Abstract
The brain contains the highest concentration of cholesterol in the human body, which emphasizes the importance of cholesterol in brain physiology. Cholesterol is involved in neurogenesis and synaptogenesis, and age-related reductions in cholesterol levels can lead to synaptic loss and impaired synaptic plasticity, which potentially contribute to neurodegeneration. The maintenance of cholesterol homeostasis in the neuronal plasma membrane is essential for normal brain function, and imbalances in cholesterol distribution are associated with various neurodegenerative disorders, including Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease. This review aims to explore the molecular and pathological mechanisms by which cholesterol imbalance can lead to neurotransmission defects and neurodegeneration, focusing on four key mechanisms: (1) synaptic dysfunction, (2) alterations in membrane structure and protein clustering, (3) oligomers of amyloid beta (Aβ) protein, and (4) α-synuclein aggregation.
Subject terms: Synaptic vesicle exocytosis, Neurodegeneration
Exploring Cholesterol Imbalance: Link to Neurodegenerative Diseases
Cholesterol, a substance crucial for the brain, can lead to diseases like Alzheimer’s and Parkinson’s when imbalanced. This review investigates how this imbalance causes brain cell degeneration, focusing on issues like communication breakdown and harmful protein build-up. The study combines findings from different experiments to understand cholesterol’s role in the brain. The review emphasizes the need for cholesterol balance for brain health and identifies potential treatment targets for neurodegenerative diseases. The main findings suggest that cholesterol imbalance disrupts brain cell communication and leads to harmful protein build-up, causing brain cell degeneration. The researchers conclude that focusing on cholesterol metabolism and distribution could lead to new treatments for these conditions. Future research may lead to treatments that correct cholesterol imbalances, possibly slowing or preventing neurodegenerative diseases.
This summary was initially drafted using artificial intelligence, then revised and fact-checked by the author.
Introduction
Cholesterol is a lipid that is critical for the structure and function of cell membranes in the brain, where it participates in neuronal signaling and synaptic transmission. The brain contains the highest concentration of cholesterol in the human body, accounting for 20–25% of the total cholesterol1,2. The blood–brain barrier (BBB) is impermeable to peripheral cholesterol3; therefore, most cholesterol in the brain is generated by de novo synthesis, mainly in the glia and to a lesser extent in neurons3. The high cholesterol concentration suggests an important role for cholesterol in brain physiology.
Cholesterol is involved in neurogenesis and synaptogenesis4,5. Age-related reductions in cholesterol levels in the plasma membrane lead to synaptic loss6,7 and impaired synaptic plasticity8, suggesting that cholesterol imbalance in the neuronal plasma membrane affects neuronal activity and contributes to neuronal degeneration9,10. The maintenance of cholesterol homeostasis is essential for normal brain functions11–13. An imbalance in cholesterol distribution can cause the pathological changes observed in various neurodegenerative diseases, such as Alzheimer’s disease (AD), Parkinson’s disease (PD), and Huntington’s disease (HD), suggesting that neurodegenerative diseases are associated with dysregulated cholesterol distribution11,12,14.
The aim of this review is to examine the molecular and pathological mechanisms by which cholesterol imbalance causes neurotransmission defects. This review focuses on four molecular mechanisms to explain how cholesterol imbalance in neurons results in neurodegeneration: (1) synaptic dysfunction, (2) membrane structure and protein clustering, (3) amyloid beta (Aβ) aggregation, and (4) α-synuclein (α-syn) aggregation.
Main Text
The molecular mechanisms of neurodegeneration induced by cholesterol imbalance
Cholesterol has a complex and multifaceted role in neurodegeneration. Given that cholesterol is an essential component of cell membranes and is involved in various physiological processes in the brain, an imbalance and dysregulation of cholesterol homeostasis can contribute to the pathogenesis of neurodegenerative diseases11,12,14. Table 1 summarizes the links between cholesterol and different neurodegenerative diseases. Several mechanisms have been proposed to explain how cholesterol imbalance may contribute to neurodegeneration:
-
Synaptic dysfunction: cholesterol is critical for the formation and function of synapses, the connections between neurons that facilitate communication in the brain. Altered cholesterol levels can affect synaptic transmission and plasticity, impairing neuronal signaling and contributing to the cognitive deficits observed in neurodegenerative diseases. The plasma membrane is enriched with cholesterol, ~80% of which is cellular cholesterol15. Therefore, reduced cholesterol levels in the plasma membrane, i.e., cholesterol imbalance, impairs synaptic transmission and plasticity and thus induces neurodegeneration9,10,16.
