Abstract
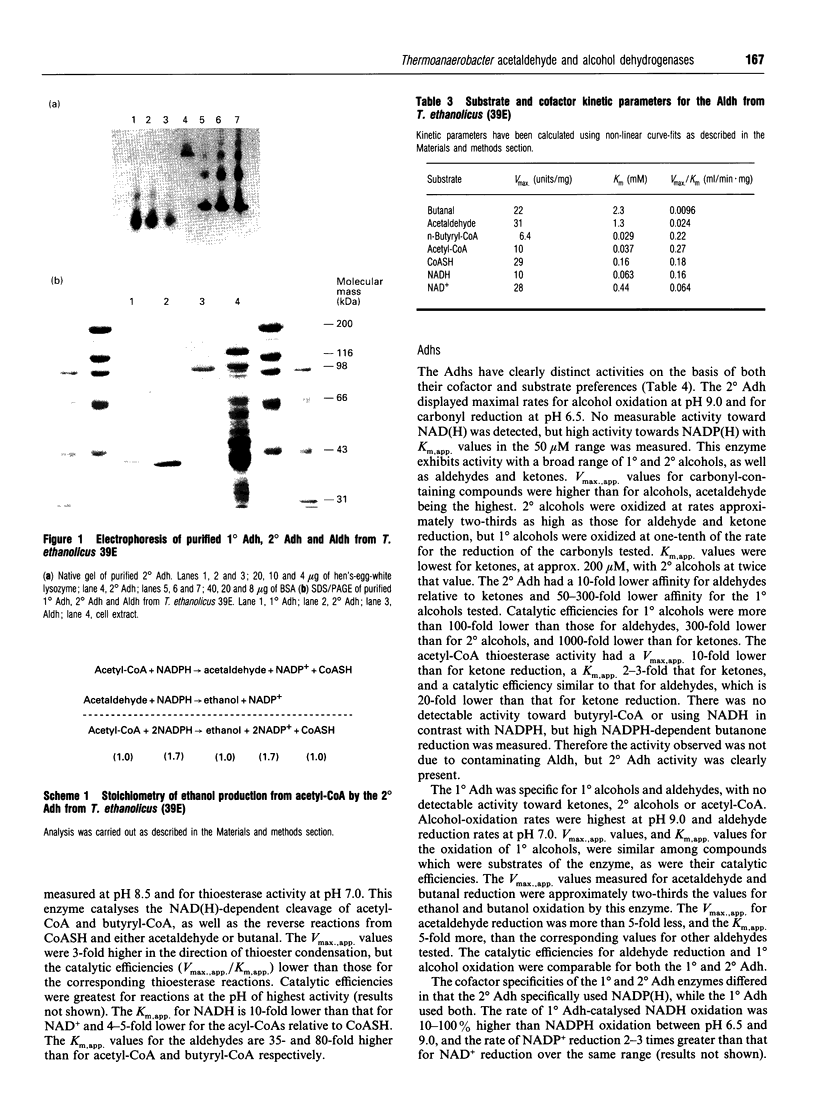
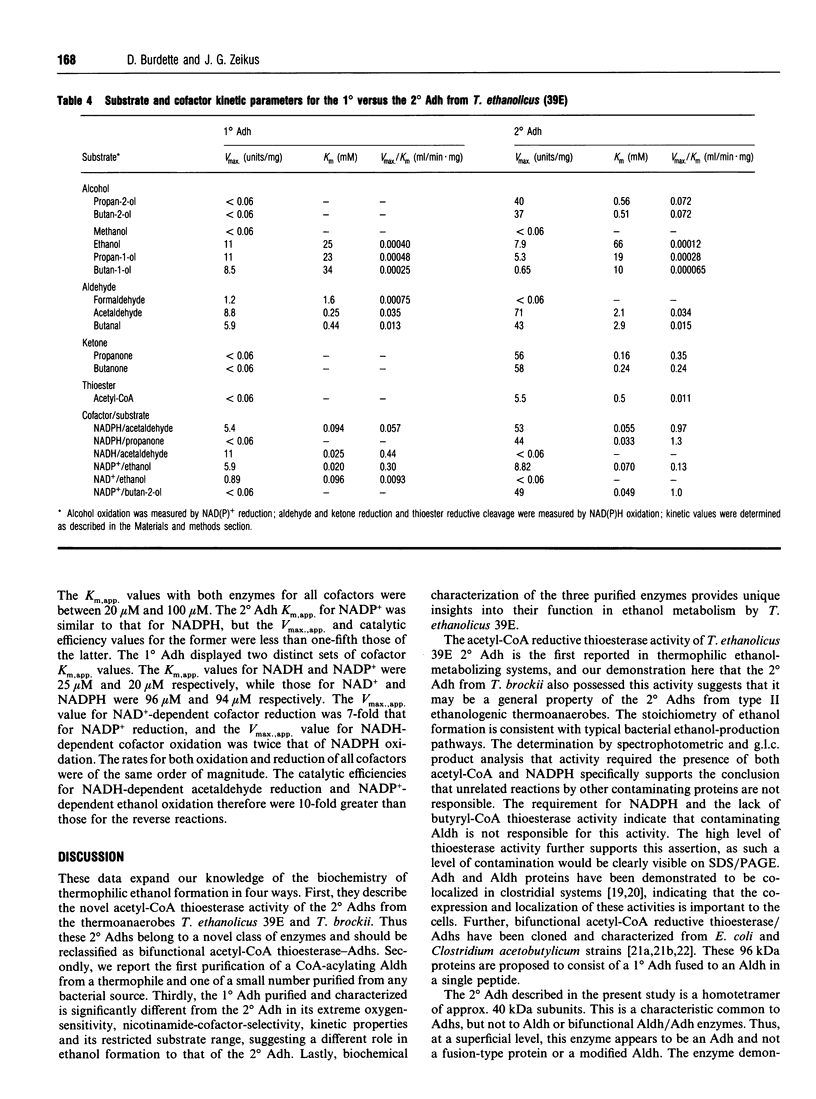
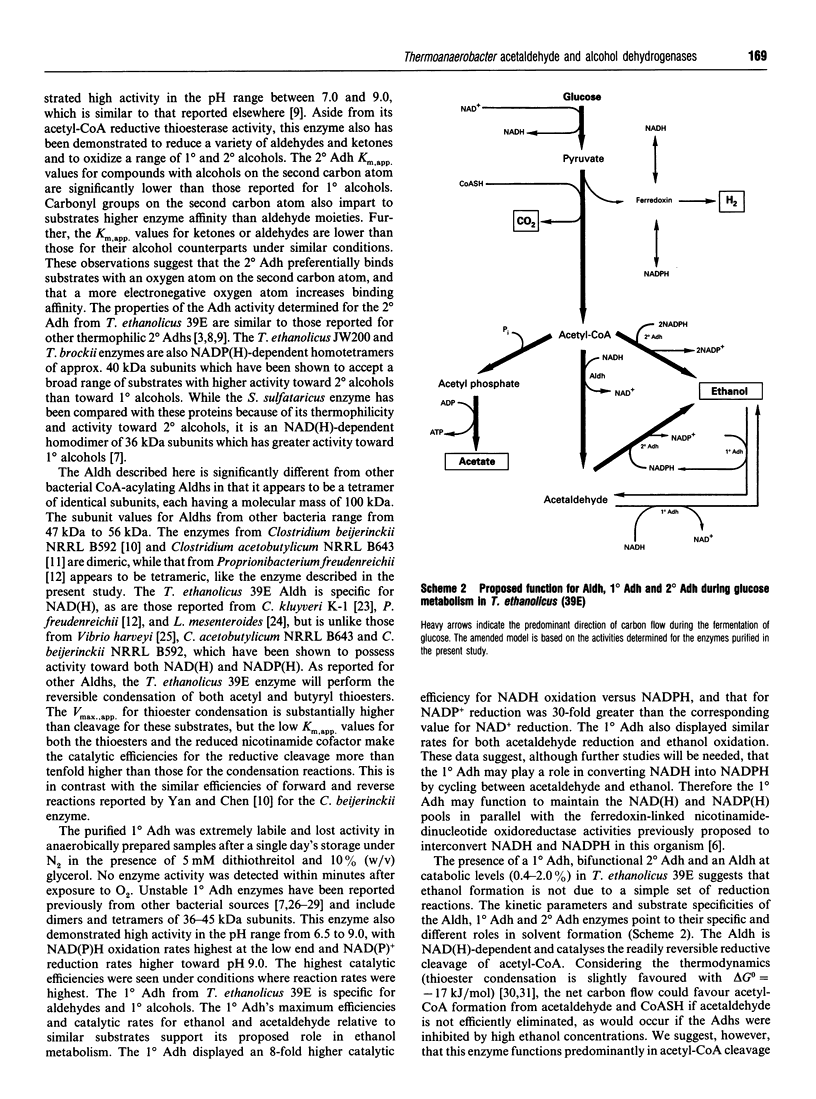
The purification and characterization of three enzymes involved in ethanol formation from acetyl-CoA in Thermoanaerobacter ethanolicus 39E (formerly Clostridium thermohydrosulfuricum 39E) is described. The secondary-alcohol dehydrogenase (2 degrees Adh) was determined to be a homotetramer of 40 kDa subunits (SDS/PAGE) with a molecular mass of 160 kDa. The 2 degrees Adh had a lower catalytic efficiency for the oxidation of 1 degree alcohols, including ethanol, than for the oxidation of secondary (2 degrees) alcohols or the reduction of ketones or aldehydes. This enzyme possesses a significant acetyl-CoA reductive thioesterase activity as determined by NADPH oxidation, thiol formation and ethanol production. The primary-alcohol dehydrogenase (1 degree Adh) was determined to be a homotetramer of 41.5 kDa (SDS/PAGE) subunits with a molecular mass of 170 kDa. The 1 degree Adh used both NAD(H) and NADP(H) and displayed higher catalytic efficiencies for NADP(+)-dependent ethanol oxidation and NADH-dependent acetaldehyde (identical to ethanal) reduction than for NADPH-dependent acetaldehyde reduction or NAD(+)-dependent ethanol oxidation. The NAD(H)-linked acetaldehyde dehydrogenase was a homotetramer (360 kDa) of identical subunits (100 kDa) that readily catalysed thioester cleavage and condensation. The 1 degree Adh was expressed at 5-20% of the level of the 2 degrees Adh throughout the growth cycle on glucose. The results suggest that the 2 degrees Adh primarily functions in ethanol production from acetyl-CoA and acetaldehyde, whereas the 1 degree Adh functions in ethanol consumption for nicotinamide-cofactor recycling.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON R. M., STADTMAN E. R. The oxidation of acetaldehyde to acetyl coenzyme A. J Biol Chem. 1953 Jun;202(2):873–890. [PubMed] [Google Scholar]
