Abstract
Regular cigarette smoking and cannabis consumption are strongly positively related to each other, yet few studies explore their underlying variation and covariation. We evaluated the genetic and environmental decomposition of variance and covariance of these two traits in twin data from three countries with different social norms and legislation. Data from the Netherlands Twin Register, FinnTwin12/16, and the Minnesota Center for Twin Family Research (total N = 21,617) were analyzed in bivariate threshold models of lifetime regular smoking initiation (RSI) and lifetime cannabis initiation (CI). We ran unstratified models and models stratified by sex and country. Prevalence of RSI was lowest in the Netherlands and prevalence of CI was highest in Minnesota. In the unstratified model, genetic (A) and common environmental factors (C) contributed substantially to the liabilities of RSI (A = 0.47, C = 0.34) and CI (A = 0.28, C = 0.51). The two liabilities were significantly phenotypically (rP = 0.56), genetically (rA = 0.74), and environmentally correlated in the unstratified model (rC = 0.47and rE = 0.48, representing correlations between common and unique environmental factors). The magnitude of phenotypic correlation between liabilities varied by country but not sex (Minnesota rP ~ 0.70, Netherlands rP ~ 0.59, Finland rP ~ 0.45). Comparisons of decomposed correlations could not be reliably tested in the stratified models. The prevalence and association of RSI and CI vary by sex and country. These two behaviors are correlated because there is genetic and environmental overlap between their underlying latent liabilities. There is heterogeneity in the genetic architecture of these traits across country.
Keywords: Genetic correlation, Substance use, Twin model, Liability threshold model
Introduction
Smoking cigarettes and cannabis use tend to co-occur. While these are two different substances with distinct effects, their relation is complex and multifaceted (Agrawal et al. 2012). Their co-occurrence can be due to multiple mechanisms, including shared genetic and environmental risk factors (common liability hypothesis; Hatoum et al. 2023; Iacono et al. 2008; Iob et al. 2021; Korhonen et al. 2012; Krueger et al. 2002; Lynskey et al. 1998; Palmer et al. 2013; van Leeuwen et al. 2011; Vanyukov et al. 2012; Young et al. 2000), as well as gateway mechanisms, where using one drug, such as cigarettes, may increase the likelihood of trying or using another drug, such as cannabis (Becker et al. 2015; Weinberger et al. 2020), or self-medication to ease symptoms of other disorders (Sumbe et al. 2022).
Studies of the genetic variability underlying tobacco and cannabis use and their association began primarily with twin studies. Twin studies are frequently conducted on each trait separately (Maes et al. 2004; Kendler et al. 2013; Smolkina et al. 2017; Hines et al. 2018), with some studies investigating their overlap (Maes et al. 1999; McGue et al. 2000; Kendler et al. 2003, 2005, 2008; Rhee et al. 2003; Fowler et al. 2007; Zellers et al. 2022). These studies investigating their overlap tend to focus on measures of substance involvement (frequency, heaviness of use, dependence, etc.) rather than initiation. The twin studies of initiation of both substances that we identified focused primarily on adolescence (Huizink et al. 2010; Agrawal et al. 2010, 2016) which is a critical period for substance use development (McGue et al. 2014). Each of these three studies found substantial positive genetic and environmental overlap in the liability to initiate tobacco and cannabis use.
Similarly, more recent molecular projects in genomics and epigenetics were sometimes carried out for each trait separately (Joehanes et al. 2016; Li et al. 2020; Xu et al. 2020; Hillmer et al. 2021; Pasman et al. 2022). Studies evaluating their overlap based on genome-wide genetic data exist and a common finding is that the genetic variants influencing the two traits are found to cluster in the same region of the genome or that the traits are genetically correlated (Agrawal et al. 2015; Stringer et al. 2016; Pasman et al. 2018; Allegrini et al. 2019; Chang et al. 2020; Johnson et al. 2020; Schaefer et al. 2023; Nannini et al. 2023; Fang et al. 2023). Both the twin and molecular studies investigating the association between tobacco and cannabis use indicate strong genetic overlap, but that genetic factors do not entirely explain the association, suggesting the importance of the environment.
Different social and cultural factors may also play a role in the use of tobacco and cannabis; for example, in the Netherlands cannabis has been available for recreational use in “coffee shops” since 1976 and is relatively destigmatized, but in Finland the sale and use of cannabis is entirely illegal. Cannabis policies vary across the United States, but policies have become increasingly permissive across the last two decades, with recreational sales permitted in about half of states. Indeed, there is evidence to show that in the USA the perceived harmfulness of cannabis use has decreased over time, social acceptability has increased, and that cannabis use has similarly increased in the same time period that these legal changes have occurred (Cerdá et al. 2012; Hasin 2018; Coughenour et al. 2021; Zellers et al. 2023). On the other hand, tobacco policies have become stricter globally across the last 40 years, but still vary between countries to some degree (Reubi and Berridge 2016). Just as the prevalence of cannabis use has increased with more permissive policies, the prevalence of tobacco use has decreased as policies become stricter (Helakorpi et al. 2007; Boardman et al. 2010; Flor et al. 2021). These results indicate that social and cultural factors influencing substance use are context dependent, and these factors differ not only across time, but also across geographic location. Therefore, studies of the cigarette and cannabis use and the relationship between them should be sensitive to cultural context.
Here we focused on lifetime initiation of regular cigarette smoking (RSI) and initiation of cannabis use (CI), in data collected from adults in large population based twin registers of European ancestry from three sites: the USA (Minnesota), the Netherlands and Finland. We evaluated the variance and covariance decomposition of lifetime smoking and lifetime cannabis use across cultural contexts. If we find differences across cultures, this could have important ramifications for the way we carry out multi-site studies of these traits.
The classical twin design is a powerful tool to address the etiology of individual differences and its bivariate extension allows to examine whether the co-occurrence of two traits is due to genetic or environmental shared risk factors. In a series of bivariate threshold twin models, we sought to address several research questions: (1) What degree of overlap is there between the underlying liabilities to RSI and CI? (2) What is the genetic correlation? (3) What is the environmental correlation and are the traits correlated due to the shared environment, unique environment, or both? (4) Do these values differ between the Netherlands, Finland, and USA? (5) Do these values differ between males and females?
We expected both traits to be heritable and we expected positive phenotypic, genetic, and environmental correlations between them, in all three countries. We also expected to replicate established sex differences, where males initiate at higher rates than females. Lastly, we anticipated some parameter differences in variance and thresholds for initiation between sexes and countries given the varied legal and social environments around cannabis and tobacco use, but we did not have directional hypotheses regarding country differences. The pre-registration is available at https://osf.io/utr6w/.
