Abstract
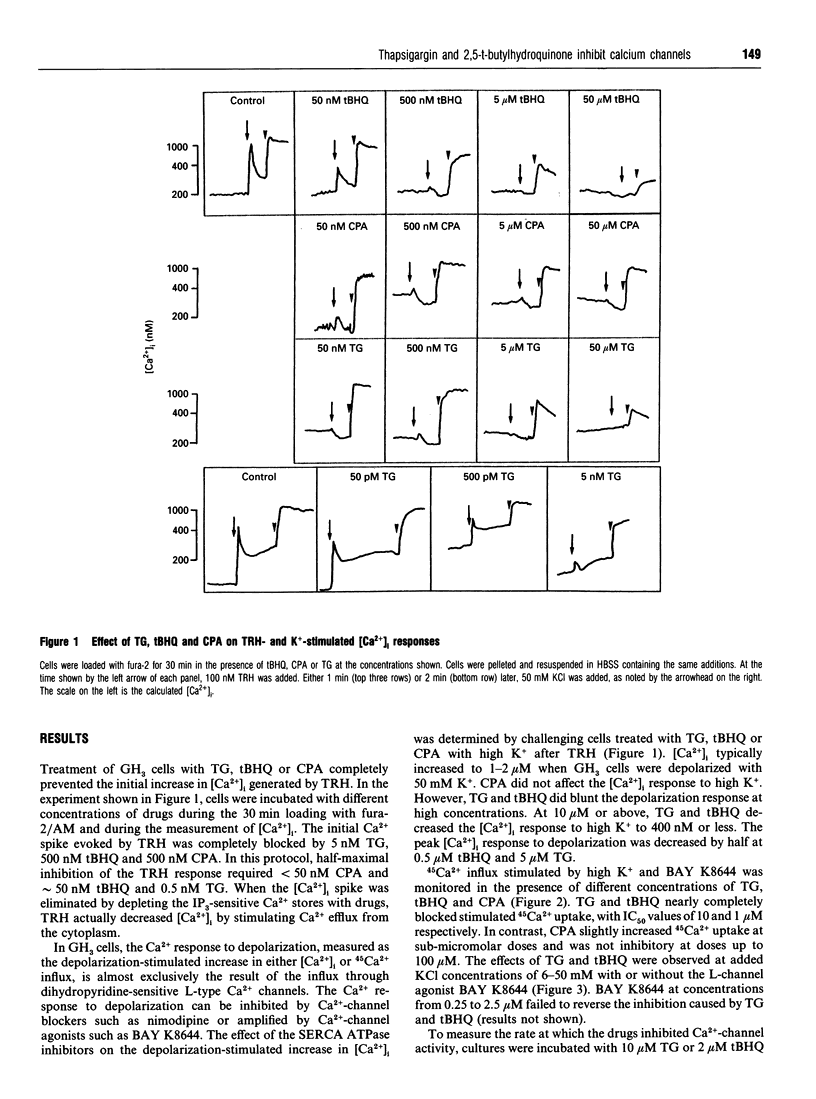
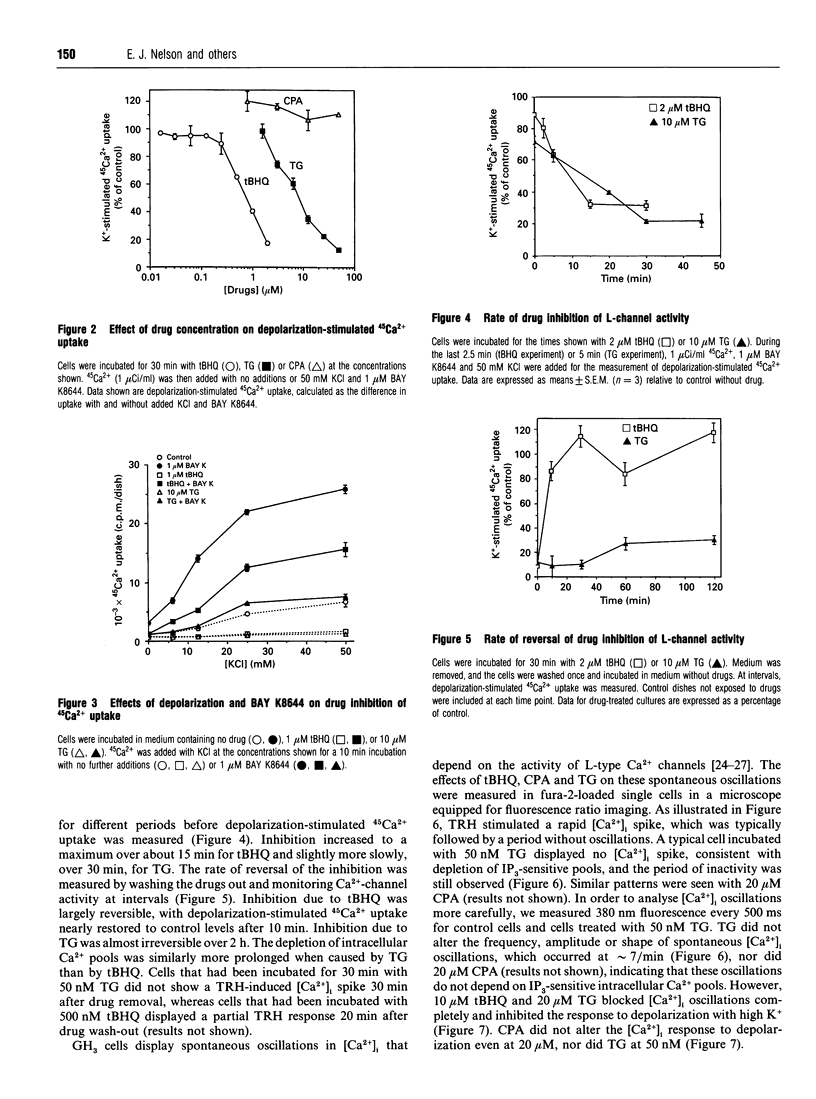
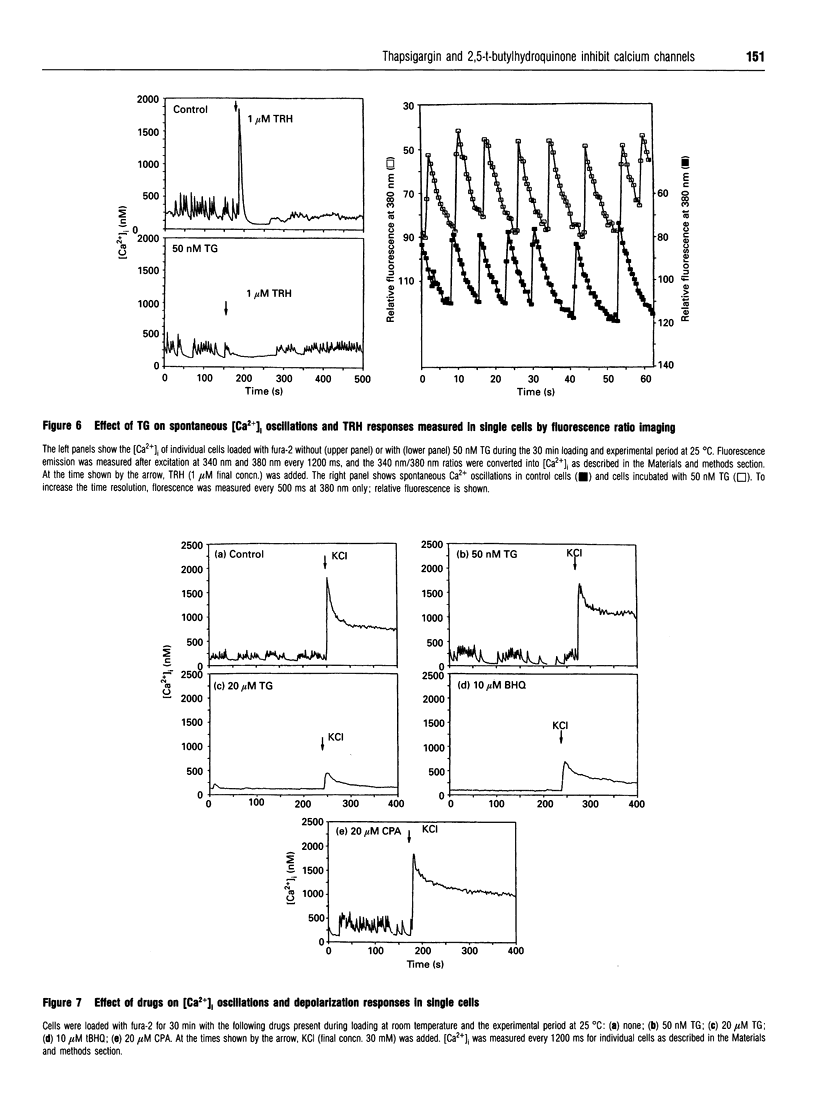
Thapsigargin (TG), 2,5-t-butylhydroquinone (tBHQ) and cyclopiazonic acid (CPA) all inhibit the initial Ca(2+)-response to thyrotropin-releasing hormone (TRH) by depleting intracellular Ca2+ pools sensitive to inositol 1,4,5-trisphosphate (IP3). Treatment of GH3 pituitary cells for 30 min with 5 nM TG, 500 nM tBHQ or 50 nM CPA completely eliminated the TRH-induced spike in intracellular free Ca2+ ([Ca2+]i). Higher concentrations of TG and tBHQ, but not CPA, were also found to inhibit strongly the activity of L-type calcium channels, as measured by the increase in [Ca2+]i or 45Ca2+ influx stimulated by depolarization. TG and tBHQ blocked high-K(+)-stimulated 45Ca2+ uptake, with IC50 values of 10 and 1 microM respectively. Maximal inhibition of L-channel activity was achieved 15-30 min after drug addition. Inhibition by tBHQ was reversible, whereas inhibition by TG was not. TG and CPA did not affect spontaneous [Ca2+]i oscillations when tested at concentrations adequate to deplete the IP3-sensitive Ca2+ pool. However, 20 microM TG and 10 microM tBHQ blocked [Ca2+]i oscillations completely. The effect of drugs on calcium currents was measured directly by using the patch-clamp technique. When added to the external bath, 10 microM CPA caused a sustained increase in the calcium-channel current amplitude over 8 min, 10 microM tBHQ caused a progressive inhibition, and 10 microM TG caused an enhancement followed by a sustained block of the calcium current over 8 min. In summary, CPA depletes IP3-sensitive Ca2+ stores and does not inhibit voltage-operated calcium channels. At sufficiently low concentrations, TG depletes IP3-sensitive stores without inhibiting L-channel activity, but, for tBHQ, inhibition of calcium channels occurs at concentrations close to those needed to block agonist mobilization of intracellular Ca2+.