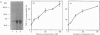Abstract
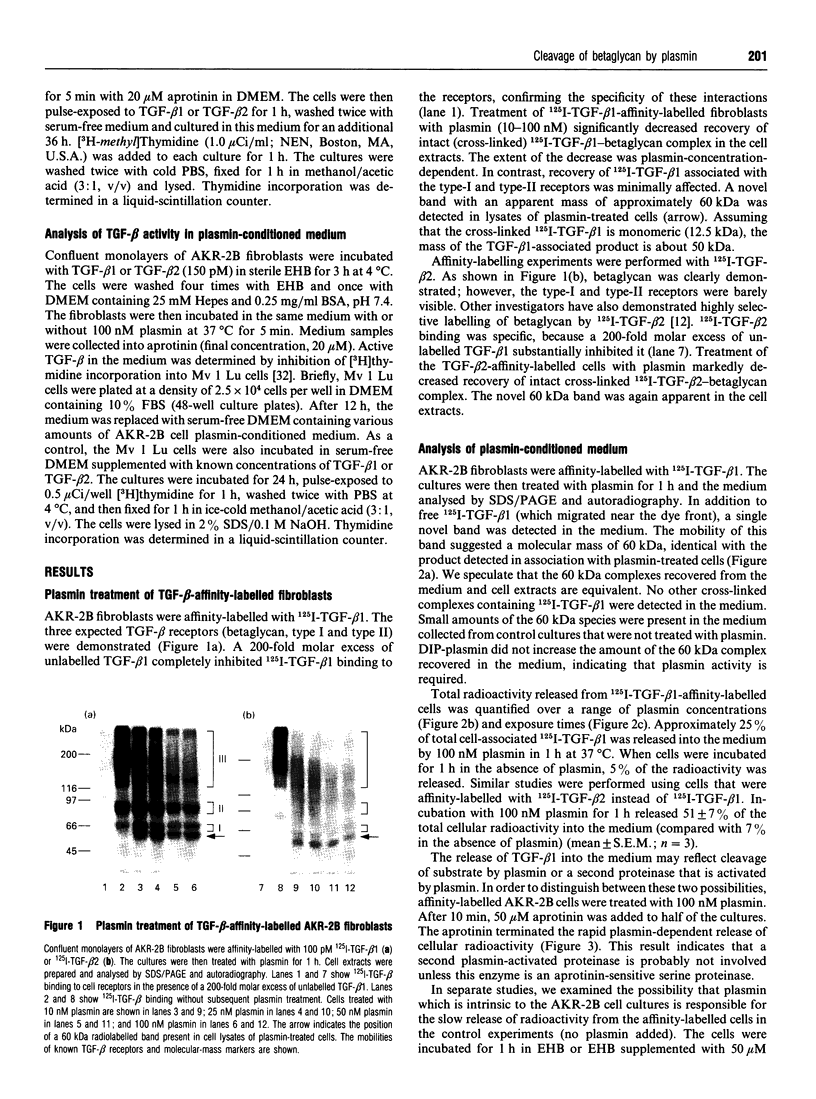
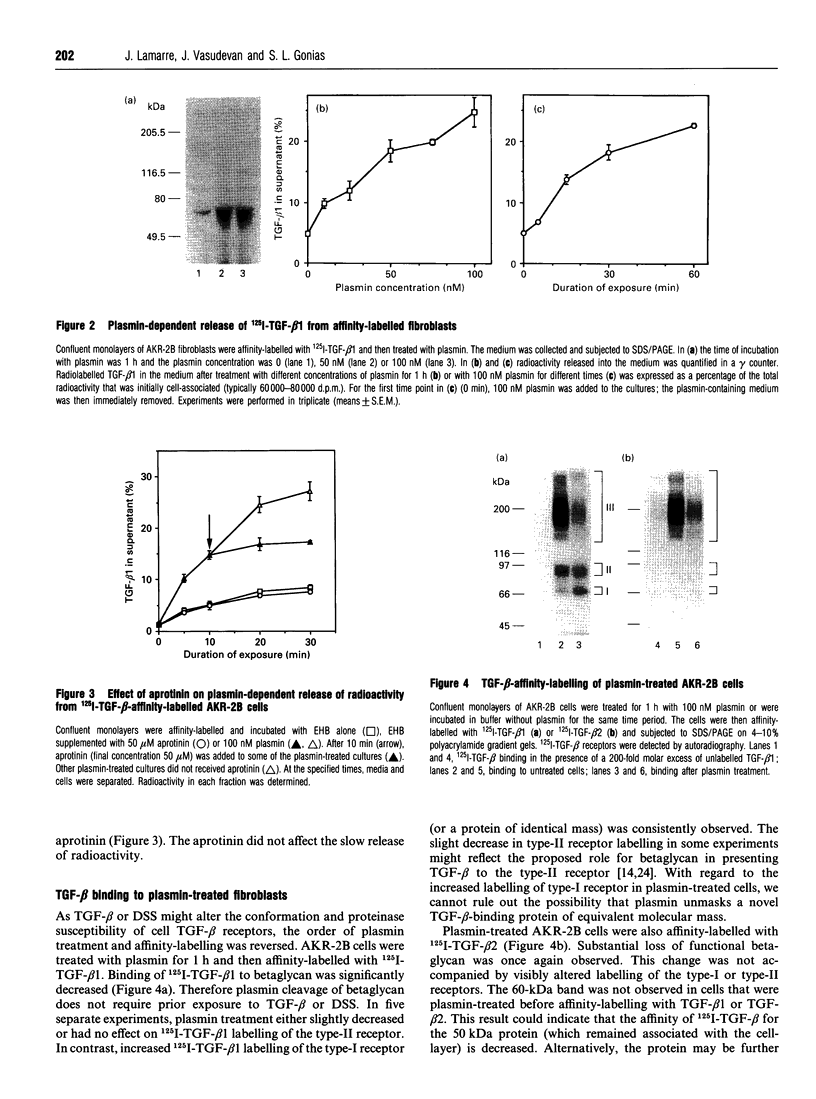
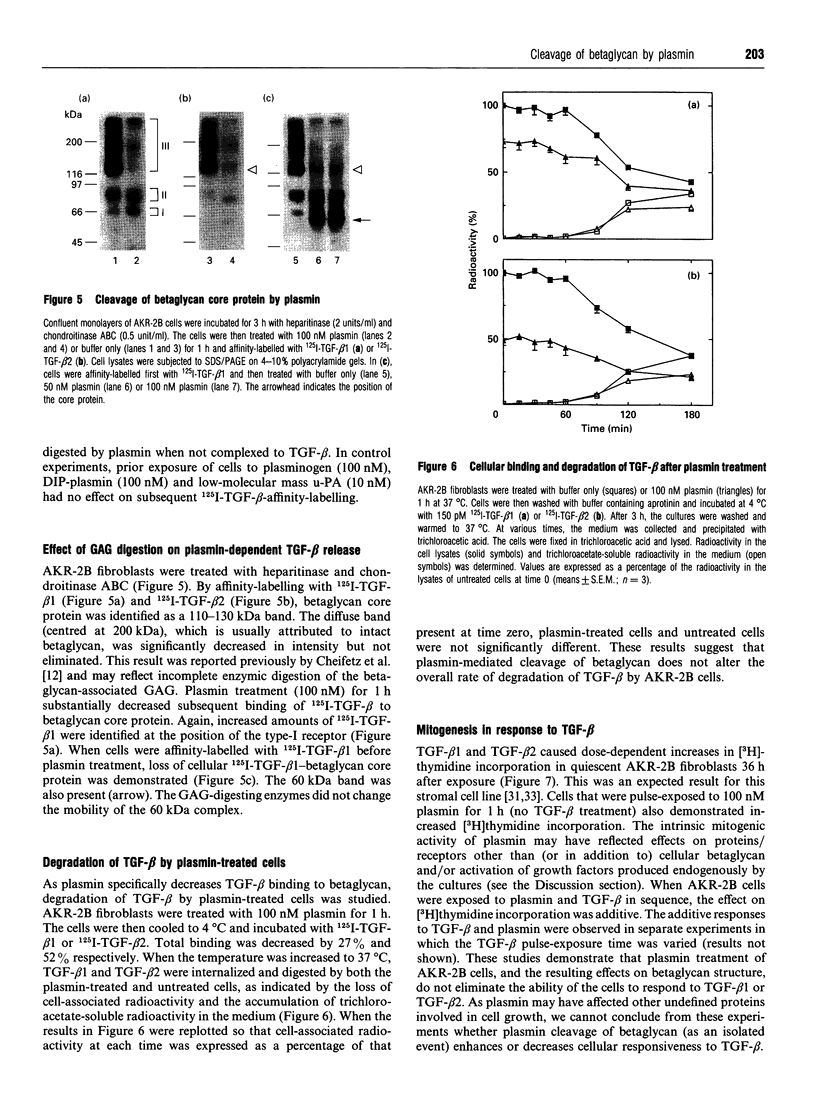
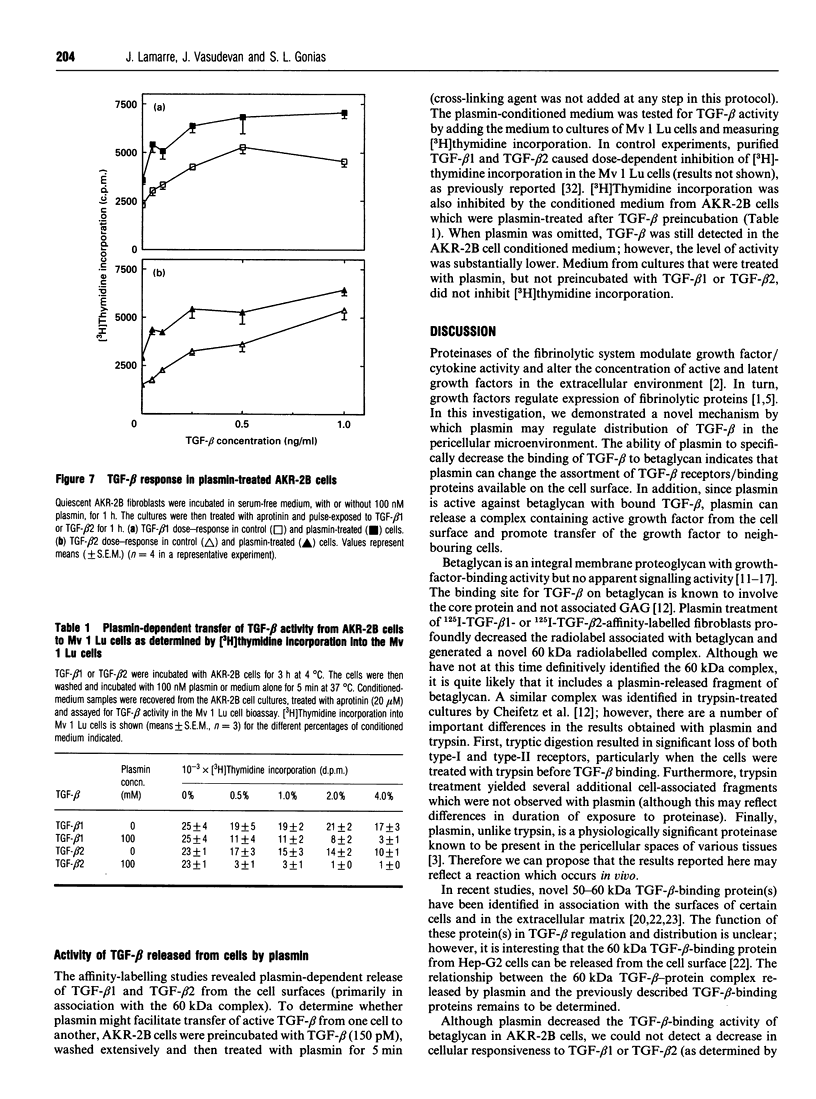
Plasmin regulates the activity and distribution of transforming growth factor beta (TGF-beta) and other growth factors. The purpose of the present investigation was to determine the effects of plasmin on cellular receptors for TGF-beta. AKR-2B fibroblasts were affinity-labelled with 125I-TGF-beta 1 and 125I-TGF-beta 2, demonstrating betaglycan, the type-I TGF-beta receptor and the type-II TGF-beta receptor. Treatment of TGF-beta-affinity-labelled cells with plasmin (10-100 nM) for 1 h profoundly and selectively decreased recovery of TGF-beta-betaglycan complex. The type-I and type-II receptors were not plasmin substrates. A radiolabelled complex with an apparent mass of 60 kDa was detected by SDS/PAGE in both the medium and cell extracts of plasmin-treated affinity-labelled cells. In order to demonstrate that plasmin cleavage of betaglycan did not require prior exposure of the betaglycan to cross-linking agent, AKR-2B cells were treated with plasmin first and then affinity-labelled. Markedly decreased TGF-beta binding to cellular betaglycan was observed. Although plasmin treatment of AKR-2B cells decreased overall binding of 125I-TGF-beta 1 and 125I-TGF-beta 2, the rate at which the cells degraded bound 125I-TGF-beta at 37 degrees C was not changed. AKR-2B cells treated with plasmin demonstrated