Abstract
The aim of this study was to evaluate the prognostic value of peripheral blood inflammation indexes in patients with metastatic Colorectal Cancer (CRC) and to establish a predictive scoring system. A total of 324 CRC patients diagnosed through pathological examination from January 2017 to July 2022 at the Third Affiliated Hospital of Kunming Medical University were included. The prognosis of patients with metastatic CRC was examined, and the correlation between IL-10 expression in pathological tissues and IL-10 expression in serum was analyzed. The results showed that the prognosis of CRC was poorer when metastasis occurred (P < 0.001). Additionally, IL-10 was highly expressed in the metastatic CRC group (P = 0.018), and the expression of IL-10 in pathological tissues of patients with metastatic CRC was positively correlated with the expression of IL-10 in serum (P = 0.037). The neutrophil-to-lymphocyte ratio (NLR), lymphocyte-to-white blood cell ratio (LWR), aggregate index of systemic inflammation (AISI), monocyte-to-lymphocyte ratio (MLR), systemic inflammatory response index (SIRI), prognostic nutritional index (PNI), advanced lung cancer inflammation index (ALI), and interleukin-10 (IL-10) were calculated and determined by ROC curve. The critical values were 2.135, 3.735, 353.745, 0.265, 1.025, 52.975, 353.635, and 11.25, respectively. Inflammatory indexes with an AUC of more than 0.6 were selected, and each colorectal cancer patient with any of these risk factors was assigned a score of one. The 324 patients were then divided into two groups: 0–4 for the low-risk group and 4–8 for the high-risk group. The occurrence of distant metastases in the two groups was statistically analyzed. The results showed that the OS and PFS of the low-risk group were significantly superior to those of the high-risk group (P < 0.05). These findings indicate that NLR, LWR, AISI, MLR, SIRI, PNI, ALI, and IL-10 are risk factors for distant metastasis in CRC patients. Therefore, the prediction scores of these indexes can be used to effectively evaluate the prognosis of patients with metastatic CRC.
Keywords: Colorectal cancer, Prognosis, Peripheral blood inflammatory index, Metastasis
Subject terms: Colorectal cancer, Prognostic markers
Introduction
CRC is one of the most common gastrointestinal malignancies, with its global incidence and mortality ranking third and second, respectively1,2. The incidence of CRC is primarily associated with poor dietary habits and lifestyle choices. Due to its large population, China has the highest number of new colorectal cancer cases globally. According to the National Cancer Center, the incidence of CRC in China in 2022 rose to the second highest among malignant tumors3,4.
Survival of patients with non-metastatic colorectal cancer is superior to that of patients with metastatic colorectal cancer, and metastasis is the most important cause of high mortality in patients with CRC5. The treatment of patients with metastatic colorectal cancer has progressed considerably over the past few decades, and multiple therapeutic options are now available for the first-line treatment of multiple colorectal cancers. In recent years, next-generation sequencing and whole-exome sequencing have been developed to reveal new predictive biomarkers for colorectal cancer, which can help to provide personalized treatment for patients, whereas targeting tumor stage, high-risk pathological features, microsatellite instability, patient age and functional status can be used to appropriately adjuvant therapy for mCRC patients6. At present, the main systemic therapies for the treatment of multiple colorectal cancer, in addition to chemotherapy and targeted therapy, immunotherapy represented by immune checkpoint inhibitors (ICIs) has also shown better efficacy in colorectal cancer patients7. The mechanism of action of ICIs is based on activating the immune system and improving the immune microenvironment of the tumor, and they are able to regulate T lymphocytes and target immune checkpoints, such as programmed cell death protein 1 (PD-1), programmed cell death ligand 1 (PD-L1) and cytotoxic T lymphocyte-associated protein 4 (CTLA-4)8. ICIs, as single agents or in combination with other anticancer drugs, have achieved unprecedented efficacy in several other cancers such as melanoma, renal cell carcinoma (RCC), non-small cell lung cancer (NSCLC), hepatocellular carcinoma (HCC), colorectal cancer, and uroepithelial carcinoma (UC)8,9.
Multiple inflammatory mediators in the tumor immune microenvironment promote the formation of metastatic ecological niches in colorectal cancer, and dysregulation of the systemic inflammatory response promotes cancer progression10. Clinically, we choose to take samples of tumor tissues and stain them with inflammatory indexes, and observe them with the help of microscope to study the etiology, pathogenesis, morphology, structure, function and metabolism, etc. Pathological diagnosis is currently the "gold standard" for the diagnosis of most of the diseases, especially tumor diseases.
However, tissue-based biomarkers have not been able to reflect the status of the immune system, which is the main driver of ICI efficacy9, and immunohistochemical staining of tumor tissues, which is a cumbersome procedure with long cycle times, and may lead to repeated sampling as a result of results such as false-negatives. In contrast, using peripheral blood inflammation indexes, such as the SII, NLR, PLR, and MLR, is a preferable method for assessing tumor aggressiveness and invasiveness in colorectal cancer. These indicators can be obtained from patients' blood samples in a simple and non-invasive manner and can be dynamically monitored during treatment to assess tumor progression. This approach effectively guides clinicians in adjusting treatment plans in a timely manner and helps optimize personalized treatment strategies for colorectal cancer. These indicators have been shown to be closely related to cancer progression11, several studies have suggested a correlation between increased NLR and PLR levels and unfavorable prognosis and survival rates across different types of cancers12,13. Elevated NLR levels have been linked to the enhanced proliferative ability of tumor cells, while the concurrent assessment of NLR and PLR is indicative of tumor invasion, recurrence, metastasis, and prognosis14. Nonetheless, there is limited research assessing the prognostic significance of these parameters in forecasting the prognosis of patients diagnosed with mCRC. Hence, this study aims to delve deeper into the inflammatory factors linked with distant metastasis in CRC patients. It seeks to identify inflammatory markers capable of early stage mCRC diagnosis and proficiently assessing its prognosis.