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benham C. D. Voltage-gated and agonist-mediated rises in intracellular Ca2+ in rat clonal pituitary cells (GH3) held under voltage clamp. J Physiol. 1989 Aug;415:143–158. doi: 10.1113/jphysiol.1989.sp017716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Cobbold P. H., Cuthbertson K. S. Spatial and temporal aspects of cell signalling. Philos Trans R Soc Lond B Biol Sci. 1988 Jul 26;320(1199):325–343. doi: 10.1098/rstb.1988.0080. [DOI] [PubMed] [Google Scholar]
- Brandl C. J., Green N. M., Korczak B., MacLennan D. H. Two Ca2+ ATPase genes: homologies and mechanistic implications of deduced amino acid sequences. Cell. 1986 Feb 28;44(4):597–607. doi: 10.1016/0092-8674(86)90269-2. [DOI] [PubMed] [Google Scholar]
- Campbell A. M., Kessler P. D., Sagara Y., Inesi G., Fambrough D. M. Nucleotide sequences of avian cardiac and brain SR/ER Ca(2+)-ATPases and functional comparisons with fast twitch Ca(2+)-ATPase. Calcium affinities and inhibitor effects. J Biol Chem. 1991 Aug 25;266(24):16050–16055. [PubMed] [Google Scholar]
- Cobbold P. H., Rink T. J. Fluorescence and bioluminescence measurement of cytoplasmic free calcium. Biochem J. 1987 Dec 1;248(2):313–328. doi: 10.1042/bj2480313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaurex N., Lew D. P., Krause K. H. Cyclopiazonic acid depletes intracellular Ca2+ stores and activates an influx pathway for divalent cations in HL-60 cells. J Biol Chem. 1992 Feb 5;267(4):2318–2324. [PubMed] [Google Scholar]
- Dolor R. J., Hurwitz L. M., Mirza Z., Strauss H. C., Whorton A. R. Regulation of extracellular calcium entry in endothelial cells: role of intracellular calcium pool. Am J Physiol. 1992 Jan;262(1 Pt 1):C171–C181. doi: 10.1152/ajpcell.1992.262.1.C171. [DOI] [PubMed] [Google Scholar]
