Abstract
Osteoanabolic-first treatment sequences are superior to oral bisphosphonates for fracture reduction and bone mineral density (BMD) gain. However, data comparing osteoanabolic medications, with the more potent antiresorptive, denosumab (DMAb), are limited. We analyzed FRAME and FRAME Extension data to assess BMD and fracture incidence in patients treated with romosozumab (Romo) followed by DMAb (Romo/DMAb) versus DMAb (DMAb/DMAb) for 24 months. In FRAME, women aged ≥55 years (total hip [TH] or femoral neck [FN] T-score: –2.5 to –3.5) were randomized to Romo or placebo for 12 months followed by DMAb for 12 months. In FRAME Extension, both cohorts received DMAb for another 12 months. This post hoc analysis compared BMD change and fracture incidence in patients on Romo/DMAb (months 0–24) versus DMAb/DMAb (months 12–36). Patient characteristics were balanced by propensity score weighting (PSW) and sensitivity analyses were conducted using PSW with multiple imputation (PSW-MI) and propensity score matching (PSM). Unmeasured confounding was addressed using E-values. After PSW, over 24 months, compared with DMAb/DMAb, treatment with Romo/DMAb produced significantly greater BMD increases at the lumbar spine [LS], TH, and FN (mean differences: 9.3%, 4.4%, and 4.1%, respectively; all p<0.001). At month 24, in women with a baseline T-score of –3.0, the probability of achieving a T-score > –2.5 was higher with Romo/DMAb versus DMAb/DMAb (LS: 92% versus 47%; TH: 50% versus 5%). In the Romo/DMAb versus DMAb/DMAb cohorts, new vertebral fractures were significantly reduced (0.62% versus 1.26% [odds ratio = 0.45; p=0.003]) and rates of clinical, nonvertebral, and hip fractures were lower (differences not significant). Similar BMD and fracture outcomes were observed with PSW-MI and PSM sensitivity analyses. The sequence of Romo/DMAb resulted in greater BMD gains and higher probability of achieving T-scores > –2.5, significantly reduced new vertebral fracture incidence, and numerically lowered the incidence (not significant) of clinical, nonvertebral, and hip fractures versus DMAb only through 24 months.
Keywords: anabolics, antiresorptives, clinical trials, menopause, osteoporosis
Introduction
Recent osteoporosis guidelines suggest that osteoanabolic agents be considered as initial therapy in patients at very high risk of fracture.1-4 These recommendations are based on studies indicating that initial treatment with osteoanabolic agents leads to faster and larger effects against fractures and greater gains in BMD than oral bisphosphonates alone.5-14 However, none of the controlled trials directly compared an osteoanabolic-first treatment sequence with the more potent antiresorptive agent, denosumab (DMAb).
Romosozumab (Romo)15 is a sclerostin inhibitor that exerts a dual effect on bone, increasing bone formation whilst decreasing bone resorption.16,17 In the FRAME clinical trial, patients received Romo or placebo for 12 months, followed by DMAb for 12 months; in the FRAME Extension, patients in both cohorts received DMAb for an additional 12 months.18,19 In FRAME, treatment with Romo for 12 months resulted in larger gains in BMD when compared with placebo, and these gains were further increased following transition to DMAb. Treatment with Romo versus placebo also reduced the relative risk of new vertebral (73%; p<0.001), clinical (36%; p=0.008), and nonvertebral (25%; p=0.096) fractures with continued fracture risk reductions of similar magnitude after transition to DMAb for 12 months.18 In FRAME Extension, fracture risk reductions were sustained in the cohort that initially received Romo versus placebo with a cumulative 36-month relative risk reduction of 66% for new vertebral, 27% for clinical, and 21% for nonvertebral fractures.19
In this post hoc analysis of FRAME and FRAME Extension, we compared the efficacy of Romo followed by DMAb with that of DMAb only over 24 months on BMD gains and fracture risk.
Methods
Patients and study design
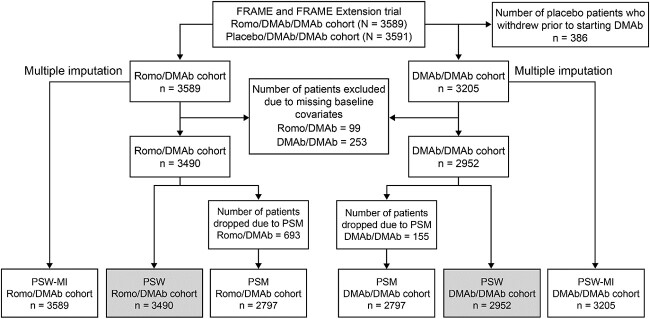
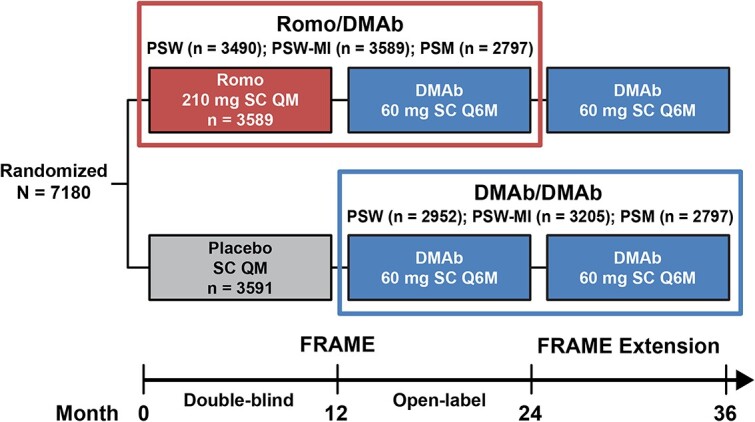
Participants enrolled in the FRAME (NCT01575834) and FRAME Extension trials were included in this analysis; both studies have been described in detail previously.18,19 In FRAME, women aged 55-90 years with a T-score of –2.5 to –3.5 at total hip (TH) or femoral neck (FN) were randomized to receive blinded Romo 210 mg s.c. once monthly (QM) or placebo s.c. QM for 12 months, after which women in both cohorts received open-label DMAb 60 mg s.c. once every 6 months (Q6M) for 12 months. In FRAME Extension, patients received DMAb 60 mg s.c. Q6M for an additional 12 months. The current post hoc analyses focused on women who received Romo for 12 months followed by DMAb for 12 months in FRAME (Romo/DMAb cohort; months 0–24; Figure 1) and those who received DMAb for 12 months in FRAME followed by DMAb for 12 months in the FRAME Extension trial (DMAb/DMAb cohort; months 12–36; Figure 1).