Materials and methods
General materials
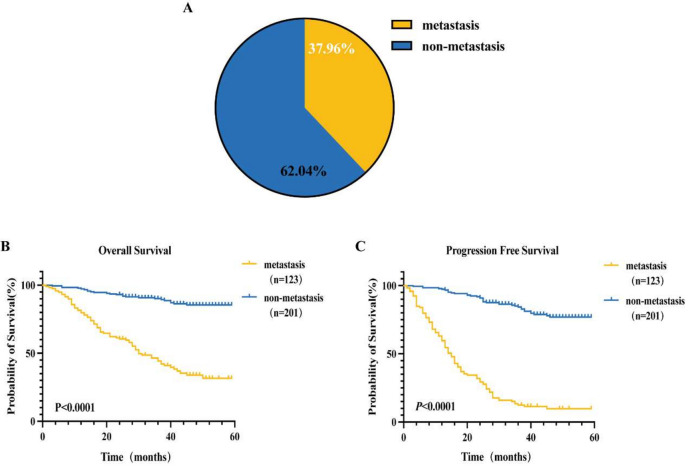
A total of 324 CRC patients admitted to the Third Affiliated Hospital of Kunming Medical University from January 2017 to July 2022 and diagnosed pathologically by biopsy or surgical resection were included in the study. Of these, 123 patients (37.96%) had distant metastases at the time of initial diagnosis, while 201 patients (62.04%) did not show distant metastases.
Ethics approval and consent to participate
All subjects signed an informed consent form before participating in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of the Third Affiliated Hospital of Kunming Medical University, Peking University Cancer Hospital Yunnan, Yunnan Cancer Hospital.
Inclusion and exclusion criteria
Inclusion criteria: (1) CRC patients with clear pathological evidence; (2) patients with complete clinical data, including imaging and genetic test results.
Exclusion criteria: (1) patients whose hematological indexes are affected by factors other than the tumor, such as infection or blood system diseases; (2) patients who received anti-tumor treatment in external hospitals; (3) patients who died due to accidents or non-tumor causes; (4) patients with double cancers.
Methods
Hematological indicators, imaging data, and survival time were collected from all newly diagnosed and untreated CRC patients. The study received approval from the Ethics Committee of the Third Affiliated Hospital of Kunming Medical University.
CRC patients included in the study were followed up via one-on-one telephone calls until December 31, 2023, or until the date of the patient's death. Metastatic indicators were defined after the diagnosis of metastatic CRC, confirmed by pathology or imaging assistance.
Statistical methods
Data analysis was performed utilizing SPSS 26.0, and graphical representation was achieved through GraphPad Prism 10.0. Optimal cut-off values were established by plotting ROC curves. Survival analysis was executed via the Kaplan–Meier method. Comparison of patient characteristics between groups was conducted employing the chi-square test for categorical variables and the t-test for continuous variables, with statistical significance set at P < 0.05.
Results
Distant metastasis of CRC patients and survival analysis between two groups
Among the 324 CRC patients included in this study, 86 cases (69.9%) presented with metastasis at the initial diagnosis, while 37 cases (30.1%) developed new metastases during the follow-up period. This resulted in a total of 123 cases (38.0%) with metastasis overall, while 201 cases (62.0%) remained without metastasis throughout the study period (Fig. 1A). OS in the CRC group with distant metastasis decreased by 19.96 months compared to the non-distant metastasis group (33.83 ± 1.92 months vs. 53.79 ± 1.01 months), with a statistical significance of P < 0.001 (Fig. 1B). In the CRC group with distant metastasis, PFS decreased by 31.83 months compared to the non-distant metastasis group (19.56 ± 1.49 months vs. 51.39 ± 1.13 months), with a statistically significant difference of P < 0.001 (Fig. 1C).
Figure 1.
Metastasis in the metastatic group versus the non-metastatic group and survival analysis between the two groups. (A)The study included 324 CRC patients, comprising 123 metastatic patients (38.0%) and 201 non-metastatic patients (62.0%). (B) The OS in the CRC group with distant metastasis decreased by 19.96 months compared to the non-distant metastasis group (33.83 ± 1.92 months vs. 53.79 ± 1.01 months), with a statistical significance of P < 0.001. (C) The PFS in the CRC group with distant metastasis decreased by 31.83 months compared to the non-distant metastasis group (19.56 ± 1.49 months vs. 51.39 ± 1.13 months), with a statistically significant difference of P < 0.001.
Evaluation of pathologic inflammatory indexes in two groups of patients
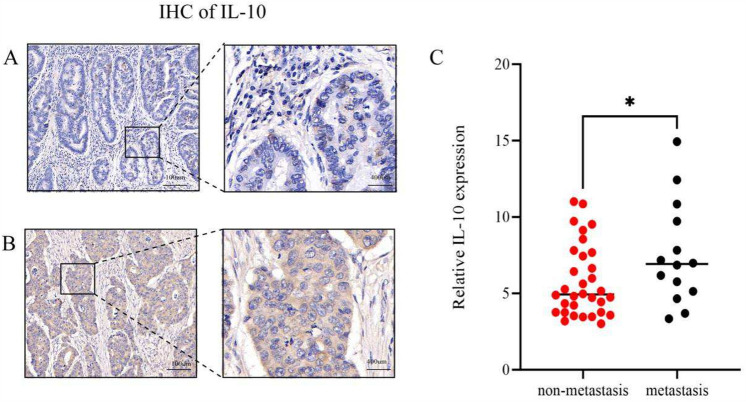
Immunohistochemical staining of IL-10 was conducted on lesions from 43 CRC patients (Fig. 2), with interpretation of tumor hot spot areas performed by a senior physician from the Department of Pathology. Six to eight non-repeated images were randomly captured from the tumor hotspot area of each pathology section, all under the same magnification. The intensity of IL-10 immunohistochemical staining was quantified using Image Pro Plus to determine the mean staining intensity of each pathology section. Subsequently, all sections were categorized into two expression groups, high and low, based on the median, for further analysis.