Methods
Participants
We harmonized data from twin cohorts across three countries (total N = 21,617): the Netherlands Twin Register (N = 12,987), FinnTwin12 and FinnTwin16 (combined N = 5,888), and the Minnesota Center for Twin Family Research (N = 2,742). All are longitudinal twin studies that have been described extensively elsewhere (Geels et al. 2013; Treur et al. 2017; Wilson et al. 2019; Rose et al. 2019; Kaidesoja et al. 2019; Ligthart et al. 2019). The Netherlands Twin Register, FinnTwin12 and FinnTwin16 assessed same-sex and opposite-sex twin pairs, whereas the Minnesota Center for Twin Family Research only recruited same-sex twin pairs. Sex is specifically sex assigned at birth and as reported on birth certificates. Furthermore, the Minnesota Center for Twin Family Research sample is composed of three cohorts with varying birth years, assessment structures, and assessment years.
We utilized one assessment per individual; assessments were selected to represent the most recent assessments and target a mean age of assessment in the twins’ 30s. Importantly, this mean age of assessment is beyond the normative ages of tobacco and cannabis initiation, which generally occurs between ages 15–19 (Richmond-Rakerd et al. 2016; Blanco et al. 2018). We utilized the most recent non-missing assessment from waves 8 and 10 in the adult Netherlands Twin Register (2009–2013), the FinnTwin12 wave 4 assessment (2006–2009), the FinnTwin16 wave 5 assessment (2010–2012), and the most recent non-missing assessment from waves 4, 5, 6, and 7 in the Minnesota Center for Twin Family Research (2013–2022).
Measures
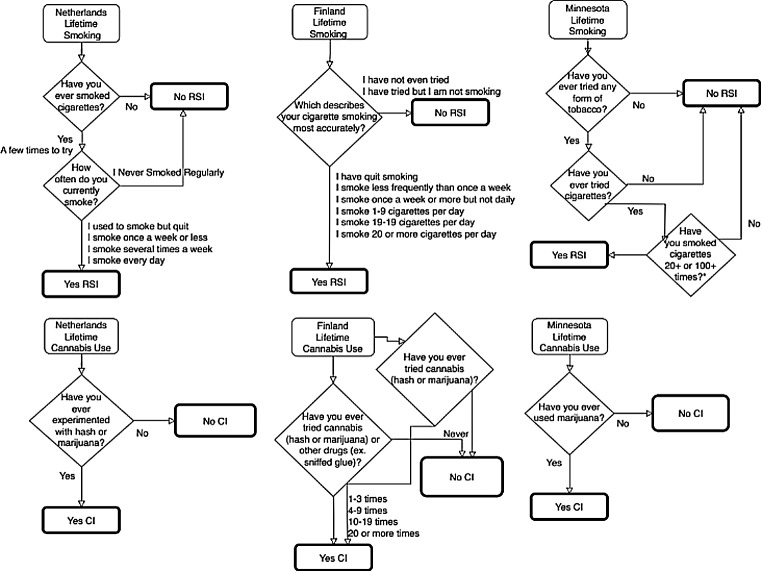
Both RSI and CI were defined as binary measures indicating whether the individual had ever been a regular cigarette smoker (yes or no) and whether the individual had ever used cannabis (yes or no). Measures were harmonized between samples, as item wording and skip-outs varied. Figure 1 provides a flowchart of the questionnaire items, response options, skip-outs, and binarization for each sample. Importantly, the FinnTwin12 and FinnTwin16 items differed slightly, as one asked only about cannabis and the other asked simultaneously about cannabis and other drugs. For participants answering yes to “have you used cannabis or other drugs” we assume they have used cannabis and code as yes for CI. Sex was operationalized as a binary variable based on sex assigned at birth as reported at intake (male or female).
Fig. 1.
Flowchart of the binary phenotype harmonization from original questionnaire items and response options in each sample. Note that the items for Finland differed slightly between the FinnTwin12 and FinnTwin16 cohorts. RSI refers to regular smoking initiation, CI refers to cannabis initiation
Analyses
All analyses were conducted in RStudio. We used the R packages ggplot2 (Wickham 2016) for visualizations, OpenMx (Boker et al. 2011; Neale et al. 2016) for twin model variance decompositions, and Psych (Revelle 2023) for tetrachoric correlations. All data (i.e. from complete and from incomplete twin pairs in which only one twin replied) were analyzed.
We first obtained descriptive statistics including prevalence of endorsement for each binary trait by country and sex. Resemblance between twins and between traits were estimated by tetrachoric correlations. Prevalences were estimated separately for each country × sex group; tetrachoric twin correlations were estimated separately for each country × sex group and stratified by zygosity (monozygotic, same-sex dizygotic, and opposite-sex dizygotic). Given the differences in item wording for the Finnish sample and cannabis use, we compared the endorsement between the two items, to ensure that the inclusion of “cannabis or other drugs” was not upwardly biasing endorsement.
We then fitted a bivariate binary threshold twin model that included additive genetic (A), shared environmental (C), and unique environmental (E) variance components. Binary threshold models assume that the observed variables (ex. having initiated cannabis use or not) are indicative of underlying, normally distributed continuous liability. This latent liability has a threshold (the parameter that depends on the prevalence) above which the trait is observed, and below which it is not. First, all data were analyzed simultaneously in one model; in other words, the model was not stratified by sex or country and produced one set of parameter estimates. We identified the binary threshold models by fixing the mean of the liability distribution to zero and the variance to one and estimated the threshold, which can be interpreted as a z-score. Additionally, we included a covariate of age on the mean of the liability distribution and computed predicted thresholds at a set of ages covering the middle of the countries’ age distribution. We retained all ACE parameters to avoid bias in the point estimates.
Next, as our research questions include the investigation of country and site differences, we ran the same bivariate binary model stratified by country and sex. This stratified model produced six sets of parameter estimates (ex. Unique parameter estimates for each country -sex group: Netherlands males, Netherlands females, Finland males, Finland females, Minnesota males, and Minnesota females). We then compared parameter estimates between these six sets to evaluate country and sex differences. The main parameter of interest was the genetic correlation between the two liability dimensions, but all possible parameter differences were investigated.
Model comparisons were conducted via likelihood ratio test, in which parameters of interest were fixed to equality in a reduced model, and the fit of this reduced model was compared to the full model where the parameter was free to vary between groups. If the equality constraint resulted in a significant worsening of model fit, the parameter was interpreted as being different between groups. If the equality constraint did not significantly worsen model fit, as indicated by the likelihood ratio test, then that parameter was interpreted as not significantly differing between groups.
Therefore, to evaluate country differences, we compared the full model to a model in which the parameter of interest was fixed to equality between males across the three countries and between females across the three countries (ex. Comparing Netherlands males to Finland males to Minnesota males in a 2-df test). To evaluate sex differences, we compared the full model to a model in which the parameter of interest was fixed to equality between males and females separately for each country (ex. Comparing Netherlands males to Netherlands females in a 1-df test).
Results
Descriptive statistics for age at data collection, year of data collection, sample sizes, sex, zygosity, and trait endorsement for each country are presented in Table 1.
Table 1.