Figure 1.
FRAME/FRAME extension study design. N = number of patients randomized in the study; n = number of patients in each study cohort. Patients included in the Romo/DMAb cohort received Romo for 12 months followed by DMAb for 12 months in FRAME (n = 3490 after PSW, n = 3589 after PSW-MI, and n = 2797 after PSM out of 3589 from the parent FRAME trial). Patients included in the DMAb/DMAb cohort received DMAb for 12 months in FRAME and for 12 months in the FRAME extension trial (n = 2952 after PSW, n = 3205 after PSW-MI, and n = 2797 after PSM out of 3205 from the parent FRAME trial). Abbreviations: DMAb = denosumab; MI = multiple imputation; PSM = propensity score matching; PSW = propensity score weighting; Q6M = every 6 months; QM = monthly; Romo = romosozumab.
Outcome measures
The study analyzed the percentage changes in BMD over 24 months (from 0 to 24) for the Romo/DMAb cohort and (from 12 to 36) for the DMAb/DMAb cohort. The least-squares mean group differences in the percentage change over 24 months between the two cohorts were calculated. Probabilities of achieving a T-score >–2.5 at the lumbar spine (LS) and TH over the 24-month treatment sequence in patients with baseline T-scores of –3.5, –3.0, and –2.7 were determined. These same baseline threshold T-score cut-points were previously used to assess probabilities of attaining target BMD levels with Romo/alendronate compared with alendronate only.20 The incidence of new vertebral, nonvertebral, clinical, and hip fractures during the 24 months of treatment was calculated in both cohorts.
Statistical analysis
Primary PSW analysis
Because patients in the DMAb/DMAb cohort started active treatment 12 months after those in the Romo/DMAb cohort, baseline characteristics differed slightly (baseline = month 0 for Romo/DMAb; baseline = month 12 for DMAb/DMAb). To address these post-randomization factors and avoid the effect of confounding and bias in this comparative analysis, we used propensity score weighting (PSW) to balance the patient characteristics. PSW with complete case analysis was chosen as the primary analytic approach because it allows the evaluation of all patients with available baseline measurements.
A propensity score (PS) was calculated by logistic regression with available baseline covariates including age, BMI, BMD T-score at multiple sites (LS, TH, FN), several key laboratory tests (serum calcium corrected by albumin, serum phosphorous, estimated glomerular filtration rate), demographic variables (ethnicity, race, geographical region), lifestyle risk factors (current smoking, alcohol use ≥3 drinks per day), fracture history (prevalent vertebral fracture, any historical fracture at age ≥ 45 years), prior use of osteoporosis medication, and parental history of hip fracture. Any individual vertebrae that were missing or not readable on baseline radiographs or any uncertainty in parental hip fracture history were also included as covariates. All baseline covariates in the DMAb/DMAb cohort were based on month 12 data, except lifestyle risk factors and parental history of hip fracture, which were based on month 0 data, and accounted for any laboratory changes, DXA changes, concomitant medicine usages, and fracture events during the 12-month placebo period before starting DMAb.
For the PSW procedure, we estimated the average treatment effect on the treated (ATT) of all treatment effects21 with weights 1 for patients in the Romo/DMAb cohort and weights PS/(1 – PS) for patients in the DMAb/DMAb cohort. We evaluated the balance of covariates before and after weighting using standardized mean difference (SMD)22 and estimated the ATT in each matched dataset. An SMD of >0.1 (>10%) between treatment groups denotes meaningful imbalance in the baseline covariate.23
Based on the PSW cohorts, percentage change from baseline in BMD was assessed in patients who had a baseline measurement and ≥1 postbaseline measurement at month 24 in the Romo/DMAb cohort and at month 36 in the DMAb/DMAb cohort. Last observation carried forward (LOCF) was used for patients who did not have BMD measurements at these endpoints but had ≥1 postbaseline measurement. The mean difference (95% confidence interval [CI]) in BMD percentage change from baseline for the Romo/DMAb cohort versus DMAb/DMAb cohort was analyzed using weighted linear regression with adjustment for machine type. The probability of achieving a TH or LS T-score > –2.5 at month 24 was estimated based on a weighted logistic regression model, adjusted for baseline LS or TH BMD T-score within each treatment cohort. The incidence of new vertebral fractures over 24 months was calculated using radiographs at month 24 for the Romo/DMAb cohort and at month 36 for the DMAb/DMAb cohort (or LOCF for patients with post-treatment radiographs before these endpoints who were missing radiographs at the 24- and 36-month endpoints). The treatment effect on new vertebral fractures was estimated using weighted logistic regression and the treatment effects of other fractures were estimated using weighted Cox proportional hazard model.
For patients with missing values of outcomes, we used all the available data to carry out imputation. These data included month-12 and month-24 DXA for all patients; month-6 and month-18 DXA for patients in imaging sub-study24; and month-6, month-12, month-18, and month-24 lumbar X-ray for all patients.
Sensitivity analyses
Sensitivity analyses were performed to evaluate the robustness of our findings and to handle missing data. First, we used a multiple imputation approach for the PSW (PSW-MI) analysis to ensure that there was no confounding bias due to missing baseline data. This approach addressed missing data for baseline covariates and outcomes. Missing values were imputed using fully conditional specification (FCS) method result in 40 complete datasets. The imputation model included all baseline covariates mentioned in the logistic regression model for PS, and selected outcomes such as new vertebral fracture at month 12 and month 24; BMD values at LS, TH, and FN at baseline, month 12 and month 24; and BMD T-scores at LS, TH, and FN at month 24. For each dataset, we calculated separate PS and performed separate PSW analyses. The results from 40 complete sets were combined, and valid inferences for the parameters of interest were derived.
