Abstract
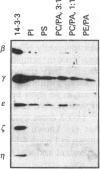
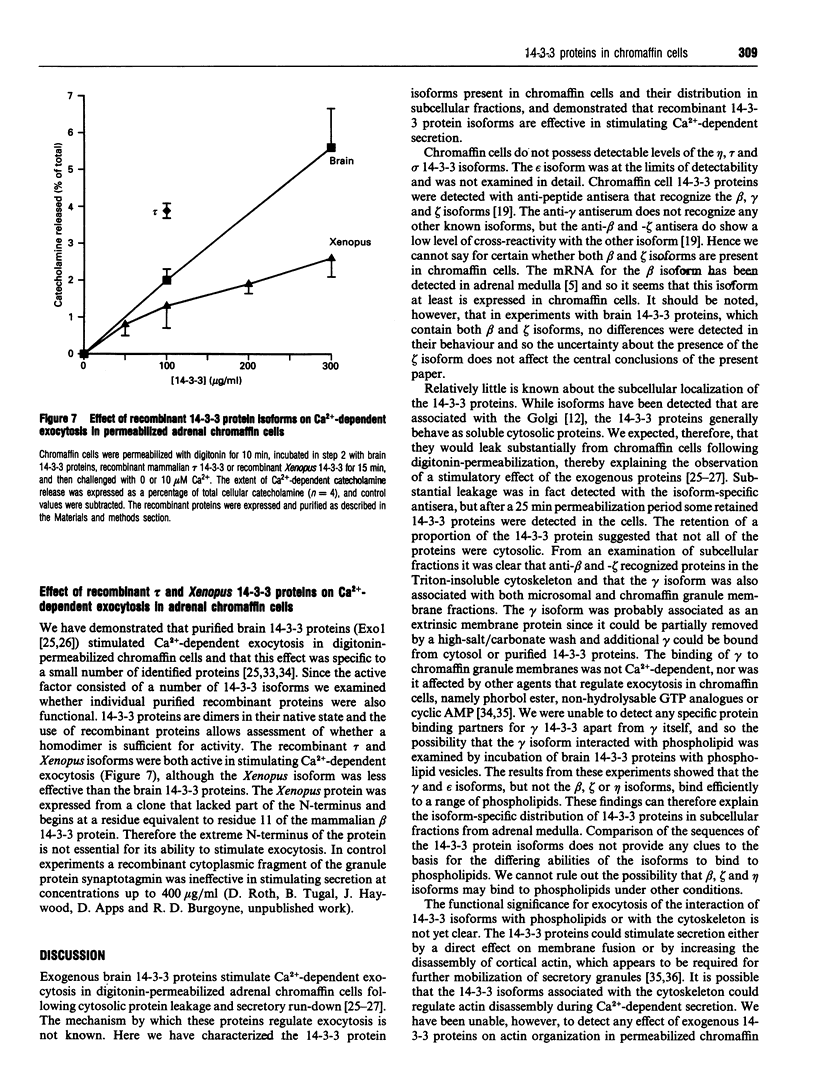
Isoform-specific antisera were used to examine which 14-3-3 isoforms were present in bovine adrenal chromaffin cells. The eta, tau and sigma isoforms were not detectable, and the epsilon isoform was present at only low levels. 14-3-3 isoforms were readily detected with antisera against the beta, gamma and zeta isoforms. The latter isoforms were found to leak from digitonin-permeabilized chromaffin cells, as expected for cytosolic proteins, but a proportion of each isoform was retained. In subcellular fractionation studies isoforms recognized by the beta and zeta antisera were found in the cytosol and Triton-insoluble cytoskeletal fractions, while the gamma isoform was found in cytosol and also in microsomal and chromaffin granule membrane fractions. The gamma 14-3-3 protein associated with granule membranes was partially removed by a high-salt/carbonate wash, and the membranes could bind further gamma from cytosol or from a purified brain 14-3-3 protein mixture. The binding of gamma 14-3-3 was not Ca(2+)-dependent, nor was it affected by phorbol ester, GTP analogues or cyclic AMP. Using pure phospholipid vesicles it was found that gamma and also epsilon 14-3-3 proteins bound directly to phospholipids. Little binding of brain beta, eta or zeta to phospholipid vesicles was detected. Brain 14-3-3 proteins were also able to aggregate phospholipid vesicles. Recombinant 14-3-3 isoforms (tau and the Xenopus protein) were able to stimulate Ca(2+)-dependent exocytosis in digitonin-permeabilized chromaffin cells. The Xenopus proteins lacks part of the extreme N-terminus, indicating that this domain is not essential for function in exocytosis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitken A., Amess B., Howell S., Jones D., Martin H., Patel Y., Robinson K., Toker A. The role of specific isoforms of 14-3-3 protein in regulating protein kinase activity in the brain. Biochem Soc Trans. 1992 Aug;20(3):607–611. doi: 10.1042/bst0200607. [DOI] [PubMed] [Google Scholar]
- Ali S. M., Geisow M. J., Burgoyne R. D. A role for calpactin in calcium-dependent exocytosis in adrenal chromaffin cells. Nature. 1989 Jul 27;340(6231):313–315. doi: 10.1038/340313a0. [DOI] [PubMed] [Google Scholar]
- Blackwood R. A., Ernst J. D. Characterization of Ca2(+)-dependent phospholipid binding, vesicle aggregation and membrane fusion by annexins. Biochem J. 1990 Feb 15;266(1):195–200. doi: 10.1042/bj2660195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt J., Thordal-Christensen H., Vad K., Gregersen P. L., Collinge D. B. A pathogen-induced gene of barley encodes a protein showing high similarity to a protein kinase regulator. Plant J. 1992 Sep;2(5):815–820. [PubMed] [Google Scholar]