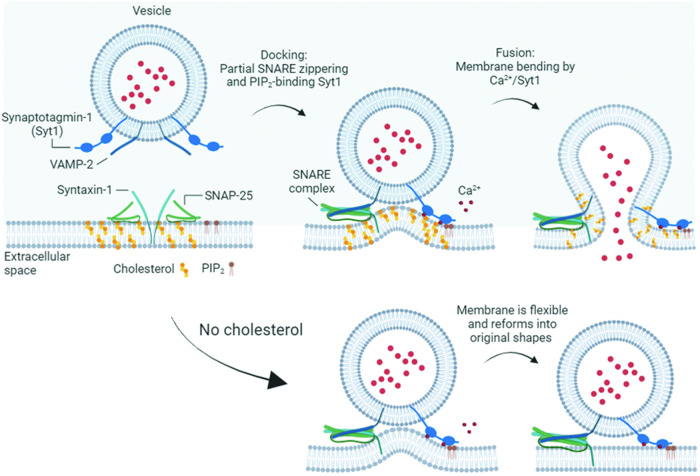
Depletion and imbalance of cholesterol in the plasma membrane cause deficits in neurotransmission; e.g., cholesterol depletion reduces Ca2+-dependent exocytosis of large dense-core vesicles (LDCVs)17, cortical secretory vesicles18, and synaptic vesicles in hippocampal neurons9,19, cortical synaptosomes20, ribbon synapses21, and motor nerve terminals22. However, unveiling the molecular pathology of cholesterol imbalance in neurodegeneration is challenging because cholesterol is involved in various cellular signaling processes and neuronal functions. The reconstitution system of vesicle fusion with purified native vesicles, including LDCVs and synaptic vesicles, can be a good model for elucidating the molecular mechanisms by which cholesterol deficiency affects vesicle fusion23 (Fig. 1).
This reconstitution of vesicle fusion shows that cholesterol has little effect on Ca2+-independent basal fusion of synaptic vesicles but is required for Ca2+-dependent fusion of LDCVs and synaptic vesicles23. It is surprising that cholesterol reduction and imbalance specifically disrupt Ca2+-dependent vesicle fusion. Cholesterol has no effect on the membrane binding or insertion of synaptotagmin-1, a Ca2+ sensor for vesicle fusion23. Once synaptotagmin-1 is inserted into the membrane, cholesterol stabilizes and strengthens the local membrane bending and deformation induced by synaptotagmin-123. The membrane is highly flexible, so it can reform into its original shape due to membrane elasticity24. Because cholesterol reduces membrane fluidity and increases membrane rigidity25, cholesterol can strengthen local membrane deformation and bending, thus lowering the energy barrier for Ca2+-dependent fusion23 (Fig. 1).
Membrane bending and curvature play crucial roles in the process of vesicle fusion by lowering the energy barrier26. The energy stored in the curvature of the membrane can be released to facilitate the merging of two separate lipid bilayers26–28. For instance, smaller vesicles, which have a greater curvature, have greater bending energy per unit surface area, leading to a more efficient fusion process26. The insertion of proteins such as the C2AB domain of synaptotagmin-1 into the plasma membrane contributes to this curvature29,30, creating a high-energy state that can drive vesicle fusion.
Cholesterol enhances membrane curvature and thus lowers the energy barrier for fusion18. It also strengthens local bending and deformation, particularly in the presence of Ca2+ and synaptotagmin-1, thereby driving Ca2+-dependent vesicle fusion23. Mechanical forces of membrane bending are critical for the dynamic process of vesicle fusion31, and cholesterol is an essential lipid for synaptic transmission because it strengthens membrane bending23.
Synaptic transmission involves the release of neurotransmitters from synaptic vesicles into synapses, where neurotransmitters bind to receptors on postsynaptic neurons, thereby transmitting signals across the neural network32. When synaptic transmission is impaired due to disruptions in Ca2+-dependent vesicle fusion, neural network formation becomes impaired33. Disruption of Ca2+-dependent vesicle fusion and synaptic transmission via a cholesterol imbalance in the plasma membrane leads to reduced neural network activity and synaptic dysfunction and ultimately contributes to neurodegeneration. The loss of the neural network caused by the dysregulation of cholesterol homeostasis can result in declines in cognitive and motor functions associated with neurodegenerative diseases.
-
Membrane structure and protein clustering: cholesterol regulates membrane structure, fluidity, and curvature25. The plasma membrane is enriched in cholesterol15, which stabilizes membrane curvature and promotes vesicle fusion17,25,34,35. The membrane curvature and deformation stabilized by cholesterol bring the two membranes close together and enable fusion. Cholesterol also contributes to vesicle fusion by stabilizing fusion pores25,36–38. Therefore, cholesterol deficiency in neurons causes defects in membrane structure, resulting in neurodegeneration.
Cholesterol also mediates the protein clustering involved in vesicle fusion. Exocytosis of neurotransmitter release is mediated by soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins39,40. Neuronal SNARE proteins consist of Q-SNARE in the plasma membrane (syntaxin-1 and SNAP-25) and R-SNARE in the vesicle membrane (synaptobrevin-2 or vesicle-associated membrane protein-2 (VAMP-2))39. Specialized microdomains within the plasma membrane, e.g., lipid rafts, detergent-resistant membranes, or liquid-ordered membrane microdomains, are enriched in cholesterol and concentrate signaling molecules41–43. Cholesterol plays an important role in the function and organization of SNARE proteins; syntaxin-1A, a neuronal SNARE protein, is concentrated in cholesterol-enriched domains in the plasma membrane44,45. The cholesterol-enriched membrane microdomains provide a specialized environment where syntaxin-1A interacts with its binding partners. In the context of synaptic transmission, the clustering of syntaxin-1A in cholesterol-enriched microdomains may enhance its interactions with other SNARE proteins, such as SNAP-25 and VAMP-2, to form the core SNARE complex necessary for vesicle fusion. This organization may influence the overall stability and efficiency of neurotransmitter release at the synapse, suggesting that cholesterol imbalance leads to defects in the clustering of the vesicle fusion machinery.
