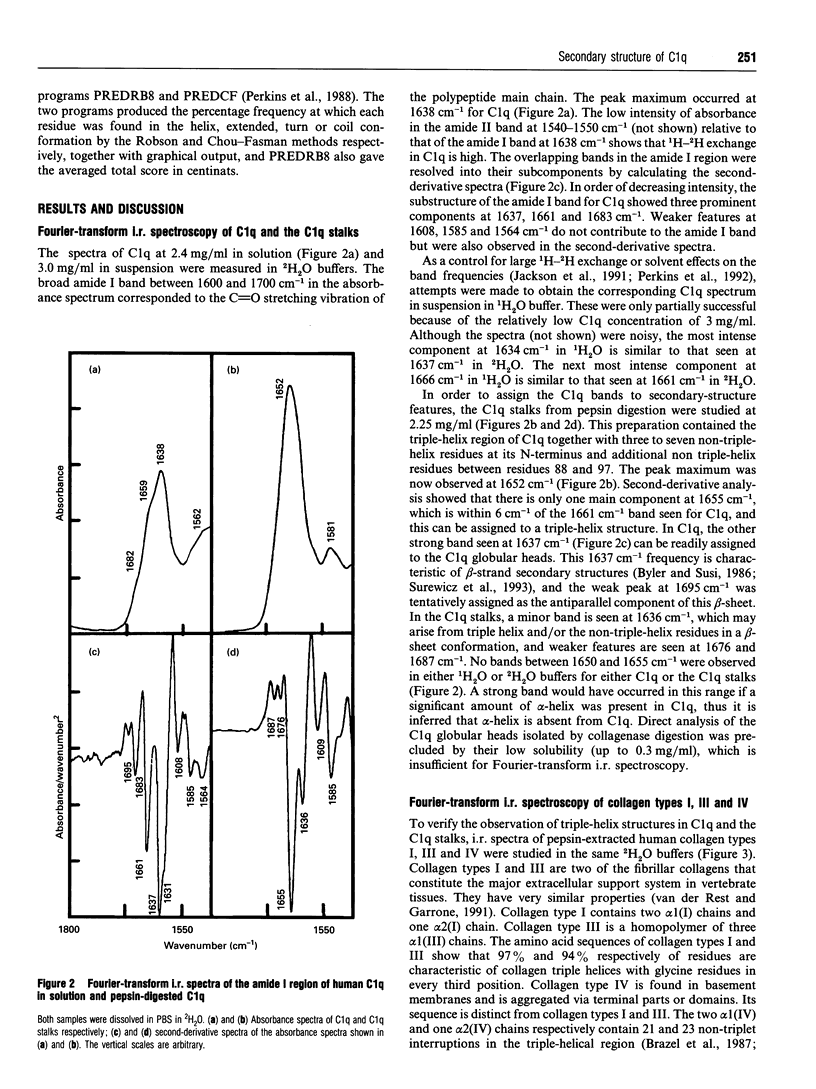
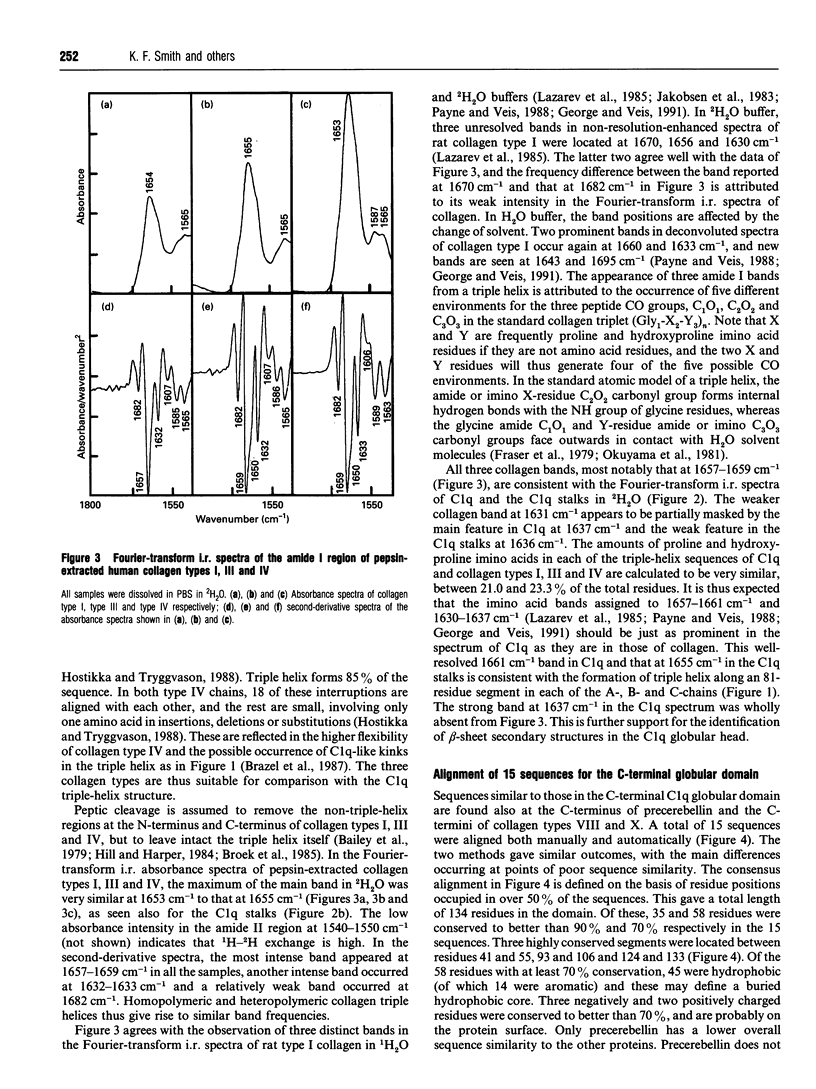
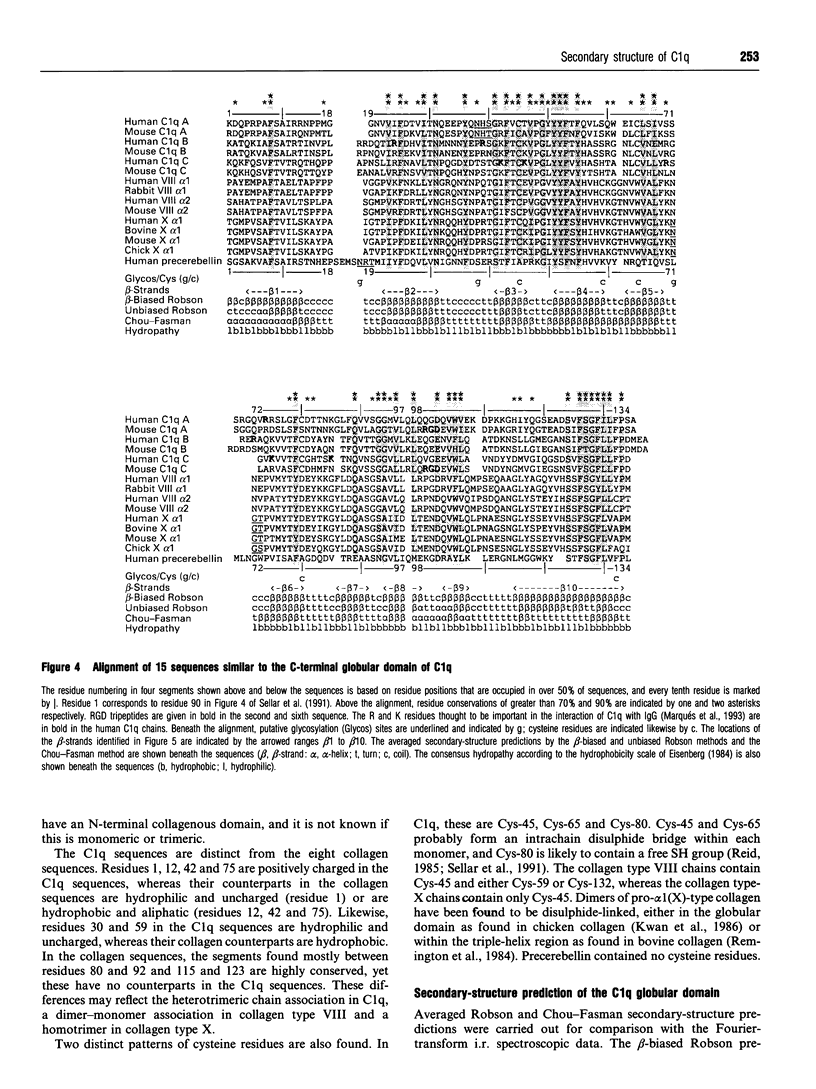
C1q plays a key role in the recognition of immune complexes, thereby initiating the classical pathway of complement activation. Although the triple-helix conformation of its N-terminal segment is well established, the secondary structure of the trimeric globular C-terminal domain is as yet unknown. The secondary structures of human C1q and C1q stalks and pepsin-extracted human collagen types I, III and IV (with no significant non-collagen-like structure) were studied by Fourier-transform i.r. spectroscopy in 2H2O buffers. After second-derivative calculation to resolve the fine structure of the broad amide I band, the Fourier-transform i.r. spectrum of C1q showed two major bands, one at 1637 cm-1, which is a characteristic frequency for beta-sheets, and one at 1661 cm-1. Both major bands were also detected for Clq in H2O buffers. Only the second major band was observed at 1655 cm-1 in pepsin-digested C1q which contains primarily the N-terminal triple-helix region. The Fourier-transform i.r. spectra of collagen in 2H2O also showed a major band at 1659 cm-1 (and minor bands at 1632 cm-1 and 1682 cm-1). It is concluded that the C1q globular heads contain primarily beta-sheet structure. The C-terminal domains of C1q show approximately 25% sequence identity with the non-collagen-like C-terminal regions of the short-chain collagen types VIII and X. To complement the Fourier-transform-i.r. spectroscopic data, averaged Robson and Chou-Fasman structure predictions on 15 similar sequences for the globular domains of C1q and collagen types VIII and X were performed. These showed a clear pattern of ten beta-strands interspersed by beta-turns and /or loops. Residues thought to be important for C1q-immune complex interactions with IgG and IgM were predicted to be at a surface-exposed loop. Sequence insertions and deletions, glycosylation sites, the free cysteine residue and RGD recognition sequences were also predicted to be at surface-exposed positions.
Full text
PDF
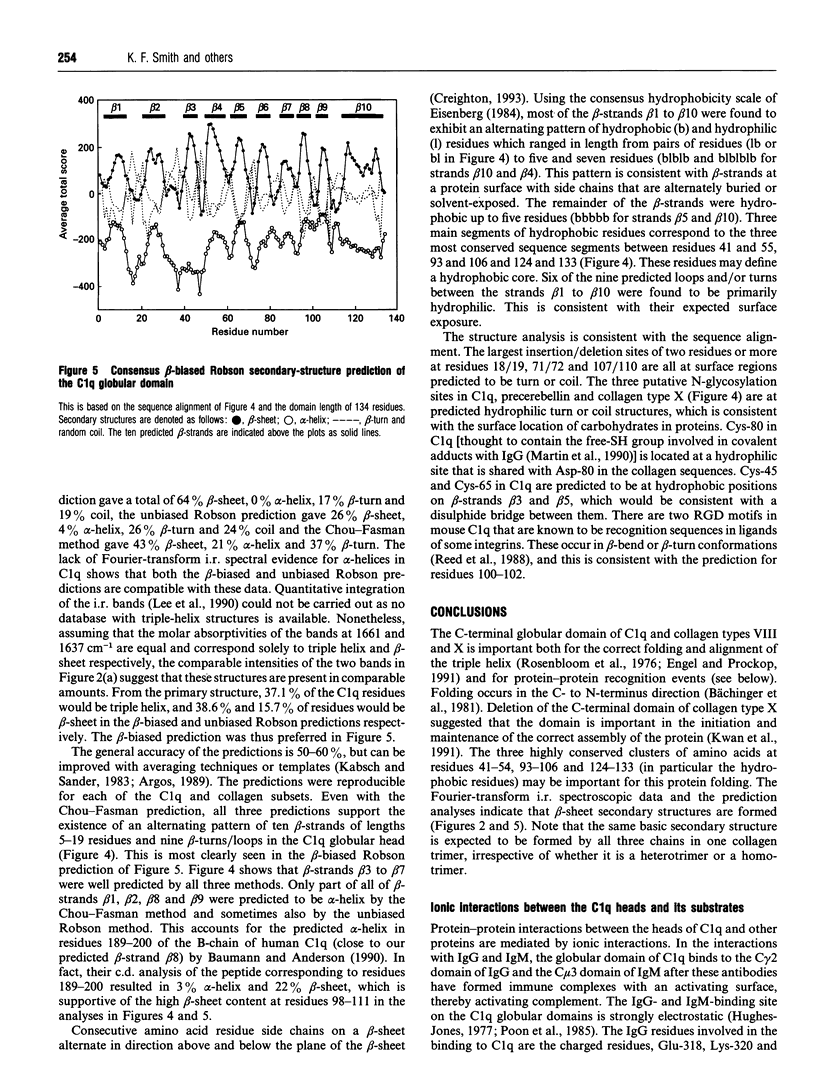


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey A. J., Sims T. J., Duance V. C., Light N. D. Partial characterization of a second basement membrane collagen in human placenta. Evidence for the existence of two type IV collagen molecules. FEBS Lett. 1979 Mar 15;99(2):361–366. doi: 10.1016/0014-5793(79)80992-8. [DOI] [PubMed] [Google Scholar]
- Baumann M. A., Anderson B. E. An immune complex selective affinity matrix utilizing a synthetic peptide. J Biol Chem. 1990 Oct 25;265(30):18414–18422. [PubMed] [Google Scholar]