Demographics and descriptive statistics by sample
| Netherlands | Finland | Minnesota | |
|---|---|---|---|
| N. Individuals | 12,987 | 5,888 | 2,742 |
| Age range | 17–97 | 22–37 | 22–49 |
| Age mean (SD) | 32.2 (14.8) | 31.3 (4.2) | 36.2 (6.9) |
| % Female | 67.2% | 56.9% | 55.2% |
| % MZ | 47.0% | 32.2% | 62.2% |
| % OS DZ | 25.3% | 35.5% | 0% |
| Years of data collection | 2009–2013 | 2006–2012 | 2013–2022 |
| % Yes RSI | 33.2% | 47.5% | 44.7% |
| % Yes CI | 29.5% | 25.7% | 62.9% |
Note N refers to number; MZ refers to monozygotic; DZ OS refers to opposite-sex pairs dizygotic pairs; RSI refers to regular smoking initiation; CI refers to cannabis initiation
All three twin cohorts have overlapping age ranges, with the age range being widest in the Netherlands and narrowest in Finland. Mean ages are comparable and in all three twin cohorts, the mean age is beyond the typical age of substance initiation. Further details on the prevalence of each trait are presented in Fig. 2.
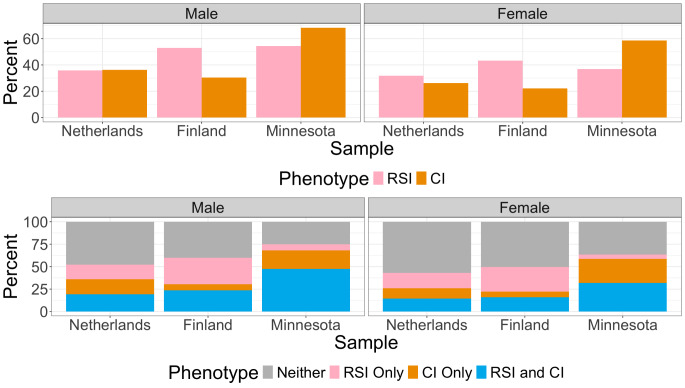
Fig. 2.
Bar charts depicting the prevalence of lifetime smoking and lifetime cannabis use stratified by sex and sample. The top bar chart presents the prevalence of each phenotype separately. The bottom bar chart presents the percentages of individuals who have endorsed neither phenotype, endorsed both phenotypes, and endorsed one phenotype but not the other. RSI refers to regular smoking initiation, CI refers to cannabis initiation
Tetrachoric correlations are presented in Table 2. In most cases and as expected, traits in monozygotic twin pairs are more strongly correlated than in same-sex dizygotic twin pairs, and same-sex dizygotic twin pairs are correlated more strongly than opposite-sex dizygotic pairs. There are some groups for which the monozygotic and dizygotic twin correlations are comparable (RSI in Finnish males, CI in Minnesota females). For the two Finnish cohorts, prevalence of CI was not higher in the cohort asked about “cannabis use or other drugs” (24.8% responded yes) as compared to those asked only about cannabis use (27.7% responded yes), suggesting that the item wording was unlikely to be biasing endorsement.
Table 2.
Tetrachoric twin correlations by sex and sample
| MZ M | DZ M | MZ F | DZ F | DZ OS | ||
|---|---|---|---|---|---|---|
| RSI | Netherlands | 0.86 | 0.52 | 0.82 | 0.55 | 0.49 |
| Finland | 0.78 | 0.71 | 0.85 | 0.67 | 0.40 | |
| Minnesota | 0.76 | 0.51 | 0.77 | 0.54 | NA | |
| CI | Netherlands | 0.73 | 0.64 | 0.73 | 0.49 | 0.51 |
| Finland | 0.74 | 0.47 | 0.83 | 0.61 | 0.33 | |
| Minnesota | 0.84 | 0.60 | 0.69 | 0.73 | NA |
Note MZ refers to monozygotic pairs; DZ refers to dizygotic pairs; M refers to same-sex male pairs; F refers to same-sex female pairs; OS refers to opposite-sex dizygotic pairs; RSI refers to regular smoking initiation; CI refers to cannabis initiation
Based on the tetrachoric correlations, we expected to see additive genetic influences on both traits. We evaluated this in the unstratified model, where we pooled across country and sex (13 estimated parameters, df = 31,325, AIC = 35026.61). As expected, RSI and CI are both heritable (RSI A = 0.47 [95% confidence interval = 0.36, 0.58], CI A = 0.28 [0.22, 0.39]) and had a substantial shared environmental component (RSI C = 0.34 [0.24, 0.44], CI C = 0.51 [0.39, 0.60]). The two traits were positively correlated at the phenotypic level (rP = 0.56 [0.54, 0.58]) and the decomposed correlations were also all moderate to strong (rA = 0.74 [0.54, 0.93], rC = 0.47 [0.45, 0.63], rE = 0.48 [0.39, 0.58]) indicating both genetic and environmental overlap in the propensity to RSI and CI. We followed this up with the stratified model to evaluate country and sex differences.
The stratified model estimates and confidence intervals are presented in Table 3 and thresholds for initiation of each substance are presented in Fig. 3 (78 estimated parameters, df = 41,484, AIC = 45,554.98). The stratified (full) model fit better than the unstratified (reduced) model (χ2 = 10,398.37, df = 10,159, p = 0.047) As we observed monozygotic and dizygotic twin correlations of comparable magnitudes for RSI in Finnish males and CI in Minnesota females, we similarly found non-significant heritability for these traits. All other traits were heritable, though notably, confidence intervals for A and C estimates were very wide despite the large sample size. In turn, the decomposed correlations could not be stably estimated in the stratified models, with confidence intervals including +/- 1.
Table 3.