slightly increased [3H]thymidine incorporation; the plasmin-treated cells retained their ability to respond to TGF-beta. Conditioned medium from plasmin-treated AKR-2B cells contained increased amounts of active TGF-beta as determined in Mv 1 Lu epithelial-cell-proliferation assays. Specific cleavage of betaglycan represents a novel mechanism whereby plasmin may regulate the assortment of receptors available for TGF-beta. In addition, plasmin may facilitate transfer of active TGF-beta between neighbouring cells by releasing the active growth factor from the cell surface.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andres J. L., DeFalcis D., Noda M., Massagué J. Binding of two growth factor families to separate domains of the proteoglycan betaglycan. J Biol Chem. 1992 Mar 25;267(9):5927–5930. [PubMed] [Google Scholar]
- Andres J. L., Stanley K., Cheifetz S., Massagué J. Membrane-anchored and soluble forms of betaglycan, a polymorphic proteoglycan that binds transforming growth factor-beta. J Cell Biol. 1989 Dec;109(6 Pt 1):3137–3145. doi: 10.1083/jcb.109.6.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anonick P. K., Gonias S. L. Soluble fibrin preparations inhibit the reaction of plasmin with alpha 2-macroglobulin. Comparison with alpha 2-antiplasmin and leupeptin. Biochem J. 1991 Apr 1;275(Pt 1):53–59. doi: 10.1042/bj2750053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assoian R. K., Komoriya A., Meyers C. A., Miller D. M., Sporn M. B. Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization. J Biol Chem. 1983 Jun 10;258(11):7155–7160. [PubMed] [Google Scholar]