- Bordin S., Page R. C. Detection of a high-affinity binding site for the globular head regions of the C1q complement protein on a human diploid fibroblast subtype. Mol Immunol. 1989 Jul;26(7):677–685. doi: 10.1016/0161-5890(89)90051-5. [DOI] [PubMed] [Google Scholar]
- Brass A., Kadler K. E., Thomas J. T., Grant M. E., Boot-Handford R. P. The fibrillar collagens, collagen VIII, collagen X and the C1q complement proteins share a similar domain in their C-terminal non-collagenous regions. FEBS Lett. 1992 Jun 1;303(2-3):126–128. doi: 10.1016/0014-5793(92)80503-9. [DOI] [PubMed] [Google Scholar]
- Brazel D., Oberbäumer I., Dieringer H., Babel W., Glanville R. W., Deutzmann R., Kühn K. Completion of the amino acid sequence of the alpha 1 chain of human basement membrane collagen (type IV) reveals 21 non-triplet interruptions located within the collagenous domain. Eur J Biochem. 1987 Nov 2;168(3):529–536. doi: 10.1111/j.1432-1033.1987.tb13450.x. [DOI] [PubMed] [Google Scholar]
- Brodsky-Doyle B., Leonard K. R., Reid K. B. Circular-dichroism and electron-microscopy studies of human subcomponent C1q before and after limited proteolysis by pepsin. Biochem J. 1976 Nov;159(2):279–286. doi: 10.1042/bj1590279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broek D. L., Madri J., Eikenberry E. F., Brodsky B. Characterization of the tissue form of type V collagen from chick bone. J Biol Chem. 1985 Jan 10;260(1):555–562. [PubMed] [Google Scholar]
- Byler D. M., Susi H. Examination of the secondary structure of proteins by deconvolved FTIR spectra. Biopolymers. 1986 Mar;25(3):469–487. doi: 10.1002/bip.360250307. [DOI] [PubMed] [Google Scholar]
- Bächinger H. P., Fessler L. I., Timpl R., Fessler J. H. Chain assembly intermediate in the biosynthesis of type III procollagen in chick embryo blood vessels. J Biol Chem. 1981 Dec 25;256(24):13193–13199. [PubMed] [Google Scholar]
- Chen Q., Linsenmayer C., Gu H., Schmid T. M., Linsenmayer T. F. Domains of type X collagen: alteration of cartilage matrix by fibril association and proteoglycan accumulation. J Cell Biol. 1992 May;117(3):687–694. doi: 10.1083/jcb.117.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Comis A., Easterbrook-Smith S. B. Binding of complement component C1q by rat adipocyte membranes. Mol Immunol. 1985 Aug;22(8):857–861. doi: 10.1016/0161-5890(85)90070-7. [DOI] [PubMed] [Google Scholar]
- Comis A., Easterbrook-Smith S. B. Binding of complement component C1q by spectrin. Biochim Biophys Acta. 1986 Apr 22;870(3):426–431. doi: 10.1016/0167-4838(86)90250-5. [DOI] [PubMed] [Google Scholar]
- Duncan A. R., Winter G. The binding site for C1q on IgG. Nature. 1988 Apr 21;332(6166):738–740. doi: 10.1038/332738a0. [DOI] [PubMed] [Google Scholar]