- Drummond A. H. Bidirectional control of cytosolic free calcium by thyrotropin-releasing hormone in pituitary cells. 1985 Jun 27-Jul 3Nature. 315(6022):752–755. doi: 10.1038/315752a0. [DOI] [PubMed] [Google Scholar]
- Enyeart J. J., Sheu S. S., Hinkle P. M. Pituitary Ca2+ channels: blockade by conventional and novel Ca2+ antagonists. Am J Physiol. 1987 Jul;253(1 Pt 1):C162–C170. doi: 10.1152/ajpcell.1987.253.1.C162. [DOI] [PubMed] [Google Scholar]
- Ferris C. D., Snyder S. H. Inositol 1,4,5-trisphosphate-activated calcium channels. Annu Rev Physiol. 1992;54:469–488. doi: 10.1146/annurev.ph.54.030192.002345. [DOI] [PubMed] [Google Scholar]
- Gershengorn M. C. Role of inositol lipid second messengers in regulation of secretion: studies of thyrotropin-releasing hormone action in pituitary cells. Soc Gen Physiol Ser. 1989;44:1–15. [PubMed] [Google Scholar]
- Hinkle P. M., Nelson E. J., Haymes A. A. Regulation of L-type voltage-gated calcium channels by epidermal growth factor. Endocrinology. 1993 Jul;133(1):271–276. doi: 10.1210/endo.133.1.7686480. [DOI] [PubMed] [Google Scholar]
- Inesi G., Cantilina T., Yu X., Nikic D., Sagara Y., Kirtley M. E. Long-range intramolecular linked functions in activation and inhibition of SERCA ATPases. Ann N Y Acad Sci. 1992 Nov 30;671:32–48. doi: 10.1111/j.1749-6632.1992.tb43782.x. [DOI] [PubMed] [Google Scholar]
- Li P., Thaw C. N., Sempowski G. D., Gershengorn M. C., Hinkle P. M. Characterization of the calcium response to thyrotropin-releasing hormone (TRH) in cells transfected with TRH receptor complementary DNA: importance of voltage-sensitive calcium channels. Mol Endocrinol. 1992 Sep;6(9):1393–1402. doi: 10.1210/mend.6.9.1279382. [DOI] [PubMed] [Google Scholar]
- Lytton J., Westlin M., Hanley M. R. Thapsigargin inhibits the sarcoplasmic or endoplasmic reticulum Ca-ATPase family of calcium pumps. J Biol Chem. 1991 Sep 15;266(26):17067–17071. [PubMed] [Google Scholar]