Second, we used propensity score matching (PSM), where subject-to-subject comparisons are simple and intuitive, but some patients are excluded because no match can be found, and sample size is therefore restricted to a subpopulation. For the PSM analysis, we matched the Romo/DMAb and DMAb/DMAb cohorts (1:1) using greedy nearest neighbor matching without replacement and a caliper of width equal to 0.25 of the standard deviation of the logit of the PS.25 The mean differences (95% CI) in BMD percentage change from baseline for the Romo/DMAb cohort versus DMAb/DMAb cohort were analyzed using a generalized estimating equation with adjustment for machine type. The probability of achieving a TH or LS T-score > –2.5 at month 24 was estimated based on a logistic regression model, adjusted for baseline LS or TH BMD T-score within each treatment cohort. For group differences in the incidence of new vertebral fractures, odds ratios with 95% CI were determined by conditional logistic regression.26 For hip, clinical, and nonvertebral fractures, treatment cohorts were compared using a Cox proportional hazard model stratified on the matched pairs.27 LOCF was used to impute any missing BMD or fracture incidence values in patients who had ≥1 postbaseline measurement.
Third, to assess how a potential unmeasured or uncontrolled confounding was associated with treatment and outcomes, we calculated the “E-value”,28 defined as the minimum strength of association, on the risk ratio scale, between an unmeasured confounder and both the treatment and outcome to fully explain a specific treatment–outcome association, conditional on the measured covariates.
For all analyses, a significance level of 0.05 (two-sided) without multiplicity adjustment was determined. All statistical analyses were carried out using SAS statistical software version 9.4 (SAS Institute).
Results
Patient disposition and baseline characteristics
Figure 2 shows the derivation of patients included in these analyses. In FRAME, 3589 and 3591 women were randomized to the Romo/DMAb and placebo/DMAb arms, respectively. Within the placebo/DMAb arm, 386 women withdrew during the year on placebo, leaving 3205 participants in the DMAb/DMAb cohort. After excluding patients who were missing baseline data (Romo/DMAb, 99 patients and DMAb/DMAb, 253 patients), the numbers of patients in the Romo/DMAb and DMAb/DMAb cohorts were 3490 and 2952, respectively. All were included in the PSW analysis.
Figure 2.
Patient disposition. The flowchart shows the number of patients included in each cohort from the FRAME and FRAME Extension trials after primary PSW and sensitivity analyses using PSW-MI and PSM. Abbreviations: DMAb = denosumab; MI = multiple imputation; PSM = propensity score matching; PSW = propensity score weighting; Romo = romosozumab.
Table 1 shows the baseline covariates before and after balancing with PSW. The cohorts were fairly comparable before PSW with some imbalances observed in a few covariates including serum phosphorus level, prior use of osteoporosis medication, and prevalent vertebral fracture. All covariates were balanced after PSW. The SMD of PS between the two cohorts before balancing was 0.36, which was reduced to –0.01 after PSW (Supplemental Figure S1).
Table 1.
Baseline characteristics before and after PSW.
| Before balancing | After PSW | |||||
|---|---|---|---|---|---|---|
| Romo/DMAb (N = 3490) |
DMAb/DMAb (N = 2952) |
Standardized mean differencea | Romo/DMAb (N = 3490) |
DMAb/DMAb (N = 2952) |
Standardized mean differencea | |
| Mean ± SD | ||||||
| Propensity score | 0.56 ± 0.08 | 0.52 ± 0.10 | 0.362 | 0.56 ± 0.08 | 0.56 ± 0.09 | −0.009 |
| Age (years) | 70.90 ± 7.02 | 71.61 ± 6.82 | −0.103 | 70.90 ± 7.02 | 70.96 ± 7.32 | −0.009 |
| BMI (kg/m2) | 24.69 ± 4.31 | 24.75 ± 4.42 | −0.013 | 24.69 ± 4.31 | 24.70 ± 4.80 | −0.002 |
| BMD T-score | ||||||
| Lumbar spine | −2.72 ± 1.04 | −2.70 ± 1.07 | −0.021 | −2.72 ± 1.04 | −2.73 ± 1.16 | 0.001 |
| Total hip | −2.48 ± 0.47 | −2.45 ± 0.49 | −0.074 | −2.48 ± 0.47 | −2.49 ± 0.52 | 0.012 |
| Femoral neck | −2.76 ± 0.28 | −2.73 ± 0.32 | −0.098 | −2.76 ± 0.28 | −2.77 ± 0.35 | 0.018 |
| Serum calcium (corrected) (mmol/L) | 2.43 ± 0.10 | 2.44 ± 0.10 | −0.089 | 2.43 ± 0.10 | 2.43 ± 0.10 | <0.001 |
| Serum phosphorus (mmol/L) | 1.22 ± 0.15 | 1.20 ± 0.15 | 0.124 | 1.22 ± 0.15 | 1.22 ± 0.16 | −0.004 |
| Estimated glomerular filtration rate (mL/min/1.73 m2) | 72.08 ± 16.73 | 71.22 ± 16.41 | 0.052 | 72.08 ± 16.73 | 72.00 ± 17.96 | 0.005 |
| n (%) | ||||||
| Ethnicity (Hispanic or Latino) | 1402 (40.17) | 1204 (40.79) | −0.013 | 1402 (40.17) | 1181 (40.02) | 0.003 |
| Race (White) | 2000 (57.31) | 1625 (55.05) | −0.046 | 2000 (57.31) | 1701 (57.62) | 0.006 |
| Geographical region | 0.077 | <0.001 | ||||
| Asia Pacific | 391 (11.20) | 366 (12.40) | 391 (11.20) | 325 (11.02) | ||
| Central and Eastern Europe | 1018 (29.17) | 816 (27.64) | 1018 (29.17) | 867 (29.36) | ||
| Central/Latin America | 1524 (43.67) | 1303 (44.14) | 1524 (43.67) | 1286 (43.55) | ||
| North America | 96 (2.75) | 68 (2.30) | 96 (2.75) | 86 (2.91) | ||
| Western Europe and Australia/New Zealand | 461 (13.21) | 399 (13.52) | 461 (13.21) | 389 (13.17) | ||
| Currently smoking | 354 (10.14) | 312 (10.57) | 0.014 | 354 (10.14) | 296 (10.02) | −0.004 |
| Alcohol use (≥3 drinks per day) | 33 (0.95) | 30 (1.02) | 0.007 | 33 (0.95) | 29 (0.98) | 0.004 |
| Prevalent vertebral fracture | 0.255 | <0.001 | ||||
| Yes | 655 (18.77) | 553 (18.73) | 655 (18.77) | 554 (18.78) | ||
| No | 2728 (78.17) | 2147 (72.73) | 2728 (78.17) | 2307 (78.14) | ||
| Missing/not readableb | 107 (3.07) | 252 (8.54) | 107 (3.07) | 91 (3.08) | ||
| Any historical fracture at age ≥ 45 | 1403 (40.20) | 1234 (41.80) | 0.033 | 1403 (40.20) | 1193 (40.43) | 0.005 |
| Any prior use of osteoporosis medication | 230 (6.59) | 281 (9.52) | 0.108 | 230 (6.59) | 195 (6.59) | <0.001 |
| Parental hip fracture | 0.033 | <0.001 | ||||
| Yes | 381 (10.92) | 313 (10.60) | 381 (10.92) | 326 (11.04) | ||
| No | 2874 (82.35) | 2444 (82.79) | 2874 (82.35) | 2428 (82.25) | ||
| Uncertain | 235 (6.73) | 195 (6.61) | 235 (6.73) | 198 (6.71) | ||
Abbreviations: DMAb = denosumab; PSW = propensity score weighting; Romo = romosozumab.