-
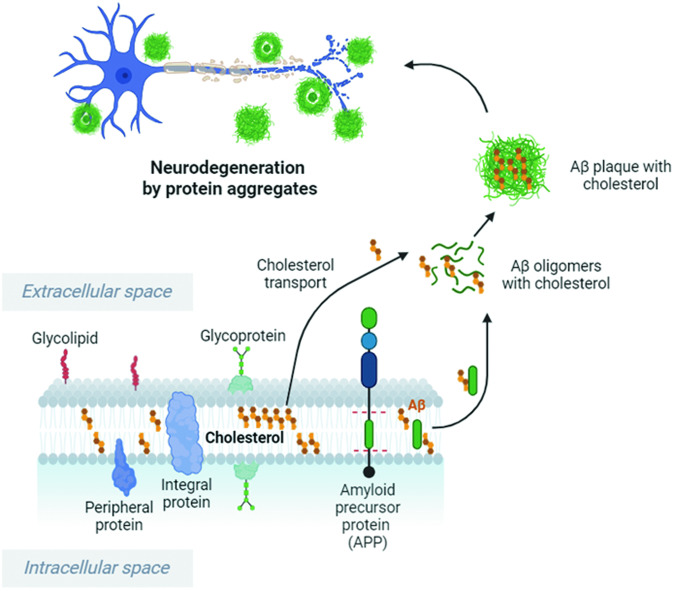
Oligomers of amyloid beta (Aβ) protein: cholesterol imbalance influences the aggregation and misfolding of proteins involved in neurodegenerative diseases, such as amyloid precursor protein (APP)46. The accumulation of Aβ plaques in the brain is a hallmark pathology of AD47,48. Aβ is derived from APP through enzymatic cleavage by the β-secretase Bace149, and cholesterol modulates the processing of APP and the generation of Aβ50,51. High cholesterol levels can promote and accelerate the cleavage of APP by Bace1, resulting in increased Aβ production and aggregation, which contributes to the formation of toxic plaques50–52.
Aβ40/Aβ42 peptides are the primary constituents of Aβ oligomers and plaques, which can be stabilized by biomolecules, including carbohydrates, nucleic acids, and lipids, e.g., cholesterol53. Extracellular cholesterol strengthens Aβ fibrils and oligomers against degradation by directly binding to Aβ53,54. Cholesterol has been implicated in the aggregation of Aβ peptides, particularly in the formation of Aβ oligomers55–57. Free cholesterol interacts with specific residues in Aβ peptides, particularly Phe1955. Cholesterol binds to the aromatic side chains of the Aβ peptide, thus increasing β-sheet formation in Aβ peptide oligomers55. A stable interaction between cholesterol and Phe19 leads to the formation of Aβ oligomers55, suggesting that the interaction of cholesterol with Aβ contributes to the formation of toxic Aβ oligomers, which play a critical role in AD pathology57.
Cholesterol dramatically enhances and accelerates the onset of Aβ42 aggregation through a heterogeneous nucleation pathway58. Cholesterol imbalance and elevated extracellular levels of cholesterol can promote the production and accumulation of Aβ peptides, which induce the formation of Aβ oligomers in the brain, thus contributing to neuronal damage and cognitive decline57 (Fig. 2). Aβ monomers misfold and form β-sheet-rich oligomers that eventually impair synaptic plasticity and neuronal survival59. The direct interaction of cholesterol with Aβ can stimulate and activate toxic Aβ oligomerization, which is a critical factor in AD pathogenesis.
The membrane curvature induced by cholesterol might contribute to Aβ aggregation60. High membrane curvature promotes Aβ nucleation, accelerating amyloid fibril formation61. Aβ aggregates can readily form on membranes with high curvature62. High membrane curvature, such as that associated with lipid rafts and membrane budding processes, might provide favorable conditions for the nucleation of Aβ peptides61–63. Given that cholesterol stabilizes high membrane curvature, cholesterol-mediated curvature of membranes may lead to altered lipid packing that engages Aβ hydrophobic groups and promotes Aβ fibrillar structures64. Lipid packing defects that occur in curved membranes may induce conformational changes in Aβ peptides, thus promoting Aβ fibrils and aggregation64. This aggregation is a hallmark of AD, and understanding the underlying mechanisms of membrane curvature is crucial for developing potential therapeutic strategies. The interplay between membrane curvature and Aβ aggregation is complex, and ongoing research continues to unveil the molecular details involved.