- Mason M. J., Garcia-Rodriguez C., Grinstein S. Coupling between intracellular Ca2+ stores and the Ca2+ permeability of the plasma membrane. Comparison of the effects of thapsigargin, 2,5-di-(tert-butyl)-1,4-hydroquinone, and cyclopiazonic acid in rat thymic lymphocytes. J Biol Chem. 1991 Nov 5;266(31):20856–20862. [PubMed] [Google Scholar]
- Missiaen L., Wuytack F., Raeymaekers L., De Smedt H., Droogmans G., Declerck I., Casteels R. Ca2+ extrusion across plasma membrane and Ca2+ uptake by intracellular stores. Pharmacol Ther. 1991;50(2):191–232. doi: 10.1016/0163-7258(91)90014-d. [DOI] [PubMed] [Google Scholar]
- Moore G. A., McConkey D. J., Kass G. E., O'Brien P. J., Orrenius S. 2,5-Di(tert-butyl)-1,4-benzohydroquinone--a novel inhibitor of liver microsomal Ca2+ sequestration. FEBS Lett. 1987 Nov 30;224(2):331–336. doi: 10.1016/0014-5793(87)80479-9. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr, Bird G. S. The inositol phosphate-calcium signaling system in nonexcitable cells. Endocr Rev. 1993 Oct;14(5):610–631. doi: 10.1210/edrv-14-5-610. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr The capacitative model for receptor-activated calcium entry. Adv Pharmacol. 1991;22:251–269. doi: 10.1016/s1054-3589(08)60037-x. [DOI] [PubMed] [Google Scholar]
- Robinson I. M., Cheek T. R., Burgoyne R. D. Ca2+ influx induced by the Ca(2+)-ATPase inhibitors 2,5-di-(t-butyl)-1,4-benzohydroquinone and thapsigargin in bovine adrenal chromaffin cells. Biochem J. 1992 Dec 1;288(Pt 2):457–463. doi: 10.1042/bj2880457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagara Y., Wade J. B., Inesi G. A conformational mechanism for formation of a dead-end complex by the sarcoplasmic reticulum ATPase with thapsigargin. J Biol Chem. 1992 Jan 15;267(2):1286–1292. [PubMed] [Google Scholar]
- Schlegel W., Winiger B. P., Mollard P., Vacher P., Wuarin F., Zahnd G. R., Wollheim C. B., Dufy B. Oscillations of cytosolic Ca2+ in pituitary cells due to action potentials. Nature. 1987 Oct 22;329(6141):719–721. doi: 10.1038/329719a0. [DOI] [PubMed] [Google Scholar]