Standardized mean difference refers to the difference of baseline characteristics between the cohorts.
Not readable was defined as at least one vertebra with missing Genant grade between T4 and L4 and all remaining vertebrae with Genant grade of 0.
PSW analysis
BMD change between Romo/DMAb and DMAb/DMAb cohorts
The least-squares mean percentage change at 24 months in BMD was higher with Romo/DMAb than with DMAb/DMAb at the LS (16.8% versus 7.5%; mean difference: 9.3%), TH (8.4% versus 4.0%; mean difference: 4.4%), and FN (7.6% versus 3.5%; mean difference: 4.1%). The difference between the cohorts was significant at all skeletal sites (p<0.001; Figure 3A). At 24 months, a small proportion of patients (Romo/DMAb, 0.2% versus DMAb/DMAb, 6.0%) had missing BMD values at each skeletal site, which were imputed by LOCF (Supplemental Table S1).
Figure 3.
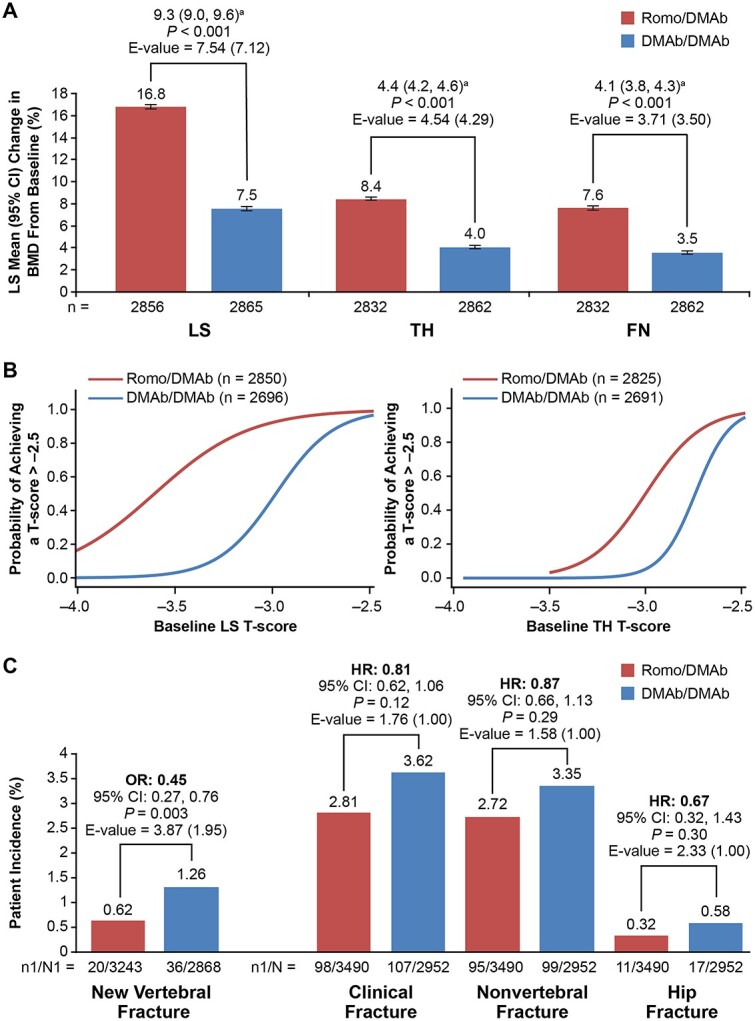
Comparison of BMD gains, probabilities of achieving T-score > –2.5, and patient incidence of fracture between Romo/DMAb and DMAb/DMAb cohorts after PSW. (A) Percentage change in BMD from baseline during the 24-month treatment period. n = number of patients with evaluable data at the time point of interest. aMean difference (95% CI) in percentage BMD change between Romo/DMAb versus DMAb/DMAb. (B) Probabilities of achieving LS and TH T-scores of >–2.5 at month 24. n = number of patients with evaluable BMD data at baseline and month 24. The results were based on observed data; no imputation method was used to handle missing outcomes. (C) Patient incidence of fracture during the 24-month period. n1 = number of patients with a fracture; N1 = number of patients in the PSW subset who had a baseline and ≥1 postbaseline evaluation of vertebral fracture at or before month 24 (Romo/DMAb cohort) or month 36 (DMAb/DMAb cohort); N = number of patients included in the PSM analysis. Abbreviations: DMAb = denosumab; FN = femoral neck; HR = hazard ratio; LS = lumbar spine; OR = odds ratio; PSW = propensity score weighting; Romo = romosozumab; TH = total hip.