- Burgoyne R. D., Morgan A., O'Sullivan A. J. A major role for protein kinase C in calcium-activated exocytosis in permeabilised adrenal chromaffin cells. FEBS Lett. 1988 Sep 26;238(1):151–155. doi: 10.1016/0014-5793(88)80246-1. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D., Morgan A., O'Sullivan A. J. The control of cytoskeletal actin and exocytosis in intact and permeabilized adrenal chromaffin cells: role of calcium and protein kinase C. Cell Signal. 1989;1(4):323–334. doi: 10.1016/0898-6568(89)90051-x. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D., Morgan A. Regulated exocytosis. Biochem J. 1993 Jul 15;293(Pt 2):305–316. doi: 10.1042/bj2930305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheek T. R., Burgoyne R. D. Nicotine-evoked disassembly of cortical actin filaments in adrenal chromaffin cells. FEBS Lett. 1986 Oct 20;207(1):110–114. doi: 10.1016/0014-5793(86)80022-9. [DOI] [PubMed] [Google Scholar]
- Dunn L. A., Holz R. W. Catecholamine secretion from digitonin-treated adrenal medullary chromaffin cells. J Biol Chem. 1983 Apr 25;258(8):4989–4993. [PubMed] [Google Scholar]
- Fu H., Coburn J., Collier R. J. The eukaryotic host factor that activates exoenzyme S of Pseudomonas aeruginosa is a member of the 14-3-3 protein family. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2320–2324. doi: 10.1073/pnas.90.6.2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch S., Aitken A., Bertsch U., Soll J. A plant homologue to mammalian brain 14-3-3 protein and protein kinase C inhibitor. FEBS Lett. 1992 Jan 20;296(2):222–224. doi: 10.1016/0014-5793(92)80384-s. [DOI] [PubMed] [Google Scholar]
- Ichimura-Ohshima Y., Morii K., Ichimura T., Araki K., Takahashi Y., Isobe T., Minoshima S., Fukuyama R., Shimizu N., Kuwano R. cDNA cloning and chromosome assignment of the gene for human brain 14-3-3 protein eta chain. J Neurosci Res. 1992 Apr;31(4):600–605. doi: 10.1002/jnr.490310403. [DOI] [PubMed] [Google Scholar]
- Ichimura T., Isobe T., Okuyama T., Takahashi N., Araki K., Kuwano R., Takahashi Y. Molecular cloning of cDNA coding for brain-specific 14-3-3 protein, a protein kinase-dependent activator of tyrosine and tryptophan hydroxylases. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7084–7088. doi: 10.1073/pnas.85.19.7084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isobe T., Hiyane Y., Ichimura T., Okuyama T., Takahashi N., Nakajo S., Nakaya K. Activation of protein kinase C by the 14-3-3 proteins homologous with Exo1 protein that stimulates calcium-dependent exocytosis. FEBS Lett. 1992 Aug 17;308(2):121–124. doi: 10.1016/0014-5793(92)81257-m. [DOI] [PubMed] [Google Scholar]
- Isobe T., Ichimura T., Sunaya T., Okuyama T., Takahashi N., Kuwano R., Takahashi Y. Distinct forms of the protein kinase-dependent activator of tyrosine and tryptophan hydroxylases. J Mol Biol. 1991 Jan 5;217(1):125–132. doi: 10.1016/0022-2836(91)90616-e. [DOI] [PubMed] [Google Scholar]
- Leffers H., Madsen P., Rasmussen H. H., Honoré B., Andersen A. H., Walbum E., Vandekerckhove J., Celis J. E. Molecular cloning and expression of the transformation sensitive epithelial marker stratifin. A member of a protein family that has been involved in the protein kinase C signalling pathway. J Mol Biol. 1993 Jun 20;231(4):982–998. doi: 10.1006/jmbi.1993.1346. [DOI] [PubMed] [Google Scholar]