Figure 2.
Differences in IL-10 expression between the two groups. (A) Non-metastatic CRC tissues exhibited low expression of IL-10. (B) Metastatic CRC tissues displayed high expression of IL-10. (C) IL-10 expression was significantly higher in the metastatic group compared to the non-metastatic group, with a statistically significant difference between the two groups. * For P < 0.05.
Statistical analysis revealed 11 cases of high IL-10 expression and 3 cases of low expression in the metastatic CRC group. Conversely, in the non-metastatic CRC group, there were 13 cases of high IL-10 expression and 19 cases of low expression. These differences between the two groups were statistically significant (Table 1).
Table 1.
IL-10 expression between the two groups at pathological level.
| Group | Metastasis, n (%) | Non-metastasis, n (%) | P |
|---|---|---|---|
| High expression | 11 (78.6%) | 13 (40.6%) | |
| Low expression | 3 (21.4%) | 19 (59.4%) | |
| total | 14 (100.0) | 32 (100.0) | 0.018 |
In the metastatic CRC group, 11 cases exhibited high expression of IL-10, while 3 cases showed low expression. Conversely, in the non-metastatic CRC group, 13 cases displayed high IL-10 expression, whereas 19 cases demonstrated low expression. The disparity between the two groups was statistically significant (P = 0.018).
Significant values are in [bold].
The correlation between IL-10 expression in pathological tissues and serum was analyzed (Table 2). Results indicated 15 cases with simultaneous high expression of IL-10 at the serum level and low expression at the pathological level, 7 cases with high expression solely at the serological level, and 9 cases with high expression solely at the pathological level. These differences were statistically significant.
Table 2.
Differences in IL-10 expression at serological and pathological levels.
| Spearman correlation analysis | sIL-10 | tIL-10 |
|---|---|---|
| tIL-10 | 0.307 (P = 0.038) | 1 |
| sIL-10 | 1 | 0.307 (P = 0.038) |
IL-10 expression in pathological tissue was positively correlated with IL-10 expression in serum, P = 0.038.
Assessment of peripheral blood validation indexes in two groups of patients
The peripheral blood inflammatory indexes of the two groups of patients initially diagnosed with CRC were analyzed and tested (Table 3). The results revealed statistically significant differences between the two groups for NEUT (P = 0.011), MON (P = 0.017), NLR (P = 0.003), LWR (P = 0.002), NWR (P = 0.014), SII (P = 0.007), SIAI (P = 0.002), dNLR (P = 0.049), MLR (P = 0.002), SIRI (P < 0.001), PNI (P < 0.001), ALI (P < 0.001), IL10 (P = 0.003), and IL6 (P = 0.049). However, the differences in the rest of the indicators were not statistically significant (P > 0.05).
Table 3.
Assessment of peripheral blood inflammation indexes in the two groups of patients.
| Index | metAstasis, n = 123 | Non-metastasis, n = 201 | P |
|---|---|---|---|
| WBC (× 109/L) | 6.72 (5.7, 8.205) | 6.21 (5.07, 7.51) | 0.082 |
| NEUT (× 109/L) | 4.17 (3.175, 5.74) | 3.655 (2.95, 4.81) | 0.011 |
| LYMP (× 109/L) | 1.62 (1.255, 2.13) | 1.835 (1.41, 2.2875) | 0.09 |
| PLT (× 109/L) | 266 (211, 342) | 253.5 (203.25, 326.25) | 0.593 |
| MON (× 109/L) | 0.43 (0.31, 0.68) | 0.37 (0.29, 0.52) | 0.017 |
| PLR | 161.45 (121.665, 235.36) | 139.04 (108.345, 183.04) | 0.221 |
| NLR | 2.56 (1.66, 4) | 1.975 (1.4925, 2.9725) | 0.003 |
| LWR | 3.52 (2.5, 5.28) | 4.945 (3.25, 6.7375) | 0.002 |
| NWR | 0.64 (0.555, 0.74) | 0.60 (0.51, 0.67) | 0.014 |
| SII | 619.62 (464.755, 1216.465) | 512.725 (363.35, 782.62) | 0.007 |
| SIAI | 305.78 (172.635, 599.355) | 202.23 (119.56, 346.8125) | 0.002 |
| dNLR | 0.64 (0.555,0.74) | 0.60 (0.51, 0.67) | 0.049 |
| MLR | 0.28 (0.19, 0.40) | 0.20 (0.15, 0.31) | 0.002 |
| NLPR | 156.40 (124.71, 239.5) | 153.305 (114.6925, 195.63) | 0.157 |
| SIRI | 1.17 (0.62, 2.21) | 0.75 (0.49,1.25) | < 0.001 |
| PNI | 51.75 (46.8, 56) | 55.175 (50.6625, 58.6625) | < 0.001 |
| ALI | 391.17 (235.4, 584.455) | 536.855 (355.1225, 731.4725) | < 0.001 |
| INF-γ (Pg/ml) | 48.2 (36.45, 68.75) | 46.65 (37.625, 64) | 0.99 |
| TNF-α (Pg/ml) | 30.9 (27.9, 35.6) | 31.3 (28.9, 34.9) | 0.386 |
| IL-10 (Pg/ml) | 12.3 (10.15, 16.05) | 11.6 (9.1, 13.9) | 0.003 |
| IL-6 (Pg/ml) | 14 (11.1, 18.6) | 13.25 (10.9, 16.375) | 0.049 |
| IL-4 (Pg/ml) | 26.4 (22.5, 37.25) | 25.9 (21.9, 31) | 0.117 |
| IL-2 (Pg/ml) | 20.6 (17.55, 23.45) | 19.7 (18.1, 21.675) | 0.189 |
The results revealed statistically significant differences between the two groups for NEUT (P = 0.011), MON (P = 0.017), NLR (P = 0.003), LWR (P = 0.002), NWR (P = 0.014), SII (P = 0.007), SIAI (P = 0.002), dNLR (P = 0.049), MLR (P = 0.002), SIRI (P < 0.001), PNI (P < 0.001), ALI (P < 0.001), IL10 (P = 0.003), and IL6 (P = 0.049).