Probabilities of achieving T-scores > –2.5
At baseline, the percentage of patients who had an LS or TH T-score < –3.5, <–3.0, and <–2.7 were similar between the two cohorts (Table 2). Over 24 months, the probabilities of achieving LS and TH T-scores > –2.5 were higher in the Romo/DMAb versus DMAb/DMAb cohorts in patients at all starting cut-points, with baseline threshold T-scores of –3.5 (LS: 60.6% versus 3.1%; TH: 3.2% versus 0%), –3.0 (LS: 92.3% versus 47.2%; TH: 49.6% versus 5.2%), and –2.7 (LS: 97.6% versus 86.9%; TH: 88.3% versus 60.8%) (Figure 3B and Table 3).
Table 2.
Proportion of patients with baseline T-scores < −2.7, <−3.0, and <−3.5 before and after PSW.
| Before balancing | After PSW | |||
|---|---|---|---|---|
| Romo/DMAb (N = 3490) |
DMAb/DMAb (N = 2952) |
Romo/DMAb (N = 3490) |
DMAb/DMAb (N = 2952) |
|
| BMD T-score, n (%) | ||||
| Lumbar spine | ||||
| <−2.7 | 1893 (54.24) | 1557 (52.74) | 1893 (54.24) | 1593 (53.96) |
| <−3.0 | 1472 (42.18) | 1224 (41.46) | 1472 (42.18) | 1257 (42.58) |
| <−3.5 | 798 (22.87) | 689 (23.34) | 798 (22.87) | 720 (24.39) |
| Total hip | ||||
| <−2.7 | 1127 (32.29) | 899 (30.45) | 1127 (32.29) | 991 (33.57) |
| <−3.0 | 433 (12.41) | 340 (11.52) | 433 (12.41) | 400 (13.55) |
| <−3.5 | 0 (0.00) | 22 (0.75) | 0 (0.00) | 29 (0.98) |
Abbreviations: DMAb = denosumab; PSW = propensity score weighting; Romo = romosozumab.
Table 3.
Probabilities of achieving a T-score>–2.5 at month 24 with Romo/DMAb or DMAb/DMAb treatment sequence after PSW.
| After PSW | ||
|---|---|---|
| Romo/ DMAb (N = 3490) |
DMAb/ DMAb (N = 2952) |
|
| Lumbar spine, n | 2850 | 2696 |
| Baseline T-score, Pt Esta (95% CI) | ||
| –3.5 | 0.606 (0.573, 0.638) | 0.031 (0.022, 0.042) |
| –3.0 | 0.923 (0.906, 0.937) | 0.472 (0.436, 0.509) |
| –2.7 | 0.976 (0.968, 0.982) | 0.869 (0.843, 0.892) |
| Total hip, n | 2825 | 2691 |
| Baseline T-score, Pt Esta (95% CI) | ||
| –3.5 | 0.032 (0.021, 0.047) | 0.000 (0.000, 0.000) |
| –3.0 | 0.496 (0.457, 0.535) | 0.052 (0.040, 0.069) |
| –2.7 | 0.883 (0.864, 0.900) | 0.608 (0.575, 0.640) |
Abbreviations: DMAb = denosumab; PSW = propensity score weighting; Romo = romosozumab.
Predicted values based on weighted logistic regression model adjusted for baseline BMD T-score. N = number of patients; n = number of patients with evaluable lumbar spine or total hip BMD value at baseline and month 24. No imputation method was used to handle missing outcomes for complete case analysis.
Incidence of fractures in Romo/DMAb and DMAb/DMAb cohorts
The 24-month incidence of new vertebral fractures was lower in the Romo/DMAb cohort than in the DMAb/DMAb cohort (0.62% versus 1.26% [odds ratio = 0.45]; p=0.003). The incidence rates of clinical fractures, nonvertebral fractures, and hip fractures were numerically but not significantly lower in the Romo/DMAb cohort than in the DMAb/DMAb cohort (Figure 3C). At month 24, 5.7% and 0.4% patients in the Romo/DMAb and DMAb/DMAb group, respectively, had missing values for new vertebral fracture, which were imputed by LOCF (Supplemental Table S1).
Sensitivity analyses
PSW-MI and PSM analyses
The PSW-MI analysis was based on 3589 patients in the Romo/DMAb cohort and 3205 patients in the DMAb/DMAb cohort. All baseline covariates were balanced after PSW-MI analysis, and the SMD of PS between the two cohorts was reduced from 0.36 to –0.004 (Supplemental Table S2). After the PSM procedure, 2797 patients in each cohort were available for the sensitivity analysis. After PSM, most of the baseline covariates were balanced except serum phosphorus, BMD T-score for TH and FN, and geographical region (Supplemental Table S3); the SMD of PS between the two cohorts was reduced from 0.36 to 0.23 (Supplemental Table S3; Supplemental Figure S1).
After balancing the treatment cohorts, results for BMD increments, probabilities of achieving non-osteoporotic T-scores, and fracture incidence were similar to those observed with the primary PSW analysis (Supplemental Tables S4 and S5; Supplemental Figures S2 and S3).
E-values
We found significant BMD gains at all skeletal sites in the Romo/DMAb cohort as compared with DMAb/DMAb cohort. The E-values for this association (E-value for the upper limit of the Cl) at LS, TH, and FN were 7.54 (7.12), 4.54 (4.29), and 3.71 (3.50), respectively (Figure 3A), which indicated that the likelihood of an unmeasured or unknown confounder having an effect on BMD gains was very small. Similar effects were observed for new vertebral fracture (E-value, 3.87 [1.95]) (Figure 3C). E-values calculated for the BMD and fracture outcomes using sensitivity analyses also demonstrated similar effects (Supplemental Figures S2 and S3).