- Brooks S. P. A simple computer program with statistical tests for the analysis of enzyme kinetics. Biotechniques. 1992 Dec;13(6):906–911. [PubMed] [Google Scholar]
- Bryant F. O., Wiegel J., Ljungdahl L. G. Purification and Properties of Primary and Secondary Alcohol Dehydrogenases from Thermoanaerobacter ethanolicus. Appl Environ Microbiol. 1988 Feb;54(2):460–465. doi: 10.1128/aem.54.2.460-465.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschhorn H., Dürre P., Gottschalk G. Production and Utilization of Ethanol by the Homoacetogen Acetobacterium woodii. Appl Environ Microbiol. 1989 Jul;55(7):1835–1840. doi: 10.1128/aem.55.7.1835-1840.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers D., Meighen E. Vibrio harveyi aldehyde dehydrogenase. Partial reversal of aldehyde oxidation and its possible role in the reduction of fatty acids for the bioluminescence reaction. J Biol Chem. 1984 Jun 10;259(11):7109–7114. [PubMed] [Google Scholar]
- Fischer R. J., Helms J., Dürre P. Cloning, sequencing, and molecular analysis of the sol operon of Clostridium acetobutylicum, a chromosomal locus involved in solventogenesis. J Bacteriol. 1993 Nov;175(21):6959–6969. doi: 10.1128/jb.175.21.6959-6969.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodlove P. E., Cunningham P. R., Parker J., Clark D. P. Cloning and sequence analysis of the fermentative alcohol-dehydrogenase-encoding gene of Escherichia coli. Gene. 1989 Dec 21;85(1):209–214. doi: 10.1016/0378-1119(89)90483-6. [DOI] [PubMed] [Google Scholar]