- Martens G. J., Piosik P. A., Danen E. H. Evolutionary conservation of the 14-3-3 protein. Biochem Biophys Res Commun. 1992 May 15;184(3):1456–1459. doi: 10.1016/s0006-291x(05)80046-4. [DOI] [PubMed] [Google Scholar]
- Martin H., Patel Y., Jones D., Howell S., Robinson K., Aitken A. Antibodies against the major brain isoforms of 14-3-3 protein. An antibody specific for the N-acetylated amino-terminus of a protein. FEBS Lett. 1993 Oct 4;331(3):296–303. doi: 10.1016/0014-5793(93)80356-y. [DOI] [PubMed] [Google Scholar]
- McConnell J. E., Hodges P. E. The alternative 5'-end of the Drosophila melanogaster epidermal growth factor receptor cDNA (DER) is part of the D14-3-3 cDNA. Gene. 1993 Apr 30;126(2):293–294. doi: 10.1016/0378-1119(93)90386-h. [DOI] [PubMed] [Google Scholar]
- Morgan A., Burgoyne R. D. Exo1 and Exo2 proteins stimulate calcium-dependent exocytosis in permeabilized adrenal chromaffin cells. Nature. 1992 Feb 27;355(6363):833–836. doi: 10.1038/355833a0. [DOI] [PubMed] [Google Scholar]
- Morgan A., Burgoyne R. D. Interaction between protein kinase C and Exo1 (14-3-3 protein) and its relevance to exocytosis in permeabilized adrenal chromaffin cells. Biochem J. 1992 Sep 15;286(Pt 3):807–811. doi: 10.1042/bj2860807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan A., Roth D., Martin H., Aitken A., Burgoyne R. D. Identification of cytosolic protein regulators of exocytosis. Biochem Soc Trans. 1993 May;21(2):401–405. doi: 10.1042/bst0210401. [DOI] [PubMed] [Google Scholar]
- Morgan A., Wilkinson M., Burgoyne R. D. Identification of Exo2 as the catalytic subunit of protein kinase A reveals a role for cyclic AMP in Ca(2+)-dependent exocytosis in chromaffin cells. EMBO J. 1993 Oct;12(10):3747–3752. doi: 10.1002/j.1460-2075.1993.tb06052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen P. J. Primary structure of a human protein kinase regulator protein. Biochim Biophys Acta. 1991 Mar 26;1088(3):425–428. doi: 10.1016/0167-4781(91)90136-a. [DOI] [PubMed] [Google Scholar]
- Prasad G. L., Valverius E. M., McDuffie E., Cooper H. L. Complementary DNA cloning of a novel epithelial cell marker protein, HME1, that may be down-regulated in neoplastic mammary cells. Cell Growth Differ. 1992 Aug;3(8):507–513. [PubMed] [Google Scholar]
- Robinson K., Jones D., Patel Y., Martin H., Madrazo J., Martin S., Howell S., Elmore M., Finnen M. J., Aitken A. Mechanism of inhibition of protein kinase C by 14-3-3 isoforms. 14-3-3 isoforms do not have phospholipase A2 activity. Biochem J. 1994 May 1;299(Pt 3):853–861. doi: 10.1042/bj2990853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth D., Morgan A., Burgoyne R. D. Identification of a key domain in annexin and 14-3-3 proteins that stimulate calcium-dependent exocytosis in permeabilized adrenal chromaffin cells. FEBS Lett. 1993 Apr 12;320(3):207–210. doi: 10.1016/0014-5793(93)80587-k. [DOI] [PubMed] [Google Scholar]