Discussion
In this post hoc analysis, we compared the efficacy of a 24-month sequence of Romo followed by DMAb versus 24 months of DMAb treatment in the FRAME and FRAME Extension populations. The treatment sequence of Romo followed by DMAb resulted in BMD gains that were more than twice as large at the LS, TH, and FN than those associated with 24 months of DMAb only. Romo followed by DMAb reduced the incidence of new vertebral fractures by approximately 50% compared with DMAb for 24 months. Results from the primary analysis using PSW were corroborated with sensitivity analyses using PSW-MI and PSM. Recent analyses from the FNIH/SABRE project show that TH BMD gain with osteoporosis treatment is an excellent surrogate for antifracture efficacy.29,30 Furthermore, achieving a TH BMD level on or after treatment that is at least above the osteoporosis range is linked to a reduced subsequent risk of fractures.20,31 Our results add to the growing body of literature showing superiority of osteoanabolic or osteoanabolic-first treatment sequences compared with even the most potent antiresorptive treatment and provide additional rationale for the evolving role of osteoanabolic therapy in osteoporosis management.5-7,9-13,32-34 Our findings also lend further support to the recent guidelines from the American Association of Clinical Endocrinologists (AACE), Endocrine Society, and The North American Menopause Society (NAMS) to consider osteoanabolic treatment as initial therapy in patients with very high fracture risk.1-3,35
The probability of achieving T-scores (>–2.5) after 24 months of treatment was also substantially higher at all starting BMD levels for the regimen beginning with Romo followed by DMAb versus DMAb only.20,36 A similar result was seen in post hoc analyses from the ARCH study, which showed that the probability of achieving LS and TH T-scores above –2.5 after 3 years with alendronate alone is much lower compared with the Romo followed by alendronate treatment sequence.20 These results indicate that for patients with very low starting BMD (T-score < –2.8 at the TH or <–3.0 at the LS), achieving BMD levels above osteoporosis range within a short period of time is much more likely when treatment begins with Romo compared with either alendronate or DMAb alone.20,36
While the incidence of nonvertebral and hip fractures was numerically lower in patients treated with Romo/DMAb versus DMAb/DMAb, the group differences were not statistically significant. This study had ample sample size to see a significant benefit against new vertebral fracture but was inadequate to see significant effects against other fracture types. This is due in part to the low osteoporosis severity level in FRAME where only a small proportion of patients had prevalent vertebral fracture (18%) or prior non-vertebral fracture history (22%).18 Furthermore, although all patients in this study ostensibly had a T-score < –2.5, it is possible that some of the patients, particularly those from the Latin American region, might have had higher T-scores if a country-specific reference population had been used to calculate T-scores, rather than the NHANES reference population.37 These challenges were faced in the full FRAME and FRAME Extension trials where an unexpectedly low background nonvertebral fracture rate was seen in the Latin American cohort.18 Based on the observed background fracture rates in the overall FRAME population, it is estimated that a sample size of over 12 000 and 21 000 patients would have been required to have enough power to see a treatment effect of Romo/DMAb versus DMAb/DMAb against nonvertebral fracture and hip fracture, respectively, in this relatively low risk population. A separate analysis focusing on the subset of postmenopausal women from FRAME who had no prior fractures but were otherwise at very high fracture risk showed that treatment with Romo/DMAb significantly increased BMD and reduced vertebral, clinical, and nonvertebral fracture risk compared with placebo/DMAb.38 Findings from the FNIH/SABRE study have suggested minimum treatment versus placebo BMD gains thought to be associated with a treatment effect against nonvertebral and hip fractures.30 In our study, the mean 4.4% group difference in TH BMD gain exceeds the surrogate threshold BMD gains associated with reductions in both nonvertebral and hip fracture (2.1% and 3.2% TH BMD gains, respectively).30 Based on these considerations, we would expect that Romo/DMAb treatment would reduce fracture risk at these sites compared with 24 months of DMAb in a population with severe osteoporosis or a study of larger sample size.
Limitations of this study included its post hoc design, the reduced sample size compared with the full FRAME population, and different baseline characteristics in the two cohorts, based on the 1-year difference between the start of active treatment in the two cohorts. One potential confounding factor not included in this analysis was the recency of previous fractures. While radiographic fractures one year prior to baseline were captured in the DMAb/DMAb cohort (while they were on placebo), radiographic vertebral fractures were not routinely captured in the Romo/DMAb cohort. Because of the imbalanced and incomplete capture of recent fracture data, we were not able to include recency of fracture as a covariate. Although the effect of unmeasured confounding cannot be ruled out, the potential impact was likely small, since many confounders were included in the analysis and evidence from our analysis of E-values confirmed its minimal effect.
Strengths of this study included that the cohorts were derived from a large randomized controlled trial with well-defined patients and baseline characteristics. Several statistical techniques (PSW and PSM) were used to provide well balanced cohorts with absolute SMDs <0.1 for almost all variables as recommended,39 and these produced BMD and fracture outcomes that were very similar. PSW-MI also produced similar outcomes, adding more robustness to our findings. In addition, E-values observed in our analysis suggest that considerable unmeasured confounding would be required to explain an effect estimate indicating minimal residual bias in our results.
In conclusion, this study is the first to compare the effects of romosozumab followed by denosumab versus denosumab therapy alone on fracture risk and BMD over a 24-month period. Our results suggest that initiating treatment with Romo followed by DMAb leads to fracture resistance, larger mean BMD gain and a greater likelihood of achieving BMD levels above the osteoporosis range compared with DMAb only. These data provide additional evidence regarding the optimal approach for managing osteoporosis in those at highest risk.
Supplementary Material
Acknowledgments
The authors thank Cynthia Deignan, PhD (Amgen) for her critical review of the data. Lisa Humphries, PhD (Amgen) and Varsha Jain, PhD (Cactus Life Sciences on behalf of Amgen) provided medical writing support.
Contributor Information
Felicia Cosman, Department of Medicine, Columbia University College of Physicians and Surgeons, New York, NY 10032, United States.
Mary Oates, Amgen Inc., Thousand Oaks, CA 91230, United States.
Donald Betah, Amgen Inc., Thousand Oaks, CA 91230, United States.
Jen Timoshanko, UCB Pharma, Slough SL1 3WE, United Kingdom.
Zhenxun Wang, Amgen Inc., Thousand Oaks, CA 91230, United States.