- Easterbrook-Smith S. B. Evidence for histidine residues in the immunoglobulin-binding site of human Clq. Biosci Rep. 1983 Feb;3(2):135–140. doi: 10.1007/BF01121944. [DOI] [PubMed] [Google Scholar]
- Eisenberg D. Three-dimensional structure of membrane and surface proteins. Annu Rev Biochem. 1984;53:595–623. doi: 10.1146/annurev.bi.53.070184.003115. [DOI] [PubMed] [Google Scholar]
- Elima K., Eerola I., Rosati R., Metsäranta M., Garofalo S., Perälä M., De Crombrugghe B., Vuorio E. The mouse collagen X gene: complete nucleotide sequence, exon structure and expression pattern. Biochem J. 1993 Jan 1;289(Pt 1):247–253. doi: 10.1042/bj2890247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J., Prockop D. J. The zipper-like folding of collagen triple helices and the effects of mutations that disrupt the zipper. Annu Rev Biophys Biophys Chem. 1991;20:137–152. doi: 10.1146/annurev.bb.20.060191.001033. [DOI] [PubMed] [Google Scholar]
- Entwistle R. A., Furcht L. T. C1q component of complement binds to fibrinogen and fibrin. Biochemistry. 1988 Jan 12;27(1):507–512. doi: 10.1021/bi00401a073. [DOI] [PubMed] [Google Scholar]
- Fraser R. D., MacRae T. P., Suzuki E. Chain conformation in the collagen molecule. J Mol Biol. 1979 Apr 15;129(3):463–481. doi: 10.1016/0022-2836(79)90507-2. [DOI] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- George A., Veis A. FTIRS in H2O demonstrates that collagen monomers undergo a conformational transition prior to thermal self-assembly in vitro. Biochemistry. 1991 Mar 5;30(9):2372–2377. doi: 10.1021/bi00223a011. [DOI] [PubMed] [Google Scholar]
- Haris P. I., Chapman D. Does Fourier-transform infrared spectroscopy provide useful information on protein structures? Trends Biochem Sci. 1992 Sep;17(9):328–333. doi: 10.1016/0968-0004(92)90305-s. [DOI] [PubMed] [Google Scholar]
- Haris P. I., Lee D. C., Chapman D. A Fourier transform infrared investigation of the structural differences between ribonuclease A and ribonuclease S. Biochim Biophys Acta. 1986 Dec 12;874(3):255–265. doi: 10.1016/0167-4838(86)90024-5. [DOI] [PubMed] [Google Scholar]
- Heinz H. P. Biological functions of C1q expressed by conformational changes. Behring Inst Mitt. 1989 Jul;(84):20–31. [PubMed] [Google Scholar]
- Higgins D. G., Bleasby A. J., Fuchs R. CLUSTAL V: improved software for multiple sequence alignment. Comput Appl Biosci. 1992 Apr;8(2):189–191. doi: 10.1093/bioinformatics/8.2.189. [DOI] [PubMed] [Google Scholar]
- Hill R. J., Harper E. Quantitation of types I and III collagens in human tissue samples and cell culture by cyanogen bromide peptide analysis. Anal Biochem. 1984 Aug 15;141(1):83–93. doi: 10.1016/0003-2697(84)90429-9. [DOI] [PubMed] [Google Scholar]