- Short A. D., Klein M. G., Schneider M. F., Gill D. L. Inositol 1,4,5-trisphosphate-mediated quantal Ca2+ release measured by high resolution imaging of Ca2+ within organelles. J Biol Chem. 1993 Dec 5;268(34):25887–25893. [PubMed] [Google Scholar]
- Stojilković S. S., Catt K. J. Calcium oscillations in anterior pituitary cells. Endocr Rev. 1992 May;13(2):256–280. doi: 10.1210/edrv-13-2-256. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Tashjian A. H., Jr Functional identification and quantitation of three intracellular calcium pools in GH4C1 cells: evidence that the caffeine-responsive pool is coupled to a thapsigargin-resistant, ATP-dependent process. Biochemistry. 1993 Nov 16;32(45):12062–12073. doi: 10.1021/bi00096a017. [DOI] [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Yasumura Y., Levine L., Sato G. H., Parker M. L. Establishment of clonal strains of rat pituitary tumor cells that secrete growth hormone. Endocrinology. 1968 Feb;82(2):342–352. doi: 10.1210/endo-82-2-342. [DOI] [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner K. A., Yacono P. W., Golan D. E., Tashjian A. H., Jr Mechanism of spontaneous intracellular calcium fluctuations in single GH4C1 rat pituitary cells. Biochem J. 1993 May 15;292(Pt 1):175–182. doi: 10.1042/bj2920175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wictome M., Michelangeli F., Lee A. G., East J. M. The inhibitors thapsigargin and 2,5-di(tert-butyl)-1,4-benzohydroquinone favour the E2 form of the Ca2+,Mg(2+)-ATPase. FEBS Lett. 1992 Jun 15;304(2-3):109–113. doi: 10.1016/0014-5793(92)80599-c. [DOI] [PubMed] [Google Scholar]
- Wuytack F., Raeymaekers L. The Ca(2+)-transport ATPases from the plasma membrane. J Bioenerg Biomembr. 1992 Jun;24(3):285–300. doi: 10.1007/BF00768849. [DOI] [PubMed] [Google Scholar]