Serge Ferrari, Division of Bone Diseases, Geneva University Hospitals and Faculty of Medicine, University of Geneva, CH-1211 Geneva, Switzerland.
Michael R McClung, Oregon Osteoporosis Center, Portland, OR 97225, United States.
Author contributions
Felicia Cosman (Conceptualization, Methodology, Writing—original draft, Writing—review & editing), Mary Oates (Conceptualization, Methodology, Writing—original draft, Writing—review & editing), Donald Betah (Conceptualization, Methodology, Writing—original draft, Writing—review & editing), Jen Timoshanko (Writing—review & editing), Zhenxun Wang (Formal analysis, Writing—review & editing), Serge Ferrari (Writing—review & editing), and Michael R. McClung (Writing—review & editing)
Funding
Amgen Inc. and UCB Pharma sponsored this study.
Conflicts of interest
FC has received grants/research support from Amgen and Radius Health; received consulting fees from Amgen, Biocon, EnteraBio, Radius Health, and UCB Pharma; served as a consultant for Myovant and Pfizer; and served on speakers’ bureaus for Amgen and Radius Health.MO, DB, and ZW are employees and stockholders of Amgen. JT is an employee and stockholder of UCB Pharma. SF has received grants and research support from Amgen, UCB Pharma, AgNovos, Alexion, and Labatec and consulting fees from Amgen, UCB Pharma, AgNovos, Flowbone, Fresenius, Galapagos, and Radius Health.MRM has received consulting fees from Amgen, Alexion, and Radius Health and honoraria from Amgen, Alexion, and UCB Pharma.
Data availability
Qualified researchers may request data from Amgen clinical studies. Complete details are available at the following: https://wwwext.amgen.com/science/clinical-trials/clinical-datatransparency-practices/clinical-trial-data-sharing-request/.
References
- 1. Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists/American College of Endocrinology Clinical Practice guidelines for the diagnosis and treatment of postmenopausal psteoporosis-2020 update. Endocr Pract. 2020;26(Suppl 1):1–46. 10.4158/GL-2020-0524SUPPL [DOI] [PubMed] [Google Scholar]
- 2. Shoback D, Rosen CJ, Black DM, Cheung AM, Murad MH, Eastell R. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society guideline update. J Clin Endocrinol Metab. 2020;105(3):587–594. 10.1210/clinem/dgaa048 [DOI] [PubMed] [Google Scholar]
- 3. Management of osteoporosis in postmenopausal women: the 2021 position statement of the North American Menopause Society. Menopause. 2021;28(9):973–997. 10.1097/GME.0000000000001831 [DOI] [PubMed] [Google Scholar]
- 4. LeBoff MS, Greenspan SL, Insogna KL, et al. The clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int. 2022;33(10):2049–2102. 10.1007/s00198-021-05900-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kendler DL, Marin F, Zerbini CAF, et al. Effects of teriparatide and risedronate on new fractures in post-menopausal women with severe osteoporosis (VERO): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet. 2018;391(10117):230–240. 10.1016/S0140-6736(17)32137-2 [DOI] [PubMed] [Google Scholar]
- 6. Saag KG, Petersen J, Brandi ML, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017;377(15):1417–1427. 10.1056/NEJMoa1708322 [DOI] [PubMed] [Google Scholar]
- 7. Leder BZ, Mitlak B, Hu MY, Hattersley G, Bockman RS. Effect of abaloparatide vs alendronate on fracture risk reduction in postmenopausal women with osteoporosis. J Clin Endocrinol Metab. 2020;105(3):938–943. 10.1210/clinem/dgz162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cosman F. Anabolic therapy and optimal treatment sequences for patients with osteoporosis at high risk for fracture. Endocr Pract. 2020;26(7):777–786. 10.4158/EP-2019-0596 [DOI] [PubMed] [Google Scholar]
- 9. Rittmaster RS, Bolognese M, Ettinger MP, et al. Enhancement of bone mass in osteoporotic women with parathyroid hormone followed by alendronate. J Clin Endocrinol Metab. 2000;85(6):2129–2134 [DOI] [PubMed] [Google Scholar]
- 10. Black DM, Bilezikian JP, Ensrud KE, et al. One year of alendronate after one year of parathyroid hormone (1-84) for osteoporosis. N Engl J Med. 2005;353(6):555–565. 10.1056/NEJMoa050336 [DOI] [PubMed] [Google Scholar]
- 11. Eastell R, Nickelsen T, Marin F, et al. Sequential treatment of severe postmenopausal osteoporosis after teriparatide: final results of the randomized, controlled European Study of Forsteo (EUROFORS). J Bone Miner Res. 2009;24(4):726–736. 10.1359/jbmr.081215 [DOI] [PubMed] [Google Scholar]
- 12. Cosman F, Miller PD, Williams GC, et al. Eighteen months of treatment with subcutaneous abaloparatide followed by 6 months of treatment with alendronate in postmenopausal women with osteoporosis: results of the ACTIVExtend trial. Mayo Clin Proc. 2017;92(2):200–210. 10.1016/j.mayocp.2016.10.009 [DOI] [PubMed] [Google Scholar]
- 13. Bone HG, Cosman F, Miller PD, et al. ACTIVExtend: 24 months of alendronate after 18 months of abaloparatide or placebo for postmenopausal osteoporosis. J Clin Endocrinol Metab. 2018;103(8):2949–2957. 10.1210/jc.2018-00163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cosman F, Kendler DL, Langdahl BL, et al. Romosozumab and antiresorptive treatment: the importance of treatment sequence. Osteoporos Int. 2022;33(6):1243–1256. 10.1007/s00198-021-06174-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. EVENITY® (Romosozumab-Aqqg) US Prescribing Information. Thousand Oaks, CA, USA: Amgen Inc.; 2019: https://www.pi.amgen.com/∼/media/amgen/repositorysites/pi-amgen-com/evenity/evenity_pi_hcp_english.ashx. Accessed 19 Sept 2022. [Google Scholar]
- 16. McClung MR, Grauer A, Boonen S, et al. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med. 2014;370(5):412–420. 10.1056/NEJMoa1305224 [DOI] [PubMed] [Google Scholar]