- Hostikka S. L., Tryggvason K. The complete primary structure of the alpha 2 chain of human type IV collagen and comparison with the alpha 1(IV) chain. J Biol Chem. 1988 Dec 25;263(36):19488–19493. [PubMed] [Google Scholar]
- Hughes-Jones N. C. Functional affinity constants of the reaction between 125I-labelled C1q and C1q binders and their use in the measurement of plasma C1q concentrations. Immunology. 1977 Feb;32(2):191–198. [PMC free article] [PubMed] [Google Scholar]
- Jackson M., Haris P. I., Chapman D. Fourier transform infrared spectroscopic studies of Ca(2+)-binding proteins. Biochemistry. 1991 Oct 8;30(40):9681–9686. doi: 10.1021/bi00104a016. [DOI] [PubMed] [Google Scholar]
- Jakobsen R. J., Brown L. L., Hutson T. B., Fink D. J., Veis A. Intermolecular interactions in collagen self-assembly as revealed by Fourier transform infrared spectroscopy. Science. 1983 Jun 17;220(4603):1288–1290. doi: 10.1126/science.6857249. [DOI] [PubMed] [Google Scholar]
- Kabsch W., Sander C. How good are predictions of protein secondary structure? FEBS Lett. 1983 May 8;155(2):179–182. doi: 10.1016/0014-5793(82)80597-8. [DOI] [PubMed] [Google Scholar]
- Kirsch T., von der Mark K. Ca2+ binding properties of type X collagen. FEBS Lett. 1991 Dec 2;294(1-2):149–152. doi: 10.1016/0014-5793(91)81363-d. [DOI] [PubMed] [Google Scholar]
- Knobel H. R., Villiger W., Isliker H. Chemical analysis and electron microscopy studies of human C1q prepared by different methods. Eur J Immunol. 1975 Jan;5(1):78–82. doi: 10.1002/eji.1830050119. [DOI] [PubMed] [Google Scholar]
- Kong R. Y., Kwan K. M., Lau E. T., Thomas J. T., Boot-Handford R. P., Grant M. E., Cheah K. S. Intron-exon structure, alternative use of promoter and expression of the mouse collagen X gene, Col10a-1. Eur J Biochem. 1993 Apr 1;213(1):99–111. doi: 10.1111/j.1432-1033.1993.tb17739.x. [DOI] [PubMed] [Google Scholar]
- Kwan A. P., Cummings C. E., Chapman J. A., Grant M. E. Macromolecular organization of chicken type X collagen in vitro. J Cell Biol. 1991 Aug;114(3):597–604. doi: 10.1083/jcb.114.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan A. P., Sear C. H., Grant M. E. Identification of disulphide-bonded type X procollagen polypeptides in embryonic chick chondrocyte cultures. FEBS Lett. 1986 Oct 6;206(2):267–272. doi: 10.1016/0014-5793(86)80994-2. [DOI] [PubMed] [Google Scholar]
- Lazarev Y. A., Grishkovsky B. A., Khromova T. B. Amide I band of IR spectrum and structure of collagen and related polypeptides. Biopolymers. 1985 Aug;24(8):1449–1478. doi: 10.1002/bip.360240804. [DOI] [PubMed] [Google Scholar]