- Battegay E. J., Raines E. W., Seifert R. A., Bowen-Pope D. F., Ross R. TGF-beta induces bimodal proliferation of connective tissue cells via complex control of an autocrine PDGF loop. Cell. 1990 Nov 2;63(3):515–524. doi: 10.1016/0092-8674(90)90448-n. [DOI] [PubMed] [Google Scholar]
- Bützow R., Fukushima D., Twardzik D. R., Ruoslahti E. A 60-kD protein mediates the binding of transforming growth factor-beta to cell surface and extracellular matrix proteoglycans. J Cell Biol. 1993 Aug;122(3):721–727. doi: 10.1083/jcb.122.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheifetz S., Andres J. L., Massagué J. The transforming growth factor-beta receptor type III is a membrane proteoglycan. Domain structure of the receptor. J Biol Chem. 1988 Nov 15;263(32):16984–16991. [PubMed] [Google Scholar]
- Danielpour D., Dart L. L., Flanders K. C., Roberts A. B., Sporn M. B. Immunodetection and quantitation of the two forms of transforming growth factor-beta (TGF-beta 1 and TGF-beta 2) secreted by cells in culture. J Cell Physiol. 1989 Jan;138(1):79–86. doi: 10.1002/jcp.1041380112. [DOI] [PubMed] [Google Scholar]
- Deutsch D. G., Mertz E. T. Plasminogen: purification from human plasma by affinity chromatography. Science. 1970 Dec 4;170(3962):1095–1096. doi: 10.1126/science.170.3962.1095. [DOI] [PubMed] [Google Scholar]
- Ellis V., Danø K. Plasminogen activation by receptor-bound urokinase. Semin Thromb Hemost. 1991 Jul;17(3):194–200. doi: 10.1055/s-2007-1002609. [DOI] [PubMed] [Google Scholar]