-
Tau aggregation: while Aβ oligomers and aggregation are primarily associated with AD, another hallmark of AD is the aggregation of hyperphosphorylated tau proteins into neurofibrillary tangles (NFTs)65. Tau is a cytoskeletal protein that stabilizes microtubules in neurons, but it is hyperphosphorylated in AD66. The interaction between tau proteins and cell membranes, particularly in cholesterol-rich regions, is a critical factor in the tau aggregation process67,68. The membrane binding of tau can induce conformational changes and aggregation with β-sheet-rich structures69. NFTs in AD brains contain cholesterol54,70, which could modulate tau-membrane interactions and affect tau aggregation68,71.
As cholesterol can influence membrane curvature, high membrane curvature caused by cholesterol can induce changes in the conformation of tau proteins and promote tau aggregation72. Membranes with high curvature that contain cholesterol induce tau fibril formation, whereas cholesterol depletion abolishes tau fibril formation72. Cholesterol-free membranes fail to induce the formation of tau fibrils, suggesting that cholesterol-mediated tau aggregation is essential for the pathology of tauopathies72. Membrane morphologies result in different hydrophobic interactions that lead to the β-sheet structures of tau proteins72. The association of tau with highly curved membranes is initiated by electrostatic attraction between the Lys sidechains of tau and the lipid headgroups; cholesterol might further strengthen this electrostatic attraction for tau fibril formation72. However, how cholesterol facilitates tau nucleation remains a topic of further study, and understanding the underlying molecular mechanisms is crucial for developing therapeutic strategies involving the disruption of tau aggregation.
α-Synuclein (α-syn) aggregation: a characteristic feature of PD is the accumulation of misfolded α-syn proteins in Lewy bodies (LBs)73. The interaction between α-syn and lipids is important for fibril formation, and the aggregation of α-syn is induced by binding to membrane lipids74. Lipids dramatically enhance the primary nucleation of α-syn to form aggregates associated with neurodegeneration74.
Table 1.
Cholesterol and neurodegenerative diseases.
| Neurodegenerative disease | Relation to cholesterol | Refs. |
|---|---|---|
| Alzheimer’s Disease | High cholesterol promotes the cleavage of APP by Bace1, resulting in Aβ production and aggregation. | 50–52 |
| Cholesterol is the primary constituent of Aβ oligomers and plaques. | 53 | |
| Cholesterol strengthens Aβ fibrils and oligomers against degradation by directly binding to Aβ. | 53,54 | |
| Cholesterol leads to the formation of Aβ oligomers through interaction with Phe19 of Aβ, thus stabilizing Aβ oligomers. | 55–57 | |
| Membrane curvature induced by cholesterol accelerates Aβ aggregation. | 60–64 | |
| ApoE4, a major risk factor for AD, is associated with dysregulation of cholesterol transport. | 46 | |
| Cholesterol stimulates tau aggregation process through tau conformational changes into β-sheet-rich structures. | 54,67–71 | |
| Cholesterol-containing membranes with high curvature induce tau fibril formation. | 72 | |
| Parkinson’s Disease | Cholesterol is a component of LBs together with α-syn. | 75 |
| Cholesterol accelerates α-syn aggregation and LB formation. | 76 | |
| Cholesterol induces toxic α-syn oligomers and fibrils by forming β-sheet structures. | 78 | |
| Huntington’s Disease | Mutant huntingtin (mHTT) aggregates in HD and reduces nuclear translocation of the sterol regulatory element-binding protein 2 (SREBP2). | 85 |
| Cholesterol modulates mHTT aggregation by regulating membrane interactions. | 86,87 | |
| Multiple Sclerosis (MS) | Disrupted cholesterol metabolism is associated with MS, and cholesterol induces misfolded protein aggregation. | 88 |
Fig. 1. Schematic illustration of the roles of cholesterol in Ca2+-dependent vesicle fusion.
Cholesterol is essential for Ca2+-dependent vesicle fusion. Synaptotagmin-1, a Ca2+ sensor that triggers fusion, induces local deformation of the plasma membrane. The plasma membrane is normally flexible and can return to its original shape due to membrane elasticity. However, cholesterol makes the membrane less fluid and more rigid, which helps to strengthen the membrane curvature and deformation, thus lowering the energy barrier for fusion. This image was created with BioRender.com.
Fig. 2. Schematic overview of cholesterol transport to promote Aβ aggregation for neurodegeneration.
Cholesterol enhances and accelerates APP cleavage by Bace1, leading to increased Aβ oligomer and plaque formation. Cholesterol binds to Aβ and increases the resistance of Aβ fibrils and oligomers to degradation. Cholesterol imbalance and high extracellular cholesterol levels can stimulate the production and accumulation of Aβ peptides, which cause Aβ oligomer formation and aggregation in the brain, resulting in neuronal damage. This image was created with BioRender.com.
Together with α-syn, cholesterol, which is a component of LBs75, accelerates α-syn aggregation and LB formation76. Cholesterol interacts with α-syn, and high cholesterol levels can promote α-syn aggregation76,77, leading to the formation of toxic LBs, which contributes to neurodegeneration in PD.
α-Syn binds to membranes through electrostatic interactions and hydrogen bonding, but cholesterol reduces the coulomb interactions and hydrophobic interactions between α-syn and membranes78. Cholesterol decreases lipid packing defects and lipid fluidity, thereby dysregulating the membrane binding of α-syn78; membrane-bound α-syn can have a β-sheet structure that induces the formation of toxic α-syn oligomers and fibrils. Together, the imbalance and dysregulated distribution of cholesterol in neurons cause neurodegeneration by accelerating α-syn aggregation and LB formation.