- Liberti P. A., Paul S. M. Gross conformation of C1q: a subcomponent of the first component of complement. Biochemistry. 1978 May 16;17(10):1952–1958. doi: 10.1021/bi00603a023. [DOI] [PubMed] [Google Scholar]
- LuValle P., Ninomiya Y., Rosenblum N. D., Olsen B. R. The type X collagen gene. Intron sequences split the 5'-untranslated region and separate the coding regions for the non-collagenous amino-terminal and triple-helical domains. J Biol Chem. 1988 Dec 5;263(34):18378–18385. [PubMed] [Google Scholar]
- Mann K., Jander R., Korsching E., Kühn K., Rauterberg J. The primary structure of a triple-helical domain of collagen type VIII from bovine Descemet's membrane. FEBS Lett. 1990 Oct 29;273(1-2):168–172. doi: 10.1016/0014-5793(90)81076-z. [DOI] [PubMed] [Google Scholar]
- Marqués G., Antón L. C., Barrio E., Sánchez A., Ruiz S., Gavilanes F., Vivanco F. Arginine residues of the globular regions of human C1q involved in the interaction with immunoglobulin G. J Biol Chem. 1993 May 15;268(14):10393–10402. [PubMed] [Google Scholar]
- Martin H., Kaul M., Loos M. Disulfide bridge formation between C1q and IgG in vitro. Eur J Immunol. 1990 Aug;20(8):1641–1645. doi: 10.1002/eji.1830200804. [DOI] [PubMed] [Google Scholar]
- McCall M. N., Easterbrook-Smith S. B. Comparison of the role of tyrosine residues in human IgG and rabbit IgG in binding of complement subcomponent C1q. Biochem J. 1989 Feb 1;257(3):845–851. doi: 10.1042/bj2570845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muragaki Y., Jacenko O., Apte S., Mattei M. G., Ninomiya Y., Olsen B. R. The alpha 2(VIII) collagen gene. A novel member of the short chain collagen family located on the human chromosome 1. J Biol Chem. 1991 Apr 25;266(12):7721–7727. [PubMed] [Google Scholar]
- Muragaki Y., Mattei M. G., Yamaguchi N., Olsen B. R., Ninomiya Y. The complete primary structure of the human alpha 1 (VIII) chain and assignment of its gene (COL8A1) to chromosome 3. Eur J Biochem. 1991 May 8;197(3):615–622. doi: 10.1111/j.1432-1033.1991.tb15951.x. [DOI] [PubMed] [Google Scholar]
- Niyibizi C., Fietzek P. P., van der Rest M. Human placenta type V collagens. Evidence for the existence of an alpha 1(V) alpha 2(V) alpha 3(V) collagen molecule. J Biol Chem. 1984 Nov 25;259(22):14170–14174. [PubMed] [Google Scholar]
- Okuyama K., Okuyama K., Arnott S., Takayanagi M., Kakudo M. Crystal and molecular structure of a collagen-like polypeptide (Pro-Pro-Gly)10. J Mol Biol. 1981 Oct 25;152(2):427–443. doi: 10.1016/0022-2836(81)90252-7. [DOI] [PubMed] [Google Scholar]
- Painter R. H. The binding of C1q to immunoglobulins. Behring Inst Mitt. 1993 Dec;(93):131–137. [PubMed] [Google Scholar]