Bivariate twin model results split by sex and sample
| Netherlands | Finland | Minnesota | |||||
|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | ||
| RSI | A |
0.83 [0.01, 0.85] |
0.66 [0.42, 0.82] |
0.07 [-0.14, 0.84] |
0.68 [0.01, 0.77] |
0.60 [-0.70, 1.00] |
0.45 [0.13, 0.54] |
| C |
-0.01 [-0.02, 0.69] |
0.15 [0.01, 0.39] |
0.68 [-0.01, 0.77] |
0.18 [-0.11, 0.70] |
0.17 [-0.25, 0.51] |
0.31 [-0.01, 0.61] |
|
| E |
0.18 [0.12, 0.24] |
0.19 [0.16, 0.24] |
0.25 [0.17, 0.42] |
0.14 [0.11, 0.22] |
0.23 [0.16, 0.33] |
0.23 [0.16, 0.33] |
|
| CI | A |
0.26 [0.01, 0.81] |
0.52 [0.01, 0.78] |
0.70 [-0.08, 0.77] |
0.41 [-0.01, 0.49] |
0.54 [0.17, 0.63] |
-0.06 [-0.37, 0.89] |
| C |
0.47 [0.11, 0.57] |
0.20 [-0.10, 0.59] |
0.04 [-0.19, 0.78] |
0.40 [-0.02, 0.60] |
0.29 [-1.00, 0.64] |
0.75 [0.48, 0.83] |
|
| E |
0.28 [0.22, 0.34] |
0.27 [0.22, 0.35] |
0.26 [0.16, 0.38] |
0.19 [0.10, 0.27] |
0.16 [0.02, 0.26] |
0.31 [0.24, 0.45] |
|
| Cor. | Phen. |
0.58 [0.54, 0.62] |
0.58 [0.55, 0.61] |
0.51 [0.45, 0.56] |
0.51 [0.46, 0.56] |
0.70 [0.57, 0.76] |
0.69 [0.64, 0.75] |
| A |
1.00 [0.89, 1.00] |
0.55 [0.51, 0.61] |
1.00 [-1.00, 1.00] |
0.69 [-0.21, 1.00] |
0.70 [-1.00, 0.75] |
NA | |
| C | NA |
0.85 [-1.00, 1.00] |
1.00 [-1.00, 1.00] |
0.31 [-1.00, 1.00] |
1.00 [-1.00, 1.00] |
0.81 [-1.00, 1.00] |
|
| E |
0.44 [0.22, 0.56] |
0.48 [0.34, 0.69] |
0.48 [-0.24, 0.70] |
0.36 [0.04, 0.47] |
0.41 [0.10, 0.98] |
0.67 [0.42, 1.00] |
|
Note A refers to additive genetic component; C refers to shared environmental component, and E refers to unique environmental component; Cor. refers to the correlation between substances; Phen. refers to the phenotypic correlation. NA indicates a correlation that cannot be estimated due to one or more negative point estimates, - indicates bound did not converge. The first number refers to the point estimate and the number in brackets is the 95% confidence interval around that point estimate. RSI refers to regular smoking initiation; CI refers to cannabis initiation
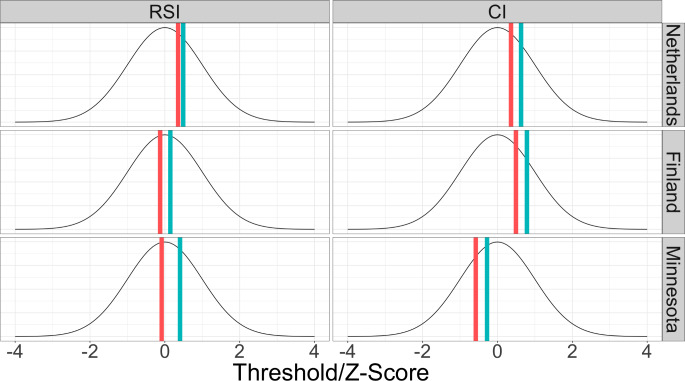
Fig. 3.
Diagram representing the thresholds for initiation of lifetime smoking and lifetime cannabis use, split by sample and sex. Blue lines represent the threshold for females and red represent the threshold for males. Thresholds can be interpreted as a z-score and are depicted here against a normal distribution with mean of 0 and variance of 1
We identified country and sex differences in the thresholds for each trait. The thresholds for RSI and CI were significantly lower for males as compared to females, i.e. reflecting the higher prevalence in men. This was also true for all three countries (out of all six comparisons, the largest p-value was 1.2 × 10− 5). Thresholds also differed between countries, and this was true for both males and females and for both substances. With respect to RSI, the threshold was higher for Netherlands males (χ2 = 202.3, df = 2, p = 1.2 × 10− 44) as compared to both Finland and Minnesota males, which did not differ from each other (χ2 = 0.91, df = 1, p = 0.34). On the other hand, for females, the threshold for RSI was different between all three twin cohorts (χ2 = 131.9, df = 2, p = 2.3 × 10− 29); the threshold was highest in the Netherlands, lowest in Finland, and Minnesota was in between. With respect to CI, the threshold differed between males from all three samples (χ2 = 339.1, df = 2, p = 2.3 × 10− 74); the threshold was highest in Finland, lowest in Minnesota, and the Netherlands threshold was in between the other two. The same pattern was observed for females (χ2 = 435.5, df = 2, p = 2.6 × 10− 95).
Given the width of confidence intervals, we generally did not identify sex differences or country differences in variance decompositions for either RSI or CI. There were two exceptions where we identified sex and country differences, as evidenced earlier by the relative magnitudes of MZ and DZ twin correlations. RSI was less heritable in Finland males as compared to males from other samples (χ2 = 16.9, df = 4, p = 2.0 × 10− 3), and as compared to Finland females (χ2 = 12.4, df = 2, p = 2.0 × 10− 3). Similarly, CI was less heritable in Minnesota females, as compared to females from other samples (χ2 = 20.9, df = 4, p = 3.3 × 10− 4) and as compared to Minnesota males (χ2 = 6.9 df = 2, p = 0.03).
We also observed country differences in the more stably estimated phenotypic correlations. Phenotypic correlations did not differ between males and females within a country, but they did differ between countries for both males (χ2 = 21.4, df = 2, p = 2.2 × 10− 5) and females (χ2 = 22.6, df = 2, p = 1.3 × 10− 5). The phenotypic correlation between RSI and CI was strongest in Minnesota (rP ~ 0.70), weakest in Finland (rP ~ 0.45), and the estimate for the Netherlands was in between the two (rP ~ 0.59). Models to evaluate sex and sample differences in the decomposed correlations did not stably converge.
Discussion
We estimated a bivariate twin decomposition of the variation underlying liability for RSI and CI. We investigated these decompositions in samples from three countries (the Netherlands, Finland, and Minnesota), which have differing policies around cigarettes and cannabis use. We identified important country and sex differences in the relationship between RSI and CI.
We replicated established sex differences in liability for RSI and CI; in all twin cohorts and for both substances, males were more likely to endorse RSI or CI as compared to females. These prevalences are captured in the twin model via the threshold for initiation, which can be interpreted as a z-score on the underlying latent risk dimension. If an individual’s latent risk is above the threshold, initiation is observed. Behaviors with higher prevalence in the population will have a lower threshold for initiation.
The thresholds for RSI and CI were higher for females as compared to males, aligning with the higher prevalence in males in all three countries. There were also country differences in the threshold for RSI for females but not males. With respect to CI, the thresholds also differed between all three countries for both males and females. The threshold for CI was lowest in Minnesota, highest in Finland, and in between for the Netherlands. These differences in threshold likely are driven by a variety of legal and cultural differences, including social norms around substance use, types of available products and administration methods, legal policies, and ease of acquiring of substances.
Legal differences are one potential source, as there are substantial differences in policies between the three countries. Cannabis use is illegal in Finland, is decriminalized for personal use in the Netherlands, and recreationally legal in about half of the US. Cannabis was not recreationally legalized in Minnesota, the birth state of all twins in the Minnesota sample, until 2023 but not all participants resided in Minnesota at the time of assessment. On the other hand, Finland and the Netherlands are considered on the forefront of tobacco control worldwide (Timberlake et al. 2020; World Health Organization 2023) as compared to the United States which lags behind (Action on Smoking and Health 2020). These important legal differences impact how easy it is to obtain cigarettes or cannabis, but also highlight the social acceptability of their use. Future work could also characterize how substance use relates to broader disinhibited behaviors in sites with different substance policies.