- Falcone D. J., McCaffrey T. A., Haimovitz-Friedman A., Vergilio J. A., Nicholson A. C. Macrophage and foam cell release of matrix-bound growth factors. Role of plasminogen activation. J Biol Chem. 1993 Jun 5;268(16):11951–11958. [PubMed] [Google Scholar]
- Flaumenhaft R., Rifkin D. B. The extracellular regulation of growth factor action. Mol Biol Cell. 1992 Oct;3(10):1057–1065. doi: 10.1091/mbc.3.10.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frolik C. A., Wakefield L. M., Smith D. M., Sporn M. B. Characterization of a membrane receptor for transforming growth factor-beta in normal rat kidney fibroblasts. J Biol Chem. 1984 Sep 10;259(17):10995–11000. [PubMed] [Google Scholar]
- Inagaki M., Moustakas A., Lin H. Y., Lodish H. F., Carr B. I. Growth inhibition by transforming growth factor beta (TGF-beta) type I is restored in TGF-beta-resistant hepatoma cells after expression of TGF-beta receptor type II cDNA. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5359–5363. doi: 10.1073/pnas.90.11.5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMarre J., Hayes M. A., Wollenberg G. K., Hussaini I., Hall S. W., Gonias S. L. An alpha 2-macroglobulin receptor-dependent mechanism for the plasma clearance of transforming growth factor-beta 1 in mice. J Clin Invest. 1991 Jan;87(1):39–44. doi: 10.1172/JCI114998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laiho M., Keski-Oja J. Growth factors in the regulation of pericellular proteolysis: a review. Cancer Res. 1989 May 15;49(10):2533–2553. [PubMed] [Google Scholar]