Significant values are in [bold].
Cut-off values and diagnostic efficacy of indicators
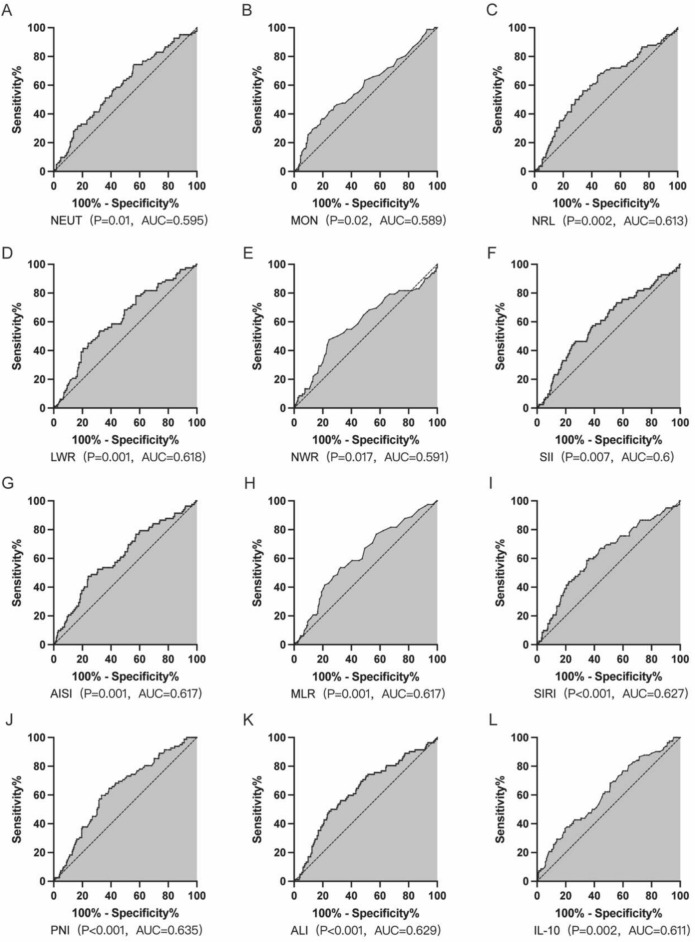
The statistically significant inflammatory indicators between the two groups were plotted on the receiver operating characteristic (ROC) curve. This curve was utilized to assess the sensitivity and specificity of these indicators for diagnosing CRC metastasis. The indicators were grouped according to the Jordon index, with the critical value chosen as the point of maximum sensitivity + specificity 1[area under the curve (AUC) maximum] for predicting metastasis occurrence (Table 4).
Table 4.
Optimal cut-off values for inflammatory indicators based on the Youden’s index of the ROC curve.
| Index | AUC (95%CI) | P | The optimal cut-off value | Sensitivity | 1-Specificity | Youden’s |
|---|---|---|---|---|---|---|
| NEUT | 0.595 (0.523–0.667) | 0.01 | 3.365 | 0.744 | 0.56 | 0.184 |
| MON | 0.589 (0.514–0.664) | 0.02 | 0.49 | 0.451 | 0.28 | 0.171 |
| NRL | 0.613 (0.54–0.685) | 0.002 | 2.135 | 0.671 | 0.444 | 0.226 |
| LWR | 0.618 (0.548–0.688) | 0.001 | 3.735 | 0.537 | 0.32 | 0.217 |
| NWR | 0.591 (0.516–0.667) | 0.017 | 0.675 | 0.476 | 0.244 | 0.231 |
| SII | 0.6 (0.527–0.672) | 0.007 | 727.595 | 0.463 | 0.267 | 0.197 |
| AISI | 0.617 (0.545–0.688) | 0.001 | 353.745 | 0.476 | 0.24 | 0.236 |
| dNLR | 0.576 (0.497–0.655) | 0.06 | 0.675 | 0.442 | 0.241 | 0.201 |
| MLR | 0.617 (0.546–0.687) | 0.001 | 0.265 | 0.537 | 0.324 | 0.212 |
| SIRI | 0.627 (0.556–0.698) | P < 0.001 | 1.025 | 0.585 | 0.347 | 0.239 |
| PNI | 0.635 (0.567–0.704) | P < 0.001 | 52.975 | 0.598 | 0.333 | 0.264 |
| ALI | 0.629 (0.557–0.7) | P < 0.001 | 353.635 | 0.448 | 0.244 | 0.243 |
| IL-10 | 0.611 (0.54–0.683) | 0.002 | 11.25 | 0.683 | 0.511 | 0.172 |
| IL-6 | 0.573 (0.499–0.648) | 0.053 | 14.65 | 0.5 | 0.356 | 0.144 |
Significant values are in [bold].