Possible therapeutic approaches for cholesterol imbalance
The apolipoprotein E (ApoE) gene is involved in the metabolism and transport of cholesterol79,80. There are three main variants or alleles of the APOE gene, namely, ApoE2, ApoE3, and ApoE481. The ApoE4 protein is the most important risk factor for late-onset AD82, i.e., the most common form of the disease that occurs after age 65. The molecular mechanisms by which ApoE4 contributes to AD are complex and not fully understood, but ApoE4 likely promotes Aβ aggregation by transporting cholesterol79,83. ApoE4 can induce cholesterol imbalance by transporting cholesterol from the plasma membrane in neurons to protein aggregates (Fig. 2). Given that cholesterol imbalance causes neurodegeneration, ApoE4 may be a possible target for mitigating Aβ aggregation and treating cholesterol imbalance.
Overall, the maintenance of cholesterol homeostasis is important for preventing or slowing the pathogenesis of neurodegenerative diseases. Strategies aimed at regulating cholesterol levels or targeting cholesterol-mediated pathways involved in protein aggregation might be potential therapeutic approaches to treating neurodegenerative diseases, although neurodegenerative diseases are complex and cholesterol imbalance is one of many contributing factors11,84.
Conclusion
Cholesterol is an essential component of the body that helps maintain the integrity of cell membranes. The role of cholesterol dysregulation in neurodegeneration is an active area of research, and the precise mechanisms involved may vary depending on the specific condition. The balance between the beneficial and detrimental effects of cholesterol remains complex. An imbalance in cholesterol regulation is a common feature of neurodegenerative conditions such as AD and PD. Therapeutic approaches for modulating cholesterol metabolism or targeting specific cholesterol-related pathways could be potential strategies for mitigating neurodegeneration. While cholesterol-lowering drugs, e.g., statins, have shown some potential in reducing the risk of certain neurodegenerative diseases, further research is required to fully understand the role of cholesterol and develop targeted therapeutic interventions.
Acknowledgements
This work was supported by grants from the Qatar Biomedical Research Institute (Project Number SF 2019 004 and IGP5-2022-001 to Y.P.) and the HBKU Thematic Research Grant (Project Number VPR-TG02-06 to Y.P.).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dietschy, J. M. & Turley, S. D. Cholesterol metabolism in the brain. Curr. Opin. Lipidol.12, 105–112 (2001). [DOI] [PubMed] [Google Scholar]
- 2.Bjorkhem, I. & Meaney, S. Brain cholesterol: long secret life behind a barrier. Arterioscler. Thromb. Vasc. Biol.24, 806–815 (2004). [DOI] [PubMed] [Google Scholar]
- 3.Dietschy, J. M. & Turley, S. D. Thematic review series: brain lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J. Lipid Res.45, 1375–1397 (2004). [DOI] [PubMed] [Google Scholar]
- 4.Pfrieger, F. W. Cholesterol homeostasis and function in neurons of the central nervous system. Cell. Mol. Life Sci.60, 1158–1171 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cartocci, V., Servadio, M., Trezza, V. & Pallottini, V. Can cholesterol metabolism modulation affect brain function and behavior? J. Cell Physiol.232, 281–286 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Svennerholm, L., Bostrom, K., Jungbjer, B. & Olsson, L. Membrane lipids of adult human brain: lipid composition of frontal and temporal lobe in subjects of age 20 to 100 years. J. Neurochem.63, 1802–1811 (1994). [DOI] [PubMed] [Google Scholar]
- 7.Martin, M., Dotti, C. G. & Ledesma, M. D. Brain cholesterol in normal and pathological aging. Biochim. Biophys. Acta1801, 934–944 (2010). [DOI] [PubMed] [Google Scholar]
- 8.Martin, M. G. et al. Constitutive hippocampal cholesterol loss underlies poor cognition in old rodents. EMBO Mol. Med.6, 902–917 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Linetti, A. et al. Cholesterol reduction impairs exocytosis of synaptic vesicles. J. Cell Sci.123, 595–605 (2010). [DOI] [PubMed] [Google Scholar]
- 10.Liu, Q. et al. Neuronal LRP1 knockout in adult mice leads to impaired brain lipid metabolism and progressive, age-dependent synapse loss and neurodegeneration. J. Neurosci.30, 17068–17078 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vance, J. E. Dysregulation of cholesterol balance in the brain: contribution to neurodegenerative diseases. Dis. Model Mech.5, 746–755 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dai, L. et al. Cholesterol metabolism in neurodegenerative diseases: molecular mechanisms and therapeutic targets. Mol. Neurobiol.58, 2183–2201 (2021). [DOI] [PubMed] [Google Scholar]