Trait correlations differed significantly by country but not by sex. RSI and CI were most strongly related in Minnesota and least strongly related in Finland. Previous developmental research has indicated that the strength of relationship between substances does vary by age (Vrieze et al. 2012; Zellers et al. 2022) and here we provide additional evidence that the strength of relationship may also vary by cultural context, though we cannot rule out age effects. Further, we cannot rule out differences by birth cohort, which could also reflect temporal changes in the use of substances over time. Smoking has become much less prevalent in the past decades, while cannabis use has increased, though trends also vary by country. Lastly, other studies have not identified cross-country differences in smoking initiation (Madden et al. 2004) and so the existence of cultural differences, or lack thereof, likely depends on a combination of age, period, and cohort effects.
This has important implications for broader research on substance use and related behaviors in the externalizing spectrum. Indeed, many studies have been published on the general genetic liability to substance use and externalizing behaviors, including some studies from Minnesota and Finland overlapping with the participants included in the present work (Young et al. 2000; Krueger et al. 2002; Dick et al. 2009; Agrawal et al. 2010; Edwards and Kendler 2012; Korhonen et al. 2012; Palmer et al. 2013; Karlsson Linnér et al. 2021). To our knowledge, only one study has harmonized and compared twin models of externalizing psychopathology and its relationship to cannabis use across samples; no sample differences were identified but the samples were American samples from different states (Zellers et al. 2020). As we have established that in adulthood, the covariation between substances differs between countries, future directions could therefore explore cultural differences in the relationships between substances and other externalizing behaviors. Lastly, we identified sex and country differences in the sources of variation and covariation underlying each trait, but these differences came with the large confidence intervals around the A and C variance estimates and should therefore be interpreted with care. Future work could attempt to identify specific cultural differences that may be driving the observed differences in prevalence and covariation between the two substances.
Limitations
One measurement limitation is that the survey items are not the same across the twin cohorts and therefore we needed to harmonize variables. Some differences therefore could be artifacts of the harmonization process, particularly for RSI, as the items to measure this differed more substantially between countries as compared to the items for CI. Secondly, the definitions for initiation differed by substance; for RSI we considered initiation of regular use but for CI we considered initiation of any use. This could contribute to explaining differences in heritability between the two substances. Additionally, age and year of data collection varies between twin cohorts and also could contribute to identified differences.
An additional measurement limitation is that co-use of cannabis and tobacco (sometimes referred to as spliff use, blunts, or mulled cigarettes; Schauer et al. 2017) was not measured. Previous works have reported meaningful differences between individuals who co-use cannabis and tobacco, as compared to individuals who use only one substance, as well as individuals who do use both substances but not concurrently (Agrawal et al. 2012; Schauer et al. 2017; Tucker et al. 2019; Akbar et al. 2019; Kumar et al. 2020; Hindocha et al. 2021). Furthermore, there could be reporting bias introduced, as it is possible that individuals regularly co-use cannabis and tobacco would not be self-reporting as lifetime smokers, because the tobacco items specified cigarette use, rather than any tobacco use. That said, the vast majority of individuals who report use of tobacco-containing cannabis products also report other tobacco use, including cigarettes (Kumar et al. 2020), so any reporting bias introduced by our lack of co-use items is likely minimal.
Lastly, in twin models utilizing binary data, the variance components underlying twin similarity (additive genetic/A and shared environmental/C) are highly correlated and therefore can be difficult to tease apart. We used a direct symmetric model parameterization and elected to interpret the full ACE model results, rather than removing components that were not significantly different from zero, as to not upwardly bias the other components’ estimates (Verhulst et al. 2019). Because of this, our model resulted in comparatively wide confidence intervals around our A and C estimates in the stratified models, despite large sample sizes. Given these wide intervals, we were unable to meaningfully compare parameters of interest, such as the genetic correlations, across samples; all comparisons were nonsignificant despite some large differences in point estimates. Future work may evaluate traits with more variability, such as age at first use, age at regular use, frequency or quantity of tobacco and cannabis consumption, to avoid the limitations of working with coarser, binary data. Additionally, measures that more accurately gauge the heaviness of use or problematic use may be better indicators of underlying genetic liability to substance use and abuse, particularly under theoretical conceptualizations that treat substance use as one manifestation of broader externalizing liability.
Conclusions
We identified meaningful sex and country differences in the liabilities RSI and CI. Our findings also have implications for the measures of substance use utilized in genetically informative studies. Whereas large genomic consortium studies benefit from coarse measures of use that can be readily harmonized across many participating studies, twin studies may benefit from including both binary and continuous measures of use. Furthermore, while the existence of general genetic vulnerability to substance (mis)use is supported across cultures and genetic ancestries (Saunders et al. 2022; Hatoum et al. 2023), the degree to which substances are phenotypically related may depend on cultural context.
Acknowledgements
We acknowledge Dr. William Iacono and Dr. Matt McGue for their roles in collection of Minnesota data, as well as Teemu Palviainen and Lannie Ligthart, data managers for the Finnish and Netherlands data respectively. DIB acknowledges her KNAW Academy Professor Award (PAH/6635) by the Netherlands Royal Academy of Arts and Sciences.
Author Contributions
Contributors: SZ data curation, investigation, formal analysis, figure and table preparation, methodology, project administration, visualization, original draft, review and editing, JvD conceptualization, review and editing, HHMM methodology, reviewing and editing, MO conceptualization, review and editing, FF conceptualization, funding acquisition, review and editing, SV resources, funding acquisition, review and editing, JK conceptualization, resources, funding acquisition, supervision, review and editing, DIB conceptualization, resources, funding acquisition, supervision, review and editing.
Funding
Open Access funding provided by University of Helsinki (including Helsinki University Central Hospital). This work was supported by the National Institutes of Health [R01DA048824 PI: Fang Fang, R01AA015416 PI: Jessica Salvatore, DA042755, DA054087, DA054987, DA044283 PI: Scott Vrieze], the Dutch Research Council [480-15-001/674, PI: Dorret I. Boomsma], the Academy of Finland [336823 and 352792 PI: Jaakko Kaprio], and the Sigrid Juselius Foundation [PI: Jaakko Kaprio].
Open Access funding provided by University of Helsinki (including Helsinki University Central Hospital).
Data Availability
The research data used in this study are confidential and are not publicly available to protect participant privacy.
Declarations
Competing Interests
The authors declare no competing interests.