The results suggested that the best cut-off value for NEUT was 3.365 × 109/L, with a sensitivity of 0.744, a 1-specificity of 0.56 and an AUC of 0.595 (P = 0.01) (Fig. 3A); the best cut-off value for MON was 0.49 × 109/L, with a sensitivity of 0.451, a 1-specificity of 0.28 and an AUC of 0.589 (P = 0.02) (Fig. 3B); and the best NRL cut-off value was 2.135, with a sensitivity of 0.671, 1-specificity of 0.444, and AUC of 0.613 (P = 0.002) (Fig. 3C); LWR optimal cut-off value was 3.735, with a sensitivity of 0.537, specificity of 0.32, and AUC of 0.618 (P < 0.001) (Fig. 3D); and NWR optimal cut-off value was 0.675, with a sensitivity of 0.476, with a specificity of 0.24 and an AUC of 0.591 (P = 0.017) (Fig. 3E); the best cut-off value for SII was 727.595, with a sensitivity of 0.463, a 1-specificity of 0.267, and an AUC of 0.6 (P = 0.007) (Fig. 3F); the best cut-off value for AISI was 353.745, with a sensitivity of 0.476, a 1-specificity of 0.24, and an AUC of 0.617 (P = 0.001) (Fig. 3G); MLR best cut-off value of 0.265, sensitivity of 0.537, 1-specificity of 0.324, AUC of 0.617 (P = 0.001) (Fig. 3H); SIRI best cut-off value of 1.025, sensitivity of 0.585, 1-specificity of 0.347, AUC of 0.627 (P < 0.001) (Fig. 3I); the best cut-off value for PNI was 52.975, with a sensitivity of 0.598, a 1-specificity of 0.333, and an AUC of 0.635 (P < 0.001) (Fig. 3J); the best cut-off value for ALI was 353.635, with a sensitivity of 0.448, a 1-specificity of 0.244, and an AUC of 0.629 (P < 0.001) (Fig. 3K); the best cut-off value for IL10 was 11.25, with a sensitivity of 0.683, a 1-specificity of 0.511 and an AUC of 0.611 (P = 0.002) (Fig. 3L). It could be concluded that NEUT ≥ 3.365 × 109/L, MON ≥ 0.49 × 109/L, NRL ≥ 2.135, LWR ≥ 3.735, NWR ≥ 0.675, SII ≥ 727.595, AISI ≥ 353.745, MLR ≥ 0.265, SIRI ≥ 1.025, and PNI ≤ 52.975, ALI ≤ 353.635, IL10 ≥ 11.25 were risk factors for distant metastasis in CRC patients.
Figure 3.
Diagnostic efficacy of each index for CRC distant metastasis. NEUT ≥ 3.365 × 109/L, MON ≥ 0.49 × 109/L, NRL ≥ 2.135, LWR ≥ 3.735, NWR ≥ 0.675, SII ≥ 727.595, AISI ≥ 353.745, MLR ≥ 0.265, SIRI ≥ 1.025, PNI ≤ 52.975, ALI ≤ 353.635, IL10 ≥ 11.25 were the risk factors for distant metastasis in CRC patients.
Scoring system
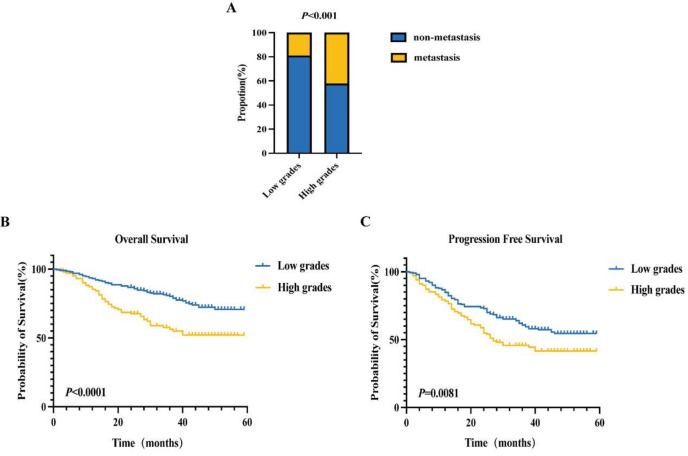
Based on the analyses, inflammation indexes with AUCs greater than 0.6 were chosen. Each CRC patient with any of the above risk factors was assigned 1 point, resulting in a scoring range of 0–8 points (Table 5). Subsequently, the 324 patients were divided into 2 groups based on their scores: those with 0–4 points were classified into the low-risk group, while those with 4–8 points were categorized into the high-risk group. The occurrence of distant metastasis in these groups underwent statistical analysis, revealing a significant difference (P < 0.05) (Fig. 4A). Survival curves for OS and PFS were then plotted for both groups (Fig. 4B,C). The average OS for the low-risk group was 49 months, with an average PFS of 41 months, whereas the average OS for the high-risk group was 40 months, with a median PFS of 27 months. These differences were statistically significant (P < 0.05).
Table 5.
Scoring system.
| Index | Score |
|---|---|
| NRL | |
| ≥ 2.135 | 1 |
| < 2.135 | 0 |
| LWR | |
| ≥ 3.735 | 1 |
| < 3.735 | 0 |
| AISI | |
| ≥ 353.745 | 1 |
| < 353.745 | 0 |
| MLR | |
| ≥ 0.265 | 1 |
| < 0.265 | 0 |
| SIRI | |
| ≥ 1.025 | 1 |
| < 1.025 | 0 |
| PNI | |
| ≤ 52.975 | 1 |
| > 52.975 | 0 |
| ALI | |
| ≤ 353.635 | 1 |
| > 353.635 | 0 |
| IL-10 | |
| ≥ 11.25 | 1 |
| < 11.25 | 0 |
Inflammation indicators with an AUC greater than 0.6 were selected, and patients with CRC were scored 1 point for each of the above risk factors, ranging from 0 to 8 points.
Figure 4.
Comparison of the occurrence of distant metastases and OS and PFS survival curves between the two groups of patients. (A) The disparity in distant metastasis between the low and high subgroups exhibits statistical significance (P < 0.001). (B) The mean OS for the low subgroup is 49 months, contrasting with 40 months for the high subgroup, both statistically significant (P < 0.05). (C) The mean PFS in the low subgroup spans 41 months, while the median PFS in the high subgroup stands at 27 months, both showing statistical significance (P < 0.05).