- 13.Yoon, J. H. et al. Brain lipidomics: from functional landscape to clinical significance. Sci. Adv.8, eadc9317 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varma, V. R. et al. Abnormal brain cholesterol homeostasis in Alzheimer’s disease-a targeted metabolomic and transcriptomic study. NPJ Aging Mech. Dis.7, 11 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lange, Y. & Steck, T. L. Active membrane cholesterol as a physiological effector. Chem. Phys. Lipids199, 74–93 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Koudinov, A. R. & Koudinova, N. V. Essential role for cholesterol in synaptic plasticity and neuronal degeneration. FASEB J.15, 1858–1860 (2001). [DOI] [PubMed] [Google Scholar]
- 17.Zhang, J., Xue, R., Ong, W. Y. & Chen, P. Roles of cholesterol in vesicle fusion and motion. Biophys. J.97, 1371–1380 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Churchward, M. A., Rogasevskaia, T., Hofgen, J., Bau, J. & Coorssen, J. R. Cholesterol facilitates the native mechanism of Ca2+-triggered membrane fusion. J. Cell Sci.118, 4833–4848 (2005). [DOI] [PubMed] [Google Scholar]
- 19.Mailman, T., Hariharan, M. & Karten, B. Inhibition of neuronal cholesterol biosynthesis with lovastatin leads to impaired synaptic vesicle release even in the presence of lipoproteins or geranylgeraniol. J. Neurochem.119, 1002–1015 (2011). [DOI] [PubMed] [Google Scholar]
- 20.Teixeira, G., Vieira, L. B., Gomez, M. V. & Guatimosim, C. Cholesterol as a key player in the balance of evoked and spontaneous glutamate release in rat brain cortical synaptosomes. Neurochem. Int.61, 1151–1159 (2012). [DOI] [PubMed] [Google Scholar]
- 21.Mercer, A. J., Szalewski, R. J., Jackman, S. L., Van Hook, M. J. & Thoreson, W. B. Regulation of presynaptic strength by controlling Ca2+ channel mobility: effects of cholesterol depletion on release at the cone ribbon synapse. J. Neurophysiol.107, 3468–3478 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tarakanova, O. I., Petrov, A. M. & Zefirov, A. L. The role of membrane cholesterol in neurotransmitter release from motor nerve terminals. Dokl. Biol. Sci.: Proc. Acad. Sci. USSR, Biol. Sci. Sect.438, 138–140 (2011). [DOI] [PubMed] [Google Scholar]
- 23.Ali Moussa, H. Y. et al. Requirement of cholesterol for calcium-dependent vesicle fusion by strengthening synaptotagmin-1-induced membrane bending. Adv. Sci.10, e2206823 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lipowsky, R. Remodeling of membrane shape and topology by curvature elasticity and membrane tension. Adv. Biol.6, e2101020 (2022). [DOI] [PubMed] [Google Scholar]
- 25.Yang, S. T., Kreutzberger, A. J. B., Lee, J., Kiessling, V. & Tamm, L. K. The role of cholesterol in membrane fusion. Chem. Phys. Lipids199, 136–143 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang, Z. & Jackson, M. B. Membrane bending energy and fusion pore kinetics in Ca(2+)-triggered exocytosis. Biophys. J.98, 2524–2534 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martens, S., Kozlov, M. M. & McMahon, H. T. How synaptotagmin promotes membrane fusion. Science316, 1205–1208 (2007). [DOI] [PubMed] [Google Scholar]
- 28.Hui, E., Johnson, C. P., Yao, J., Dunning, F. M. & Chapman, E. R. Synaptotagmin-mediated bending of the target membrane is a critical step in Ca(2+)-regulated fusion. Cell138, 709–721 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herrick, D. Z., Sterbling, S., Rasch, K. A., Hinderliter, A. & Cafiso, D. S. Position of synaptotagmin I at the membrane interface: cooperative interactions of tandem C2 domains. Biochemistry45, 9668–9674 (2006). [DOI] [PubMed] [Google Scholar]
- 30.Ali Moussa, H. Y. & Park, Y. Electrostatic regulation of the cis- and trans-membrane interactions of synaptotagmin-1. Sci. Rep.12, 22407 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McMahon, H. T., Kozlov, M. M. & Martens, S. Membrane curvature in synaptic vesicle fusion and beyond. Cell140, 601–605 (2010). [DOI] [PubMed] [Google Scholar]
- 32.Sudhof, T. C. Calcium control of neurotransmitter release. Cold Spring Harb. Perspect. Biol.4, a011353 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Melland, H., Arvell, E. H. & Gordon, S. L. Disorders of synaptic vesicle fusion machinery. J. Neurochem.157, 130–164 (2021). [DOI] [PubMed] [Google Scholar]
- 34.Chernomordik, L., Kozlov, M. M. & Zimmerberg, J. Lipids in biological membrane fusion. J. Membr. Biol.146, 1–14 (1995). [DOI] [PubMed] [Google Scholar]
- 35.Chen, Z. & Rand, R. P. The influence of cholesterol on phospholipid membrane curvature and bending elasticity. Biophys. J.73, 267–276 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ivankin, A., Kuzmenko, I. & Gidalevitz, D. Cholesterol mediates membrane curvature during fusion events. Phys. Rev. Lett.108, 238103 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kreutzberger, A. J., Kiessling, V. & Tamm, L. K. High cholesterol obviates a prolonged hemifusion intermediate in fast SNARE-mediated membrane fusion. Biophys. J.109, 319–329 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu, L., Courtney, K. C. & Chapman, E. R. Cholesterol stabilizes recombinant exocytic fusion pores by altering membrane bending rigidity. Biophys. J.120, 1367–1377 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jahn, R. & Scheller, R. H. SNAREs-engines for membrane fusion. Nat. Rev. Mol. Cell Biol.7, 631–643 (2006). [DOI] [PubMed] [Google Scholar]