- Hensgens C. M., Vonck J., Van Beeumen J., van Bruggen E. F., Hansen T. A. Purification and characterization of an oxygen-labile, NAD-dependent alcohol dehydrogenase from Desulfovibrio gigas. J Bacteriol. 1993 May;175(10):2859–2863. doi: 10.1128/jb.175.10.2859-2863.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungermann K., Thauer R. K., Leimenstoll G., Decker K. Function of reduced pyridine nucleotide-ferredoxin oxidoreductases in saccharolytic Clostridia. Biochim Biophys Acta. 1973 May 30;305(2):268–280. doi: 10.1016/0005-2728(73)90175-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamed R. J., Zeikus J. G. Novel NADP-linked alcohol--aldehyde/ketone oxidoreductase in thermophilic ethanologenic bacteria. Biochem J. 1981 Apr 1;195(1):183–190. doi: 10.1042/bj1950183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovitt R. W., Longin R., Zeikus J. G. Ethanol Production by Thermophilic Bacteria: Physiological Comparison of Solvent Effects on Parent and Alcohol-Tolerant Strains of Clostridium thermohydrosulfuricum. Appl Environ Microbiol. 1984 Jul;48(1):171–177. doi: 10.1128/aem.48.1.171-177.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovitt R. W., Shen G. J., Zeikus J. G. Ethanol production by thermophilic bacteria: biochemical basis for ethanol and hydrogen tolerance in Clostridium thermohydrosulfuricum. J Bacteriol. 1988 Jun;170(6):2809–2815. doi: 10.1128/jb.170.6.2809-2815.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynd L., Kerby R., Zeikus J. G. Carbon monoxide metabolism of the methylotrophic acidogen Butyribacterium methylotrophicum. J Bacteriol. 1982 Jan;149(1):255–263. doi: 10.1128/jb.149.1.255-263.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie K. F., Eddy C. K., Ingram L. O. Modulation of alcohol dehydrogenase isoenzyme levels in Zymomonas mobilis by iron and zinc. J Bacteriol. 