Discussion
In recent years, the incidence rate of CRC ranks third globally, following lung cancer and breast cancer. CRC stands as the second leading cause of cancer-related deaths1. With shifts in dietary habits and lifestyles among China's residents, the incidence and mortality rates of CRC in China have seen a consistent rise4. Early symptoms of CRC often manifest insidiously, typically progressing to intermediate or advanced stages with metastasis by the time of diagnosis, which remains the primary contributor to the high mortality rate among CRC patients5. Our findings reveal that the CRC patients with distant metastasis exhibit shorter OS and PFS durations compared to those without distant metastasis, underscoring metastasis as a determinant of poor prognosis in CRC patients. Hence, identifying prognostic markers associated with metastasis holds significant clinical relevance in CRC.
Currently, treatment options for colorectal cancer include surgery, chemotherapy, targeted therapy, immunotherapy, and combinations of these approaches. Surgery is the main option for early-stage colorectal cancer because it allows complete resection (R0 resection) and potentially cures the disease. Immunotherapy based on the immune microenvironment has made significant progress in the treatment of colorectal cancer in recent decades6,15. The interaction of various cell populations within the tumor microenvironment (TME) promotes tumor progression. In addition to tumor cells, this microenvironment includes stromal cells, endothelial cells, tumor-associated fibroblasts, immune cells, and inflammatory cells, and these diverse cell populations secrete a range of signaling molecules, including growth factors, cytokines, and chemokines, which interact with each other to create a microenvironment that supports tumor cell invasion and metastasis15.
Inflammation plays a pivotal role in tumor initiation, progression, and prognosis. Alterations in inflammatory cells profoundly influence tumor development by fostering tumor cell growth and proliferation, inducing angiogenesis within the tumor microenvironment, facilitating tumor invasion and metastasis, while concurrently exerting immunosuppressive effects against tumors16. Malnutrition is a common condition in cancer patients and is usually associated with functional limitations as well as increased morbidity and mortality17. In addition, malnourished cancer patients may be less tolerant to chemotherapy, have a lower quality of life, and have a lower overall survival rate17. Malignant inflammation and malnutrition play a key role in cancer patients, leading to poor treatment outcomes. There is growing evidence that peripheral blood parameters serve as alternative markers to reflect inflammatory status and nutritional status in cancer patients18. Due to the importance of inflammatory and nutritional status on cancer prognosis, several peripheral blood parameters including prognostic nutritional index (PNI), neutrophil/lymphocyte ratio (NLR) and pan-immuno-inflammatory value (PIV) have been widely used in clinical practice to guide the prognosis of cancer patients18.
Traditional pathological diagnosis serves as the cornerstone for tumor diagnosis. In this study, initial immunohistochemical staining of IL-10, an inflammation indicator in CRC lesions, revealed its heightened expression in the metastatic CRC group, establishing a significant correlation between IL-10 expression in metastatic CRC tissues and serum levels. In addition to IL-10, several serum inflammatory markers such as NLR, PLR, LMR, SII, and SIRI, derived from peripheral blood neutrophil, monocyte, lymphocyte, and platelet counts, have demonstrated prognostic implications across various malignancies. Further investigations have been undertaken to evaluate the prognostic and therapeutic implications of serum inflammatory markers in cancer patients. Notably, the NLR, derived from routine hematological tests by dividing the neutrophil count by the lymphocyte count, stands as an easily accessible metric19. NLR, recognized as one of the most extensively investigated inflammatory markers in malignant tumors, has consistently demonstrated prognostic relevance across various cancers. For instance, Zhang et al. reported that in patients with locally advanced rectal cancer (LARC), elevated NLR scores correlated with inferior DFS and OS outcomes. Moreover, patients with higher NLR scores in LARC following neoadjuvant radiotherapy (NCRT) might benefit from intensified adjuvant therapy20. Studies have demonstrated that LWR serves as a valuable preoperative marker in colorectal cancer patients. Additionally, LWR has been identified as an independent risk factor for overall incidence, multiple occurrences, and severe presentations of the disease21. The MLR, utilized as a biomarker of inflammatory response, is determined by dividing the monocyte count by the lymphocyte count. An investigation in the United States revealed that elevated levels of MLR, along with NLR, PLR, SII, and AISI, heightened the risk of developing prostate cancer (PCa) in adults. Notably, MLR exhibited potential superiority over other inflammatory biomarkers (NLR, SIRI, AISI, PLR, and SII) in predicting PCa22. The PNI serves as an indicator of both nutritional and immune status in patients. In a study concerning non-small cell lung cancer (NSCLC), researchers identified PNI, along with gender, as independent prognostic factors for NSCLC patients. Notably, patients with low PNI generally exhibited a poorer prognosis. Similar findings were observed in breast cancer patients, where studies revealed that individuals with high PNI experienced longer DFS and OS compared to those with low PNI across both early and advanced breast cancer stages, particularly in the advanced setting. Moreover, irrespective of molecular subtypes, breast cancer patients with a high PNI demonstrated prolonged mean DFS and OS compared to their counterparts with a low PNI23. The ALI amalgamates conventional nutritional status indicators such as body mass with blood albumin and NLR to provide a more comprehensive reflection of tumor progression in cancer patients. Studies have revealed that gastric cancer (GC) patients with a low ALI exhibit larger tumor volumes, higher TNM stages, and shorter OS. Moreover, preoperative ALI emerged as an independent prognostic factor for radical gastrectomy outcomes in GC patients24. The SIRI, calculated from peripheral blood neutrophils, monocytes, and lymphocytes, elucidates the interplay between the pro-tumor effects of inflammatory cells and anti-tumor immunity. Elevated neutrophil and monocyte levels typically foster tumor progression, whereas diminished lymphocyte counts signify suppression of the body’s anti-tumor immune response. Consequently, an elevation in neutrophils, monocytes, and/or a reduction in lymphocytes correlates with an increased SIRI, often indicative of a poorer prognosis25. Elevated SII levels in cancer patients signify an aberrantly heightened systemic inflammatory response, characterized by neutrophilia or thrombocytosis alongside reduced lymphocyte counts. These alterations are implicated in bolstering tumor angiogenesis, adhesion, and metastasis, while concurrently impeding immune clearance26.Significant differences in NLR, PLR, SII, and SIRI levels were observed between the recurrent and non-recurrent colorectal cancer groups. Subsequent investigations indicated that SII, along with lymph node metastasis, could serve as predictive factors for colorectal cancer recurrence26.