- Swanson K. D., Ganguly R. Characterization of a Drosophila melanogaster gene similar to the mammalian genes encoding the tyrosine/tryptophan hydroxylase activator and protein kinase C inhibitor proteins. Gene. 1992 Apr 15;113(2):183–190. doi: 10.1016/0378-1119(92)90394-5. [DOI] [PubMed] [Google Scholar]
- Toker A., Ellis C. A., Sellers L. A., Aitken A. Protein kinase C inhibitor proteins. Purification from sheep brain and sequence similarity to lipocortins and 14-3-3 protein. Eur J Biochem. 1990 Jul 31;191(2):421–429. doi: 10.1111/j.1432-1033.1990.tb19138.x. [DOI] [PubMed] [Google Scholar]
- Toker A., Sellers L. A., Amess B., Patel Y., Harris A., Aitken A. Multiple isoforms of a protein kinase C inhibitor (KCIP-1/14-3-3) from sheep brain. Amino acid sequence of phosphorylated forms. Eur J Biochem. 1992 Jun 1;206(2):453–461. doi: 10.1111/j.1432-1033.1992.tb16946.x. [DOI] [PubMed] [Google Scholar]
- Watanabe M., Isobe T., Ichimura T., Kuwano R., Takahashi Y., Kondo H. Molecular cloning of rat cDNAs for beta and gamma subtypes of 14-3-3 protein and developmental changes in expression of their mRNAs in the nervous system. Brain Res Mol Brain Res. 1993 Jan;17(1-2):135–146. doi: 10.1016/0169-328x(93)90082-z. [DOI] [PubMed] [Google Scholar]
- Watanabe M., Isobe T., Okuyama T., Ichimura T., Kuwano R., Takahashi Y., Kondo H. Molecular cloning of cDNA to rat 14-3-3 eta chain polypeptide and the neuronal expression of the mRNA in the central nervous system. Brain Res Mol Brain Res. 1991 May;10(2):151–158. doi: 10.1016/0169-328x(91)90105-7. [DOI] [PubMed] [Google Scholar]
- Wilson S. P., Kirshner N. Calcium-evoked secretion from digitonin-permeabilized adrenal medullary chromaffin cells. J Biol Chem. 1983 Apr 25;258(8):4994–5000. [PubMed] [Google Scholar]
- Wu Y. N., Vu N. D., Wagner P. D. Anti-(14-3-3 protein) antibody inhibits stimulation of noradrenaline (norepinephrine) secretion by chromaffin-cell cytosolic proteins. Biochem J. 1992 Aug 1;285(Pt 3):697–700. doi: 10.1042/bj2850697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T., Nakata H., Fujisawa H. A new activator protein that activates tryptophan 5-monooxygenase and tyrosine 3-monooxygenase in the presence of Ca2+-, calmodulin-dependent protein kinase. Purification and characterization. J Biol Chem. 1981 Jun 10;256(11):5404–5409. [PubMed] [Google Scholar]
- Zupan L. A., Steffens D. L., Berry C. A., Landt M., Gross R. W. Cloning and expression of a human 14-3-3 protein mediating phospholipolysis. Identification of an arachidonoyl-enzyme intermediate during catalysis. J Biol Chem. 1992 May 5;267(13):8707–8710. [PubMed] [Google Scholar]
- van Heusden G. P., Wenzel T. J., Lagendijk E. L., de Steensma H. Y., van den Berg J. A. Characterization of the yeast BMH1 gene encoding a putative protein homologous to mammalian protein kinase II activators and protein kinase C inhibitors. FEBS Lett. 1992 May 11;302(2):145–150. doi: 10.1016/0014-5793(92)80426-h. [DOI] [PubMed] [Google Scholar]