- 40.Brunger, A. T., Choi, U. B., Lai, Y., Leitz, J. & Zhou, Q. Molecular mechanisms of fast neurotransmitter release. Annu Rev. Biophys.47, 469–497 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laude, A. J. & Prior, I. A. Plasma membrane microdomains: organization, function and trafficking. Mol. Membr. Biol.21, 193–205 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Willmann, R. et al. Cholesterol and lipid microdomains stabilize the postsynapse at the neuromuscular junction. EMBO J.25, 4050–4060 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Korade, Z. & Kenworthy, A. K. Lipid rafts, cholesterol, and the brain. Neuropharmacology55, 1265–1273 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murray, D. H. & Tamm, L. K. Clustering of syntaxin-1A in model membranes is modulated by phosphatidylinositol 4,5-bisphosphate and cholesterol. Biochemistry48, 4617–4625 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sieber, J. J. et al. Anatomy and dynamics of a supramolecular membrane protein cluster. Science317, 1072–1076 (2007). [DOI] [PubMed] [Google Scholar]
- 46.Feringa, F. M. & van der Kant, R. Cholesterol and Alzheimer’s disease; from risk genes to pathological effects. Front. Aging Neurosci.13, 690372 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hampel, H. et al. The amyloid-beta pathway in Alzheimer’s disease. Mol. Psychiatry26, 5481–5503 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jack, C. R. Jr et al. 11 C PiB and structural MRI provide complementary information in imaging of Alzheimer’s disease and amnestic mild cognitive impairment. Brain131, 665–680 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao, J., Liu, X., Xia, W., Zhang, Y. & Wang, C. Targeting amyloidogenic processing of APP in Alzheimer’s disease. Front. Mol. Neurosci.13, 137 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Capitini, C. et al. APP and Bace1: differential effect of cholesterol enrichment on processing and plasma membrane mobility. iScience26, 106611 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cordy, J. M., Hussain, I., Dingwall, C., Hooper, N. M. & Turner, A. J. Exclusively targeting beta-secretase to lipid rafts by GPI-anchor addition up-regulates beta-site processing of the amyloid precursor protein. Proc. Natl Acad. Sci. USA100, 11735–11740 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang, H. et al. Regulation of beta-amyloid production in neurons by astrocyte-derived cholesterol. Proc. Natl Acad. Sci. USA10.1073/pnas.2102191118 (2021). [DOI] [PMC free article] [PubMed]
- 53.Stewart, K. L. & Radford, S. E. Amyloid plaques beyond Abeta: a survey of the diverse modulators of amyloid aggregation. Biophys. Rev.9, 405–419 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gellermann, G. P., Appel, T. R., Davies, P. & Diekmann, S. Paired helical filaments contain small amounts of cholesterol, phosphatidylcholine and sphingolipids. Biol. Chem.387, 1267–1274 (2006). [DOI] [PubMed] [Google Scholar]
- 55.Zhou, X. & Xu, J. Free cholesterol induces higher beta-sheet content in Abeta peptide oligomers by aromatic interaction with Phe19. PLoS ONE7, e46245 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fernandez-Perez, E. J. et al. Effect of cholesterol on membrane fluidity and association of abeta oligomers and subsequent neuronal damage: a double-edged sword. Front. Aging Neurosci.10, 226 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rudajev, V. & Novotny, J. Cholesterol as a key player in amyloid beta-mediated toxicity in Alzheimer’s disease. Front. Mol. Neurosci.15, 937056 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Habchi, J. et al. Cholesterol catalyses Abeta42 aggregation through a heterogeneous nucleation pathway in the presence of lipid membranes. Nat. Chem.10, 673–683 (2018). [DOI] [PubMed] [Google Scholar]
- 59.Michaels, T. C. T. et al. Dynamics of oligomer populations formed during the aggregation of Alzheimer’s Abeta42 peptide. Nat. Chem.12, 445–451 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Terakawa, M. S. et al. Impact of membrane curvature on amyloid aggregation. Biochim. Biophys. Acta Biomembr.1860, 1741–1764 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sugiura, Y., Ikeda, K. & Nakano, M. High membrane curvature enhances binding, conformational changes, and fibrillation of amyloid-beta on lipid bilayer surfaces. Langmuir31, 11549–11557 (2015). [DOI] [PubMed] [Google Scholar]
- 62.Tahirbegi, B. et al. A novel abeta(40) assembly at physiological concentration. Sci. Rep.10, 9477 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fabiani, C. & Antollini, S. S. Alzheimer’s disease as a membrane disorder: spatial cross-talk among beta-amyloid peptides, nicotinic acetylcholine receptors and lipid rafts. Front. Cell. Neurosci.13, 309 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sahoo, A. & Matysiak, S. Effects of applied surface-tension on membrane-assisted Abeta aggregation. Phys. Chem. Chem. Phys.23, 20627–20633 (2021). [DOI] [PubMed] [Google Scholar]