In this study, we examined the disparity in peripheral blood inflammation indexes between metastatic and non-metastatic groups, and the results suggested that NEUT, MON, NLR, LWR, NWR, SII, SIAI, dNLR, MLR, SIRI, PNI, ALI, IL10, IL6 were significantly higher than that of the non-metastatic group, and the differences were statistically significant with P < 0.05. At the same time, the above inflammatory indexes were further plotted on the ROC curve and the best cut-off value was obtained, suggesting that NEUT ≥ 3.365 × 109/L, MON ≥ 0.49 × 109/L, NRL ≥ 2.135, LWR ≥ 3.735, NWR ≥ 0.675, SII ≥ 727.595, AISI ≥ 353.745, MLR ≥ 0.265, SIRI ≥ 0.265, and MLR ≥ 0.265, and SIRI ≥ 0.695, respectively. 0.265, SIRI ≥ 1.025, PNI ≤ 52.975, ALI ≤ 353.635, and IL10 ≥ 11.25 were risk factors for distant metastasis in CRC patients. Inflammation indicators with AUC greater than 0.6 were selected, and the included patients were scored and grouped according to the above risk factors, and after statistical analysis, it was found that the risk of occurrence of metastasis in the low group was significantly lower than that in the high group, and the OS and PFS of the low group were significantly better than those of the high group.
In this study, we established a predictive scoring system by exploring the relationship between peripheral blood inflammation indexes and the prognosis of patients with colorectal cancer metastases, providing clinicians with a simple, economical and non-invasive tool for prognostic assessment. This is an important guidance for the development and adjustment of personalized treatment plans. Moreover, this study revealed the association between several peripheral blood inflammation indicators and colorectal cancer metastasis, which have the potential to be used as biomarkers for the early diagnosis of colorectal cancer metastasis, which can help to take interventions at the early stage of the disease and improve the therapeutic efficacy and patient survival. Given the strong association between inflammatory markers and response to immunotherapy, the results of this study provide a scientific basis for evaluating patients' sensitivity to immunotherapy and help optimize immunotherapy regimens.
However, this study was only conducted in the Third Affiliated Hospital of Kunming Medical University, and the sample size is relatively limited. Multi-center and large sample size validation is needed in the future to further confirm the generality and reliability of the results. The study mainly focuses on the correlation between peripheral blood inflammation indexes and metastasis and prognosis of colorectal cancer, but the specific mechanism of its role is not sufficiently explored, and further in-depth studies are needed to investigate how these inflammation indexes affect the biological behavior of colorectal cancer. In addition, this study did not fully explore the value of dynamically monitoring the changes of these inflammatory markers during treatment to predict the response to treatment and prognosis. Based on this, in the future, we would like to consider expanding the sample size and conducting a multicenter, prospective study in conjunction with multiple medical institutions at home and abroad to validate the generalizability of the current study results. And we will actively use advanced technologies such as molecular biology and gene sequencing to explore the specific molecular mechanisms between peripheral blood inflammation indicators and colorectal cancer development, to provide new targets for targeted therapy. During patient treatment, the changes of peripheral blood inflammatory indexes are regularly monitored, and the value of these indexes in predicting treatment response and prognosis is analyzed in conjunction with clinical data to provide real-time and accurate basis for clinical decision-making.
With the in-depth research on the relationship between peripheral blood inflammatory markers and colorectal cancer prognosis, the personalized treatment plan for tumor patients will be more accurate, and the therapeutic effect as well as the survival rate of patients will be further improved. Moreover, through the application of advanced technologies such as high-throughput sequencing and proteomics, more peripheral blood biomarkers related to colorectal cancer prognosis will be discovered, providing more options for early diagnosis and prognosis assessment. We believe that in the future, the close connection between inflammatory indicators and immunotherapy response will be further revealed, which will provide a scientific basis for the precise application of immunotherapy and promote the wide application of immunotherapy in colorectal cancer treatment.
In conclusion, this study has significant potential in the prognostic assessment of colorectal cancer, but it still needs to make up for its shortcomings by expanding the sample size, in-depth mechanism study and dynamic monitoring.
Acknowledgements
The author thanks all the staff of the Department of Gastrointestinal Oncology, the Third Affiliated Hospital of Kunming Medical University, Peking University Cancer Hospital Yunnan, Yunnan Cancer Hospital, Kunming, Yunnan Province, People's Republic of China.This study was supported by the Yunnan Province's ‘Xingdian Talent Support Programme’ for Famous Doctors (No. XDYC-MY-2022-0011).
Author contributions
X.S. was involved in the statistics and drafting of the paper, M.Y.X. was involved in the collection of clinical data and statistical graphing, J.D.T. was involved in the statistics and revision of the paper, T.R.X., Z.T.L. and S.Q.Y. were involved in the collection and organization of the clinical data, X.Y.C. and Y.H. were involved in the statistical graphing. All authors have read and approved the final manuscript. All authors contributed to the analysis of the data, drafting and revision of the paper and agreed to take responsibility for all aspects of the work.
Data availability
Data generated or analyzed during this study are available from the corresponding authors upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Xin Shen, Mengying Xiang and Jiadai Tang.