- 17. Padhi D, Jang G, Stouch B, Fang L, Posvar E. Single-dose, placebo-controlled, randomized study of AMG 785, a sclerostin monoclonal antibody. J Bone Miner Res. 2011;26(1):19–26. 10.1002/jbmr.173 [DOI] [PubMed] [Google Scholar]
- 18. Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375(16):1532–1543. 10.1056/NEJMoa1607948 [DOI] [PubMed] [Google Scholar]
- 19. Lewiecki EM, Dinavahi RV, Lazaretti-Castro M, et al. One year of romosozumab followed by two years of denosumab maintains fracture risk reductions: results of the FRAME extension study. J Bone Miner Res. 2019;34(3):419–428. 10.1002/jbmr.3622 [DOI] [PubMed] [Google Scholar]
- 20. Cosman F, Libanati C, Deignan C, et al. Romosozumab followed by antiresorptive treatment increases the probability of achieving bone mineral density treatment goals. JBMR Plus. 2021;5(11):e10546. 10.1002/jbm4.10546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Austin PC. Variance estimation when using inverse probability of treatment weighting (IPTW) with survival analysis. Stat Med. 2016;35(30):5642–5655. 10.1002/sim.7084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang D, Dalton JE. A unified approach to measuring the effect size between two groups using SAS®. Orlando, FL: SAS Institute Inc., SAS Global Forum, 2012;Paper 335:1–6. https://support.sas.com/resources/papers/proceedings12/335-2012.pdf [Google Scholar]
- 23. Normand ST, Landrum MB, Guadagnoli E, et al. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54(4):387–398. 10.1016/S0895-4356(00)00321-8 [DOI] [PubMed] [Google Scholar]
- 24. Cosman F, Crittenden DB, Ferrari S, et al. FRAME study: the foundation effect of building bone with 1 year of romosozumab leads to continued lower fracture risk after transition to denosumab. J Bone Miner Res. 2018;33(7):1219–1226. 10.1002/jbmr.3427 [DOI] [PubMed] [Google Scholar]
- 25. Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985;39(1):33–38. 10.1080/00031305.1985.10479383 [DOI] [Google Scholar]
- 26. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424. 10.1080/00273171.2011.568786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Austin PC. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med. 2014;33(7):1242–1258. 10.1002/sim.5984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167(4):268–274. 10.7326/M16-2607 [DOI] [PubMed] [Google Scholar]
- 29. Black DM, Bauer DC, Vittinghoff E, et al. Treatment-related changes in bone mineral density as a surrogate biomarker for fracture risk reduction: meta-regression analyses of individual patient data from multiple randomised controlled trials. Lancet Diabetes Endocrinol. 2020;8(8):672–682. 10.1016/S2213-8587(20)30159-5 [DOI] [PubMed] [Google Scholar]
- 30. Eastell R, Vittinghoff E, Lui LY, et al. Validation of the surrogate threshold effect for change in bone mineral density as a surrogate endpoint for fracture outcomes: the FNIH-ASBMR SABRE project. J Bone Miner Res. 2022;37(1):29–35. 10.1002/jbmr.4433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cummings SR, Cosman F, Eastell R, Reid IR, Mehta M, Lewiecki EM. Goal-directed treatment of osteoporosis. J Bone Miner Res. 2013;28(3):433–438. 10.1002/jbmr.1854 [DOI] [PubMed] [Google Scholar]
- 32. Saag KG, Shane E, Boonen S, et al. Teriparatide or alendronate in glucocorticoid-induced osteoporosis. N Engl J Med. 2007;357(20):2028–2039. 10.1056/NEJMoa071408 [DOI] [PubMed] [Google Scholar]
- 33. Hadji P, Zanchetta JR, Russo L, et al. The effect of teriparatide compared with risedronate on reduction of back pain in postmenopausal women with osteoporotic vertebral fractures. Osteoporos Int. 2012;23(8):2141–2150. 10.1007/s00198-011-1856-y [DOI] [PubMed] [Google Scholar]
- 34. Händel MN, Cardoso I, von Bülow C, et al. Fracture risk reduction and safety by osteoporosis treatment compared with placebo or active comparator in postmenopausal women: systematic review, network meta-analysis, and meta-regression analysis of randomised clinical trials. BMJ. 2023;381:e068033. 10.1136/bmj-2021-068033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kanis JA, Harvey NC, McCloskey E, et al. Algorithm for the management of patients at low, high and very high risk of osteoporotic fractures. Osteoporos Int. 2020;31(1):1–12. 10.1007/s00198-019-05176-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cosman F, Huang S, Chines A, Cummings SR. Probability of achieving bone mineral density treatment goals with denosumab treatment in women with osteoporosis. 2022 Annual Meeting of the American Society for Bone and Mineral Research. Austin, Texas, USA. September 09–12, 2022. J Bone Miner Res. 2023;38(S1):S1–S364-FRI-580. [Google Scholar]
- 37. Cosman F, Crittenden DB, Ferrari S, et al. Romosozumab FRAME study: a post hoc analysis of the role of regional background fracture risk on nonvertebral fracture outcome. J Bone Miner Res. 2018;33(8):1407–1416. 10.1002/jbmr.3439 [DOI] [PubMed] [Google Scholar]
- 38. McClung MR, Betah D, Deignan C, et al. Romosozumab efficacy in postmenopausal women without prior fracture who fulfill AACE criteria for osteoanabolic therapy: post-hoc analysis of clinical trial data. Annual Meeting of the American Society for Bone and Mineral Research, Austin, Texas, USA. September 9–12, 2022. J Bone Miner Res. 2023;38(S1):S1-S364–1076. [Google Scholar]
- 39. Rubin DB. Using propensity scores to help design observational studies: application to the tobacco litigation. Health Serv Outcome Res Methodol. 2001;2(3/4):169–188. 10.1023/A:1020363010465 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Qualified researchers may request data from Amgen clinical studies. Complete details are available at the following: https://wwwext.amgen.com/science/clinical-trials/clinical-datatransparency-practices/clinical-trial-data-sharing-request/.