- 65.Hernandez, F. et al. Tau aggregation. Neuroscience518, 64–69 (2023). [DOI] [PubMed] [Google Scholar]
- 66.Thijssen, E. H. et al. Plasma phosphorylated tau 217 and phosphorylated tau 181 as biomarkers in Alzheimer’s disease and frontotemporal lobar degeneration: a retrospective diagnostic performance study. Lancet Neurol.20, 739–752 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wray, S. & Noble, W. Linking amyloid and tau pathology in Alzheimer’s disease: the role of membrane cholesterol in Abeta-mediated tau toxicity. J. Neurosci.29, 9665–9667 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bok, E. et al. Role of the lipid membrane and membrane proteins in tau pathology. Front. Cell Dev. Biol.9, 653815 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sallaberry, C. A. et al. Tau and membranes: interactions that promote folding and condensation. Front. Cell Dev. Biol.9, 725241 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goux, W. J., Rodriguez, S. & Sparkman, D. R. Analysis of the core components of Alzheimer paired helical filaments. A gas chromatography/mass spectrometry characterization of fatty acids, carbohydrates and long-chain bases. FEBS Lett.366, 81–85 (1995). [DOI] [PubMed] [Google Scholar]
- 71.Tuck, B. J. et al. Cholesterol determines the cytosolic entry and seeded aggregation of tau. Cell Rep.39, 110776 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.El Mammeri, N., Gampp, O., Duan, P. & Hong, M. Membrane-induced tau amyloid fibrils. Commun. Biol.6, 467 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mahul-Mellier, A. L. et al. The process of Lewy body formation, rather than simply alpha-synuclein fibrillization, is one of the major drivers of neurodegeneration. Proc. Natl Acad. Sci. USA117, 4971–4982 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Galvagnion, C. et al. Lipid vesicles trigger alpha-synuclein aggregation by stimulating primary nucleation. Nat. Chem. Biol.11, 229–234 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.den Jager, W. A. Sphingomyelin in Lewy inclusion bodies in Parkinson’s disease. Arch. Neurol.21, 615–619 (1969). [DOI] [PubMed] [Google Scholar]
- 76.Bosco, D. A. et al. Elevated levels of oxidized cholesterol metabolites in Lewy body disease brains accelerate alpha-synuclein fibrilization. Nat. Chem. Biol.2, 249–253 (2006). [DOI] [PubMed] [Google Scholar]
- 77.Jakubec, M. et al. Cholesterol-containing lipid nanodiscs promote an alpha-synuclein binding mode that accelerates oligomerization. FEBS J.288, 1887–1905 (2021). [DOI] [PubMed] [Google Scholar]
- 78.Qi, Z., Wan, M., Zhang, J. & Li, Z. Influence of cholesterol on the membrane binding and conformation of alpha-synuclein. J. Phys. Chem. B127, 1956–1964 (2023). [DOI] [PubMed] [Google Scholar]
- 79.Husain, M. A., Laurent, B. & Plourde, M. APOE and Alzheimer’s disease: from lipid transport to physiopathology and therapeutics. Front. Neurosci.15, 630502 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marais, A. D. Apolipoprotein E in lipoprotein metabolism, health and cardiovascular disease. Pathology51, 165–176 (2019). [DOI] [PubMed] [Google Scholar]
- 81.Huang, Y. A., Zhou, B., Wernig, M. & Sudhof, T. C. ApoE2, ApoE3, and ApoE4 differentially stimulate APP transcription and abeta secretion. Cell168, 427–441.e421 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Di Battista, A. M., Heinsinger, N. M. & Rebeck, G. W. Alzheimer’s disease genetic risk factor APOE-epsilon4 also affects normal brain function. Curr. Alzheimer Res.13, 1200–1207 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Raulin, A. C. et al. ApoE in Alzheimer’s disease: pathophysiology and therapeutic strategies. Mol. Neurodegener.17, 72 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dugger, B. N. & Dickson, D. W. Pathology of neurodegenerative diseases. Cold Spring Harb. Perspect. Biol.10.1101/cshperspect.a028035 (2017). [DOI] [PMC free article] [PubMed]
- 85.Kacher, R., Mounier, C., Caboche, J. & Betuing, S. Altered cholesterol homeostasis in Huntington’s disease. Front Aging Neurosci.14, 797220 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gao, X. et al. Cholesterol modifies huntingtin binding to, disruption of, and aggregation on lipid membranes. Biochemistry55, 92–102 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stonebraker, A. R. et al. Cholesterol impacts the formation of huntingtin/lipid complexes and subsequent aggregation. Protein Sci.32, e4642 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pineda-Torra, I., Siddique, S., Waddington, K. E., Farrell, R. & Jury, E. C. Disrupted lipid metabolism in multiple sclerosis: a role for liver X receptors? Front. Endocrinol.12, 639757 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]