Footnotes
Edited by: Yoon-Mi Hur
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Action on Smoking and Health (2020) Tobacco control in the United States: failure to protect the right to health. Tob Prev Cessat 6:34. 10.18332/tpc/122543 10.18332/tpc/122543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A, Silberg JL, Lynskey MT et al (2010) Mechanisms underlying the lifetime co-occurrence of tobacco and cannabis use in adolescent and young adult twins. Drug Alcohol Depend 108:49–55. 10.1016/j.drugalcdep.2009.11.016 10.1016/j.drugalcdep.2009.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A, Budney AJ, Lynskey MT (2012) The co-occurring use and misuse of cannabis and tobacco: a review. Addict Abingdon Engl 107:1221–1233. 10.1111/j.1360-0443.2012.03837.x 10.1111/j.1360-0443.2012.03837.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A, Lynskey MT, Kapoor M et al (2015) Are genetic variants for tobacco smoking associated with cannabis involvement? Drug Alcohol Depend 150:183–187. 10.1016/j.drugalcdep.2015.02.029 10.1016/j.drugalcdep.2015.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A, Grant JD, Lynskey MT et al (2016) The genetic relationship between cannabis and tobacco cigarette use in european- and African-American female twins and siblings. Drug Alcohol Depend 163:165–171. 10.1016/j.drugalcdep.2016.04.011 10.1016/j.drugalcdep.2016.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbar SA, Tomko RL, Salazar CA et al (2019) Tobacco and cannabis co-use and interrelatedness among adults. Addict Behav 90:354–361. 10.1016/j.addbeh.2018.11.036 10.1016/j.addbeh.2018.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allegrini AG, Verweij KJH, Abdellaoui A et al (2019) Genetic vulnerability for smoking and Cannabis Use: associations with E-Cigarette and Water Pipe Use. Nicotine Tob Res off J Soc Res Nicotine Tob 21:723–730. 10.1093/ntr/nty150 10.1093/ntr/nty150 [DOI] [PubMed] [Google Scholar]
- Becker J, Schaub MP, Gmel G, Haug S (2015) Cannabis use and other predictors of the onset of daily cigarette use in young men: what matters most? Results from a longitudinal study. BMC Public Health 15:843. 10.1186/s12889-015-2194-3 10.1186/s12889-015-2194-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco C, Flórez-Salamanca L, Secades-Villa R et al (2018) Predictors of initiation of nicotine, alcohol, cannabis, and cocaine use: results of the national epidemiologic survey on Alcohol and related conditions (NESARC). Am J Addict 27:477–484. 10.1111/ajad.12764 10.1111/ajad.12764 [DOI] [PubMed] [Google Scholar]
- Boardman JD, Blalock CL, Pampel FC (2010) Trends in the genetic influences on smoking. J Health Soc Behav 51:108–123. 10.1177/0022146509361195 10.1177/0022146509361195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boker S, Neale M, Maes H et al (2011) OpenMx: an Open source extended Structural equation modeling Framework. Psychometrika 76:306–317. 10.1007/s11336-010-9200-6 10.1007/s11336-010-9200-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerdá M, Wall M, Keyes KM et al (2012) Medical marijuana laws in 50 states: investigating the relationship between state legalization of medical marijuana and marijuana use, abuse and dependence. Drug Alcohol Depend 120:22–27. 10.1016/j.drugalcdep.2011.06.011 10.1016/j.drugalcdep.2011.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L-H, Ong J-S, An J et al (2020) Investigating the genetic and causal relationship between initiation or use of alcohol, caffeine, cannabis and nicotine. Drug Alcohol Depend 210:107966. 10.1016/j.drugalcdep.2020.107966 10.1016/j.drugalcdep.2020.107966 [DOI] [PubMed] [Google Scholar]
- Coughenour P, Sadicario JS, Karjane N et al (2021) Prevalence and Social Acceptability of Cannabis, Tobacco, and Alcohol Use in Adult Women. Womens Health Rep 2:452–458. 10.1089/whr.2021.0042 10.1089/whr.2021.0042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Bernard M, Aliev F et al (2009) The role of Socioregional Factors in moderating genetic influences on early adolescent behavior problems and Alcohol Use. Alcohol Clin Exp Res 33:1739–1748. 10.1111/j.1530-0277.2009.01011.x 10.1111/j.1530-0277.2009.01011.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards AC, Kendler KS (2012) Twin study of the relationship between adolescent Attention-Deficit/Hyperactivity disorder and adult alcohol dependence. J Stud Alcohol Drugs 73:185–194 10.15288/jsad.2012.73.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang F, Quach B, Lawrence KG et al (2023) Trans-ancestry epigenome-wide association meta-analysis of DNA methylation with lifetime cannabis use. Mol Psychiatry 1–10. 10.1038/s41380-023-02310-w [DOI] [PMC free article] [PubMed]
- Flor LS, Reitsma MB, Gupta V et al (2021) The effects of tobacco control policies on global smoking prevalence. Nat Med 27:239–243. 10.1038/s41591-020-01210-8 10.1038/s41591-020-01210-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler T, Lifford K, Shelton K et al (2007) Exploring the relationship between genetic and environmental influences on initiation and progression of substance use. 10.1111/j.1360-0443.2006.01694.x. Addiction [DOI] [PMC free article] [PubMed]
- Geels LM, Vink JM, van Beek JHDA et al (2013) Increases in alcohol consumption in women and elderly groups: evidence from an epidemiological study. BMC Public Health 13:207. 10.1186/1471-2458-13-207 10.1186/1471-2458-13-207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS (2018) US Epidemiology of Cannabis Use and Associated problems. Neuropsychopharmacology 43:195–212. 10.1038/npp.2017.198 10.1038/npp.2017.198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatoum AS, Colbert SMC, Johnson EC et al (2023) Multivariate genome-wide association meta-analysis of over 1 million subjects identifies loci underlying multiple substance use disorders. Nat Ment Health 1:210–223. 10.1038/s44220-023-00034-y 10.1038/s44220-023-00034-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helakorpi S, Martelin T, Torppa J et al (2007) Did the Tobacco Control Act Amendment in 1995 affect daily smoking in Finland? Effects of a restrictive workplace smoking policy |. J Public Health | Oxf Acad J Public Health 30:407–414. 10.1093/pubmed/fd051 10.1093/pubmed/fd051 [DOI] [PubMed] [Google Scholar]
- Hillmer A, Chawar C, Sanger S et al (2021) Genetic basis of cannabis use: a systematic review. BMC Med Genomics 14:203. 10.1186/s12920-021-01035-5 10.1186/s12920-021-01035-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindocha C, Brose LS, Walsh H, Cheeseman H (2021) Cannabis use and co-use in tobacco smokers and non-smokers: prevalence and associations with mental health in a cross-sectional, nationally representative sample of adults in Great Britain, 2020. Addiction 116:2209–2219. 10.1111/add.15381 10.1111/add.15381 [DOI] [PubMed] [Google Scholar]
- Hines LA, Morley KI, Rijsdijk F et al (2018) Overlap of heritable influences between cannabis use disorder, frequency of use and opportunity to use cannabis: Trivariate twin modelling and implications for genetic design. Psychol Med 48:2786–2793. 10.1017/S0033291718000478 10.1017/S0033291718000478 [DOI] [PubMed] [Google Scholar]