- Payne K. J., Veis A. Fourier transform IR spectroscopy of collagen and gelatin solutions: deconvolution of the amide I band for conformational studies. Biopolymers. 1988 Nov;27(11):1749–1760. doi: 10.1002/bip.360271105. [DOI] [PubMed] [Google Scholar]
- Perkins S. J., Haris P. I., Sim R. B., Chapman D. A study of the structure of human complement component factor H by Fourier transform infrared spectroscopy and secondary structure averaging methods. Biochemistry. 1988 May 31;27(11):4004–4012. doi: 10.1021/bi00411a017. [DOI] [PubMed] [Google Scholar]
- Perkins S. J. Molecular modelling of human complement subcomponent C1q and its complex with C1r2C1s2 derived from neutron-scattering curves and hydrodynamic properties. Biochem J. 1985 May 15;228(1):13–26. doi: 10.1042/bj2280013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins S. J., Nealis A. S., Sutton B. J., Feinstein A. Solution structure of human and mouse immunoglobulin M by synchrotron X-ray scattering and molecular graphics modelling. A possible mechanism for complement activation. J Mol Biol. 1991 Oct 20;221(4):1345–1366. doi: 10.1016/0022-2836(91)90937-2. [DOI] [PubMed] [Google Scholar]
- Perkins S. J., Smith K. F., Nealis A. S., Haris P. I., Chapman D., Bauer C. J., Harrison R. A. Secondary structure changes stabilize the reactive-centre cleaved form of SERPINs. A study by 1H nuclear magnetic resonance and Fourier transform infrared spectroscopy. J Mol Biol. 1992 Dec 20;228(4):1235–1254. doi: 10.1016/0022-2836(92)90329-i. [DOI] [PubMed] [Google Scholar]
- Perkins S. J., Villiers C. L., Arlaud G. J., Boyd J., Burton D. R., Colomb M. G., Dwek R. A. Neutron scattering studies of subcomponent C1q of first component C1 of human complement and its association with subunit C1r2C1s2 within C1. J Mol Biol. 1984 Nov 5;179(3):547–557. doi: 10.1016/0022-2836(84)90079-2. [DOI] [PubMed] [Google Scholar]
- Petry F., Reid K. B., Loos M. Gene expression of the A- and B-chain of mouse C1q in different tissues and the characterization of the recombinant A-chain. J Immunol. 1991 Dec 1;147(11):3988–3993. [PubMed] [Google Scholar]
- Petry F., Reid K. B., Loos M. Isolation, sequence analysis and characterization of cDNA clones coding for the C chain of mouse C1q. Sequence similarity of complement subcomponent C1q, collagen type VIII and type X and precerebellin. Eur J Biochem. 1992 Oct 1;209(1):129–134. doi: 10.1111/j.1432-1033.1992.tb17269.x. [DOI] [PubMed] [Google Scholar]
- Petry F., Reid K. B., Loos M. Molecular cloning and characterization of the complementary DNA coding for the B-chain of murine Clq. FEBS Lett. 1989 Nov 20;258(1):89–93. doi: 10.1016/0014-5793(89)81622-9. [DOI] [PubMed] [Google Scholar]