References
- 1.Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin.10.3322/caac.21834 (2024). 10.3322/caac.21834 [DOI] [PubMed] [Google Scholar]
- 2.Song, M. Global epidemiology and prevention of colorectal cancer. Lancet Gastroenterol. Hepatol.7, 588–590 (2022). 10.1016/S2468-1253(22)00089-9 [DOI] [PubMed] [Google Scholar]
- 3.Wu, W. et al. Cancer trends and risk factors in China over the past 30 years (1990–2019). J. Cancer14, 1935–1945 (2023). 10.7150/jca.83162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang, Y. et al. Epidemiology and risk factors of colorectal cancer in China. Chin. J. Cancer Res.32, 729–741 (2020). 10.21147/j.issn.1000-9604.2020.06.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li, F. et al. Molecular targeted therapy for metastatic colorectal cancer: Current and evolving approaches. Front. Pharmacol.14, 1165666 (2023). 10.3389/fphar.2023.1165666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leowattana, W., Leowattana, P. & Leowattana, T. Systemic treatment for metastatic colorectal cancer. World J. Gastroenterol.29, 1569–1588 (2023). 10.3748/wjg.v29.i10.1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He, X., Lan, H., Jin, K. & Liu, F. Can immunotherapy reinforce chemotherapy efficacy? A new perspective on colorectal cancer treatment. Front. Immunol.14, 1237764 (2023). 10.3389/fimmu.2023.1237764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rizzo, A. et al. Hypertransaminasemia in cancer patients receiving immunotherapy and immune-based combinations: the MOUSEION-05 study. Cancer Immunol Immunother72, 1381–1394 (2023). 10.1007/s00262-023-03366-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dall’Olio, F. G. et al. Immortal time bias in the association between toxicity and response for immune checkpoint inhibitors: A meta-analysis. Immunotherapy13, 257–270 (2021). 10.2217/imt-2020-0179 [DOI] [PubMed] [Google Scholar]
- 10.Bhat, A. A. et al. Cytokine- and chemokine-induced inflammatory colorectal tumor microenvironment: Emerging avenue for targeted therapy. Cancer Commun.42, 689–715 (2022). 10.1002/cac2.12295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feier, C. V. I., Muntean, C., Bolboacă, S. D. & Olariu, S. Exploratory evaluation of pre-treatment inflammation profiles in patients with colorectal cancer. Diseases12, 61 (2024). 10.3390/diseases12030061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen, W., Xin, S. & Xu, B. Value research of NLR, PLR, and RDW in prognostic assessment of patients with colorectal cancer. J. Healthc. Eng.2022, 1–6 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qin, L. The predictive value of NLR, PLR and MLR in the differential diagnosis of benign uterine diseases and endometrial malignant tumors. Discov. Oncol.15, 91 (2024). 10.1007/s12672-024-00956-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang, H., Gong, H., Tang, A. & Cui, Y. Neutrophil/lymphocyte ratio predicts lymph node metastasis in patients with gastric cancer. Am. J. Transl. Res.15, 1412 (2023). [PMC free article] [PubMed] [Google Scholar]
- 15.Ding, K. et al. The next bastion to be conquered in immunotherapy: Microsatellite stable colorectal cancer. Front. Immunol.14, 1298524 (2023). 10.3389/fimmu.2023.1298524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He, R. et al. Unveiling the immune symphony: Decoding colorectal cancer metastasis through immune interactions. Front. Immunol.15, 1362709 (2024). 10.3389/fimmu.2024.1362709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marano, L. et al. Clinical nutrition in surgical oncology: Young AIOM-AIRO-SICO multidisciplinary national survey on behalf of NutriOnc research group. Front. Nutr.10, 1045022 (2023). 10.3389/fnut.2023.1045022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sahin, T. K., Rizzo, A., Aksoy, S. & Guven, D. C. Prognostic significance of the Royal Marsden Hospital (RMH) score in patients with cancer: A systematic review and meta-analysis. Cancers16, 1835 (2024). 10.3390/cancers16101835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suh, J. et al. Clinical significance of pre-treated neutrophil-lymphocyte ratio in the management of urothelial carcinoma: A systemic review and meta-analysis. Front. Oncol.9, 1365 (2019). 10.3389/fonc.2019.01365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang, Y. et al. Prognostic value of pretreatment systemic inflammatory markers in patients with locally advanced rectal cancer following neoadjuvant chemoradiotherapy. Sci. Rep.10, 8017 (2020). 10.1038/s41598-020-64684-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ang, J. J., Chia, D. K. A. & Chan, D. K. H. Lymphocyte-white cell ratio is a novel marker of morbidity following colorectal cancer surgery. J. Surg. Res.259, 71–78 (2021). 10.1016/j.jss.2020.11.027 [DOI] [PubMed] [Google Scholar]
- 22.Wang, L., Li, X., Liu, M., Zhou, H. & Shao, J. Association between monocyte-to-lymphocyte ratio and prostate cancer in the U.S. population: A population-based study. Front. Cell Dev. Biol.12, 1372731 (2024). 10.3389/fcell.2024.1372731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen, L. et al. Prognostic nutritional index (PNI) in patients with breast cancer treated with neoadjuvant chemotherapy as a useful prognostic indicator. Front. Cell Dev. Biol.9, 656741 (2021). 10.3389/fcell.2021.656741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang, X., Wang, D., Sun, T., Li, W. & Dang, C. Advanced lung cancer inflammation index (ALI) predicts prognosis of patients with gastric cancer after surgical resection. BMC Cancer22, 684 (2022). 10.1186/s12885-022-09774-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou, Q. et al. Systemic inflammation response index as a prognostic marker in cancer patients: A systematic review and meta-analysis of 38 cohorts. Dose Response19, 155932582110647 (2021). 10.1177/15593258211064744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakamoto, S. et al. Systemic immune-inflammation index predicts tumor recurrence after radical resection for colorectal cancer. Tohoku J. Exp. Med.261, 229–238 (2023). 10.1620/tjem.2023.J074 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data generated or analyzed during this study are available from the corresponding authors upon reasonable request.