- Huizink AC, Levälahti E, Korhonen T et al (2010) Tobacco, cannabis, and other illicit drug use among Finnish adolescent twins: causal relationship or correlated liabilities? J Stud Alcohol Drugs. 10.15288/jsad.2010.71.5 10.15288/jsad.2010.71.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono WG, Malone SM, McGue M (2008) Behavioral disinhibition and the development of early-onset addiction: common and specific influences. Annu Rev Clin Psychol 4:325–348. 10.1146/annurev.clinpsy.4.022007.141157 10.1146/annurev.clinpsy.4.022007.141157 [DOI] [PubMed] [Google Scholar]
- Iob E, Schoeler T, Cecil CM et al (2021) Identifying risk factors involved in the common versus specific liabilities to substance use: a genetically informed approach. Addict Biol 26:e12944. 10.1111/adb.12944 10.1111/adb.12944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joehanes R, Just AC, Marioni RE et al (2016) Epigenetic signatures of cigarette smoking. Circ Cardiovasc Genet 9:436–447. 10.1161/CIRCGENETICS.116.001506 10.1161/CIRCGENETICS.116.001506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EC, Demontis D, Thorgeirsson TE et al (2020) A large-scale genome-wide association study meta-analysis of cannabis use disorder. Lancet Psychiatry 7:1032–1045. 10.1016/S2215-0366(20)30339-4 10.1016/S2215-0366(20)30339-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaidesoja M, Aaltonen S, Bogl LH et al (2019) FinnTwin16: a longitudinal study from Age 16 of a Population-based Finnish twin cohort. Twin Res Hum Genet 22:530–539. 10.1017/thg.2019.106 10.1017/thg.2019.106 [DOI] [PubMed] [Google Scholar]
- Karlsson Linnér R, Mallard TT, Barr PB et al (2021) Multivariate analysis of 1.5 million people identifies genetic associations with traits related to self-regulation and addiction. Nat Neurosci 24:1367–1376. 10.1038/s41593-021-00908-3 10.1038/s41593-021-00908-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Jacobson KC, Prescott CA, Neale MC (2003) Specificity of genetic and environmental risk factors for use and abuse/dependence of cannabis, cocaine, hallucinogens, sedatives, stimulants, and opiates in male twins. Am J Psychiatry 160:687–695. 10.1176/appi.ajp.160.4.687 10.1176/appi.ajp.160.4.687 [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Jacobson KC et al (2005) Genetic and environmental influences on illicit drug use and tobacco use across birth cohorts. Psychol Med 35:1349–1356. 10.1017/S0033291705004964 10.1017/S0033291705004964 [DOI] [PubMed] [Google Scholar]
- Kendler KS, Schmitt E, Aggen SH, Prescott CA (2008) Genetic and environmental influences on alcohol, caffeine, cannabis, and nicotine use from early adolescence to middle adulthood. Arch Gen Psychiatry 65:674–682. 10.1001/archpsyc.65.6.674 10.1001/archpsyc.65.6.674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Myers J, Damaj MI, Chen X (2013) Early Smoking Onset and Risk for subsequent nicotine dependence: a monozygotic Co-twin Control Study. Am J Psychiatry 170:408–413. 10.1176/appi.ajp.2012.12030321 10.1176/appi.ajp.2012.12030321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen T, Latvala A, Dick DM et al (2012) Genetic and environmental influences underlying externalizing behaviors, cigarette smoking and illicit drug use across adolescence. Behav Genet 42:614–625. 10.1007/s10519-012-9528-z 10.1007/s10519-012-9528-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ et al (2002) Etiologic connections among substance dependence, antisocial behavior, and personality: modeling the externalizing spectrum. J Abnorm Psychol 111:411–424. 10.1037/0021-843X.111.3.411 10.1037/0021-843X.111.3.411 [DOI] [PubMed] [Google Scholar]
- Kumar N, Puljević C, Ferris J et al (2020) The intersection between Spliff usage, Tobacco Smoking, and having the First Joint after Waking. Sci Rep 10:7650. 10.1038/s41598-020-64110-4 10.1038/s41598-020-64110-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Chen Y, Yao J et al (2020) Genome-Wide Association Study of Smoking Behavior Traits in a Chinese Han Population. Front Psychiatry 11 [DOI] [PMC free article] [PubMed]
- Ligthart L, van Beijsterveldt CEM, Kevenaar ST et al (2019) The Netherlands Twin Register: Longitudinal Research based on twin and twin-family designs. Twin Res Hum Genet 22:623–636. 10.1017/thg.2019.93 10.1017/thg.2019.93 [DOI] [PubMed] [Google Scholar]
- Lynskey MT, Fergusson DM, Horwood LJ (1998) The origins of the correlations between tobacco, alcohol, and cannabis use during adolescence. J Child Psychol Psychiatry 39:995–1005 10.1111/1469-7610.00402 [DOI] [PubMed] [Google Scholar]
- Madden PAF, Pedersen NL, Kaprio J et al (2004) The Epidemiology and Genetics of Smoking initiation and persistence: crosscultural comparisons of Twin Study results. Twin Res Hum Genet 7:82–97. 10.1375/twin.7.1.82 10.1375/twin.7.1.82 [DOI] [PubMed] [Google Scholar]
- Maes HH, Woodard CE, Murrelle L et al (1999) Tobacco, alcohol and drug use in eight- to sixteen-year-old twins: the Virginia Twin Study of adolescent behavioral development. J Stud Alcohol 60:293–305. 10.15288/jsa.1999.60.293 10.15288/jsa.1999.60.293 [DOI] [PubMed] [Google Scholar]
- Maes HH, Sullivan PF, Bulik CM et al (2004) A twin study of genetic and environmental influences on tobacco initiation, regular tobacco use and nicotine dependence. Psychol Med 34:1251–1261. 10.1017/S0033291704002405 10.1017/S0033291704002405 [DOI] [PubMed] [Google Scholar]
- McGue M, Elkins I, Iacono WG (2000) Genetic and environmental influences on adolescent substance use and abuse. Am J Med Genet 96:671–677. 10.1002/1096-8628(20001009)96:5%3C671::aid-ajmg14%3E3.0.co;2-w [DOI] [PubMed] [Google Scholar]
- McGue M, Irons D, Iacono WG (2014) The adolescent origins of substance use disorders: a behavioral genetic perspective. Nebr Symp Motiv 61:31–50. 10.1007/978-1-4939-0653-6_3 10.1007/978-1-4939-0653-6_3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nannini DR, Zheng Y, Joyce BT et al (2023) Genome-wide DNA methylation association study of recent and cumulative marijuana use in middle aged adults. Mol Psychiatry 1–11. 10.1038/s41380-023-02106-y [DOI] [PMC free article] [PubMed]
- Neale MC, Hunter MD, Pritikin JN et al (2016) OpenMx 2.0: extended structural equation and statistical modeling. Psychometrika 81:535–549. 10.1007/s11336-014-9435-8 10.1007/s11336-014-9435-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer RHC, Knopik VS, Rhee SH et al (2013) Prospective effects of adolescent indicators of behavioral disinhibition on DSM-IV alcohol, tobacco, and illicit drug dependence in young adulthood. Addict Behav 38:2415–2421. 10.1016/j.addbeh.2013.03.021 10.1016/j.addbeh.2013.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasman JA, Verweij KJH, Gerring Z et al (2018) GWAS of lifetime cannabis use reveals new risk loci, genetic overlap with psychiatric traits, and a causal influence of schizophrenia. Nat Neurosci 21:1161–1170. 10.1038/s41593-018-0206-1 10.1038/s41593-018-0206-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasman JA, Demange PA, Guloksuz S et al (2022) Genetic risk for smoking: disentangling interplay between genes and socioeconomic status. Behav Genet 52:92–107. 10.1007/s10519-021-10094-4 10.1007/s10519-021-10094-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reubi D, Berridge V (2016) The internationalisation of Tobacco Control, 1950–2010. Med Hist 60:453–472. 10.1017/mdh.2016.97 10.1017/mdh.2016.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revelle W (2023) psych: Procedures for Psychological, Psychometric, and Personality Research. https://CRAN.R-project.org/package=psych