- Poon P. H., Phillips M. L., Schumaker V. N. Immunoglobulin M possesses two binding sites for complement subcomponent C1q, and soluble 1:1 and 2:1 complexes are formed in solution at reduced ionic strength. J Biol Chem. 1985 Aug 5;260(16):9357–9365. [PubMed] [Google Scholar]
- Reed J., Hull W. E., von der Lieth C. W., Kübler D., Suhai S., Kinzel V. Secondary structure of the Arg-Gly-Asp recognition site in proteins involved in cell-surface adhesion. Evidence for the occurrence of nested beta-bends in the model hexapeptide GRGDSP. Eur J Biochem. 1988 Dec 1;178(1):141–154. doi: 10.1111/j.1432-1033.1988.tb14439.x. [DOI] [PubMed] [Google Scholar]
- Reichenberger E., Beier F., LuValle P., Olsen B. R., von der Mark K., Bertling W. M. Genomic organization and full-length cDNA sequence of human collagen X. FEBS Lett. 1992 Oct 26;311(3):305–310. doi: 10.1016/0014-5793(92)81126-7. [DOI] [PubMed] [Google Scholar]
- Reid K. B. Activation and control of the complement system. Essays Biochem. 1986;22:27–68. [PubMed] [Google Scholar]
- Reid K. B., Day A. J. Ig-binding domains of C1q. Immunol Today. 1990 Nov;11(11):387–388. doi: 10.1016/0167-5699(90)90150-8. [DOI] [PubMed] [Google Scholar]
- Reid K. B. Isolation, by partial pepsin digestion, of the three collagen-like regions present in subcomponent Clq of the first component of human complement. Biochem J. 1976 Apr 1;155(1):5–17. doi: 10.1042/bj1550005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid K. B. Molecular cloning and characterization of the complementary DNA and gene coding for the B-chain of subcomponent C1q of the human complement system. Biochem J. 1985 Nov 1;231(3):729–735. doi: 10.1042/bj2310729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remington M. C., Bashey R. I., Brighton C. T., Jimenez S. A. Biosynthesis of a disulphide-bonded short-chain collagen by calf growth-plate cartilage. Biochem J. 1984 Nov 15;224(1):227–233. doi: 10.1042/bj2240227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbloom J., Endo R., Harsch M. Termination of procollagen chain synthesis by puromycin. Evidence that assembly and secretion require a COOH-terminal extension. J Biol Chem. 1976 Apr 10;251(7):2070–2076. [PubMed] [Google Scholar]
- Sawada H., Konomi H., Hirosawa K. Characterization of the collagen in the hexagonal lattice of Descemet's membrane: its relation to type VIII collagen. J Cell Biol. 1990 Jan;110(1):219–227. doi: 10.1083/jcb.110.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumaker V. N., Hanson D. C., Kilchherr E., Phillips M. L., Poon P. H. A molecular mechanism for the activation of the first component of complement by immune complexes. Mol Immunol. 1986 May;23(5):557–565. doi: 10.1016/0161-5890(86)90119-7. [DOI] [PubMed] [Google Scholar]