1989 Feb;171(2):1063–1067. doi: 10.1128/jb.171.2.1063-1067.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair R. V., Bennett G. N., Papoutsakis E. T. Molecular characterization of an aldehyde/alcohol dehydrogenase gene from Clostridium acetobutylicum ATCC 824. J Bacteriol. 1994 Feb;176(3):871–885. doi: 10.1128/jb.176.3.871-885.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale A. D., Scopes R. K., Kelly J. M., Wettenhall R. E. The two alcohol dehydrogenases of Zymomonas mobilis. Purification by differential dye ligand chromatography, molecular characterisation and physiological roles. Eur J Biochem. 1986 Jan 2;154(1):119–124. doi: 10.1111/j.1432-1033.1986.tb09366.x. [DOI] [PubMed] [Google Scholar]
- Palosaari N. R., Rogers P. Purification and properties of the inducible coenzyme A-linked butyraldehyde dehydrogenase from Clostridium acetobutylicum. J Bacteriol. 1988 Jul;170(7):2971–2976. doi: 10.1128/jb.170.7.2971-2976.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rella R., Raia C. A., Pensa M., Pisani F. M., Gambacorta A., De Rosa M., Rossi M. A novel archaebacterial NAD+-dependent alcohol dehydrogenase. Purification and properties. Eur J Biochem. 1987 Sep 15;167(3):475–479. doi: 10.1111/j.1432-1033.1987.tb13361.x. [DOI] [PubMed] [Google Scholar]
- Smith L. T., Kaplan N. O. Purification, properties, and kinetic mechanism of coenzyme A-linked aldehyde dehydrogenase from Clostridium kluyveri. Arch Biochem Biophys. 1980 Sep;203(2):663–675. doi: 10.1016/0003-9861(80)90224-6. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan R. T., Chen J. S. Coenzyme A-acylating aldehyde dehydrogenase from Clostridium beijerinckii NRRL B592. Appl Environ Microbiol. 1990 Sep;56(9):2591–2599. doi: 10.1128/aem.56.9.2591-2599.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]