- Rhee SH, Hewitt JK, Young SE et al (2003) Genetic and environmental influences on substance initiation, use, and problem use in adolescents. Arch Gen Psychiatry 60:1256–1264. 10.1001/archpsyc.60.12.1256 10.1001/archpsyc.60.12.1256 [DOI] [PubMed] [Google Scholar]
- Richmond-Rakerd LS, Slutske WS, Lynskey MT et al (2016) Age at first use and later substance use disorder: Shared genetic and environmental pathways for nicotine, alcohol, and cannabis. J Abnorm Psychol 125:946–959. 10.1037/abn0000191 10.1037/abn0000191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose RJ, Salvatore JE, Aaltonen S et al (2019) FinnTwin12 cohort: an updated review. Twin Res Hum Genet 22:302–311. 10.1017/thg.2019.83 10.1017/thg.2019.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders GRB, Wang X, Chen F et al (2022) Genetic diversity fuels gene discovery for tobacco and alcohol use. Nature 612:720–724. 10.1038/s41586-022-05477-4 10.1038/s41586-022-05477-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer JD, Jang S-K, Clark DA et al (2023) Associations between polygenic risk of substance use and use disorder and alcohol, cannabis, and nicotine use in adolescence and young adulthood in a longitudinal twin study. Psychol Med 53:2296–2306. 10.1017/S0033291721004116 10.1017/S0033291721004116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer GL, Rosenberry ZR, Peters EN (2017) Marijuana and tobacco co-administration in blunts, spliffs, and mulled cigarettes: a systematic literature review. Addict Behav 64:200–211. 10.1016/j.addbeh.2016.09.001 10.1016/j.addbeh.2016.09.001 [DOI] [PubMed] [Google Scholar]
- Smolkina M, Morley KI, Rijsdijk F et al (2017) Cannabis and Depression: a Twin Model Approach to Co-morbidity. Behav Genet 47:394–404. 10.1007/s10519-017-9848-0 10.1007/s10519-017-9848-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer S, Minică CC, Verweij KJH et al (2016) Genome-wide association study of lifetime cannabis use based on a large meta-analytic sample of 32 330 subjects from the International Cannabis Consortium. Transl Psychiatry 6:e769–e769. 10.1038/tp.2016.36 10.1038/tp.2016.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumbe A, Wilkinson AV, Clendennen SL et al (2022) Association of tobacco and marijuana use with symptoms of depression and anxiety among adolescents and young adults in Texas. Tob Prev Cessat 8:1–11. 10.18332/tpc/144500 10.18332/tpc/144500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timberlake DS, Laitinen U, Kinnunen JM, Rimpela AH (2020) Strategies and barriers to achieving the goal of Finland’s tobacco endgame. Tob Control 29:398–404. 10.1136/tobaccocontrol-2018-054779 10.1136/tobaccocontrol-2018-054779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treur JL, Taylor AE, Ware JJ et al (2017) Smoking and caffeine consumption: a genetic analysis of their association. Addict Biol 22:1090–1102. 10.1111/adb.12391 10.1111/adb.12391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker JS, Pedersen ER, Seelam R et al (2019) Types of cannabis and tobacco/nicotine co-use and associated outcomes in young adulthood. Psychol Addict Behav 33:401–411. 10.1037/adb0000464 10.1037/adb0000464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leeuwen AP, Verhulst FC, Reijneveld SA et al (2011) Can the gateway hypothesis, the common liability model and/or, the route of administration model predict initiation of cannabis use during adolescence? A survival analysis–the TRAILS study. J Adolesc Health off Publ Soc Adolesc Med 48:73–78. 10.1016/j.jadohealth.2010.05.008 10.1016/j.jadohealth.2010.05.008 [DOI] [PubMed] [Google Scholar]
- Vanyukov MM, Tarter RE, Kirillova GP et al (2012) Common liability to addiction and gateway hypothesis: theoretical, empirical and evolutionary perspective. Drug Alcohol Depend 123:S3. 10.1016/j.drugalcdep.2011.12.018 10.1016/j.drugalcdep.2011.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhulst B, Prom-Wormley E, Keller M et al (2019) Type I Error Rates and Parameter Bias in multivariate behavioral genetic models. Behav Genet 49:99–111. 10.1007/s10519-018-9942-y 10.1007/s10519-018-9942-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrieze SI, Hicks BM, Iacono WG, McGue M (2012) Decline in genetic influence on the co-occurrence of alcohol, marijuana, and nicotine dependence symptoms from age 14 to 29. Am J Psychiatry 169:1073–1081. 10.1176/appi.ajp.2012.11081268 10.1176/appi.ajp.2012.11081268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger AH, Delnevo CD, Wyka K et al (2020) Cannabis Use is Associated with increased risk of cigarette smoking initiation, persistence, and relapse among adults in the United States. Nicotine Tob Res 22:1404–1408. 10.1093/ntr/ntz085 10.1093/ntr/ntz085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer-, New York [Google Scholar]
- Wilson S, Haroian K, Iacono WG et al (2019) Minnesota Center for Twin and Family Research. Twin Res Hum Genet 22:746–752 10.1017/thg.2019.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2023) The Netherlands at the forefront of tobacco control. https://www.who.int/europe/news/item/31-07-2023-the-netherlands-at-the-forefront-of-tobacco-control. Accessed 27 Dec 2023
- Xu K, Li B, McGinnis KA et al (2020) Genome-wide association study of smoking trajectory and meta-analysis of smoking status in 842,000 individuals. Nat Commun 11:5302. 10.1038/s41467-020-18489-3 10.1038/s41467-020-18489-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SE, Stallings MC, Corley RP et al (2000) Behavioral disinhibition. Am J Med Genet 695:684–695 [DOI] [PubMed] [Google Scholar]
- Zellers S, Corley R, Thibodeau E et al (2020) Adolescent externalizing psychopathology and its prospective relationship to Marijuana Use Development from Age 14 to 30: Replication across Independent Longitudinal Twin Samples. Behav Genet 50:139–151. 10.1007/s10519-020-09994-8 10.1007/s10519-020-09994-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zellers S, Iacono WG, McGue M, Vrieze S (2022) Developmental and etiological patterns of substance use from adolescence to middle age: a longitudinal twin study. Drug Alcohol Depend 233:109378. 10.1016/j.drugalcdep.2022.109378 10.1016/j.drugalcdep.2022.109378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zellers S, Ross JM, Saunders GRB et al (2023) Impacts of recreational Cannabis legalization on Cannabis Use: a Longitudinal Discordant Twin Study. Addiction 118:110–118. 10.1111/add.16016 10.1111/add.16016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The research data used in this study are confidential and are not publicly available to protect participant privacy.