- Sellar G. C., Blake D. J., Reid K. B. Characterization and organization of the genes encoding the A-, B- and C-chains of human complement subcomponent C1q. The complete derived amino acid sequence of human C1q. Biochem J. 1991 Mar 1;274(Pt 2):481–490. doi: 10.1042/bj2740481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surewicz W. K., Mantsch H. H., Chapman D. Determination of protein secondary structure by Fourier transform infrared spectroscopy: a critical assessment. Biochemistry. 1993 Jan 19;32(2):389–394. doi: 10.1021/bi00053a001. [DOI] [PubMed] [Google Scholar]
- Thomas J. T., Cresswell C. J., Rash B., Nicolai H., Jones T., Solomon E., Grant M. E., Boot-Handford R. P. The human collagen X gene. Complete primary translated sequence and chromosomal localization. Biochem J. 1991 Dec 15;280(Pt 3):617–623. doi: 10.1042/bj2800617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. T., Kwan A. P., Grant M. E., Boot-Handford R. P. Isolation of cDNAs encoding the complete sequence of bovine type X collagen. Evidence for the condensed nature of mammalian type X collagen genes. Biochem J. 1991 Jan 1;273(Pt 1):141–148. doi: 10.1042/bj2730141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urade Y., Oberdick J., Molinar-Rode R., Morgan J. I. Precerebellin is a cerebellum-specific protein with similarity to the globular domain of complement C1q B chain. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):1069–1073. doi: 10.1073/pnas.88.3.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wines B. D., Easterbrook-Smith S. B. Carbodiimide crosslinking of human C1q and rabbit IgG. Mol Immunol. 1990 Mar;27(3):221–226. doi: 10.1016/0161-5890(90)90133-k. [DOI] [PubMed] [Google Scholar]
- Yamaguchi N., Benya P. D., van der Rest M., Ninomiya Y. The cloning and sequencing of alpha 1(VIII) collagen cDNAs demonstrate that type VIII collagen is a short chain collagen and contains triple-helical and carboxyl-terminal non-triple-helical domains similar to those of type X collagen. J Biol Chem. 1989 Sep 25;264(27):16022–16029. [PubMed] [Google Scholar]
- Yamaguchi N., Mayne R., Ninomiya Y. The alpha 1 (VIII) collagen gene is homologous to the alpha 1 (X) collagen gene and contains a large exon encoding the entire triple helical and carboxyl-terminal non-triple helical domains of the alpha 1 (VIII) polypeptide. J Biol Chem. 1991 Mar 5;266(7):4508–4513. [PubMed] [Google Scholar]
- van der Rest M., Garrone R. Collagen family of proteins. FASEB J. 1991 Oct;5(13):2814–2823. [PubMed] [Google Scholar]