- Lin H. Y., Wang X. F., Ng-Eaton E., Weinberg R. A., Lodish H. F. Expression cloning of the TGF-beta type II receptor, a functional transmembrane serine/threonine kinase. Cell. 1992 Feb 21;68(4):775–785. doi: 10.1016/0092-8674(92)90152-3. [DOI] [PubMed] [Google Scholar]
- Lyons R. M., Keski-Oja J., Moses H. L. Proteolytic activation of latent transforming growth factor-beta from fibroblast-conditioned medium. J Cell Biol. 1988 May;106(5):1659–1665. doi: 10.1083/jcb.106.5.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons R. M., Moses H. L. Transforming growth factors and the regulation of cell proliferation. Eur J Biochem. 1990 Feb 14;187(3):467–473. doi: 10.1111/j.1432-1033.1990.tb15327.x. [DOI] [PubMed] [Google Scholar]
- López-Casillas F., Cheifetz S., Doody J., Andres J. L., Lane W. S., Massagué J. Structure and expression of the membrane proteoglycan betaglycan, a component of the TGF-beta receptor system. Cell. 1991 Nov 15;67(4):785–795. doi: 10.1016/0092-8674(91)90073-8. [DOI] [PubMed] [Google Scholar]
- López-Casillas F., Wrana J. L., Massagué J. Betaglycan presents ligand to the TGF beta signaling receptor. Cell. 1993 Jul 2;73(7):1435–1444. doi: 10.1016/0092-8674(93)90368-z. [DOI] [PubMed] [Google Scholar]
- Massagué J. Identification of receptors for type-beta transforming growth factor. Methods Enzymol. 1987;146:174–195. doi: 10.1016/s0076-6879(87)46020-5. [DOI] [PubMed] [Google Scholar]
- Massagué J. Receptors for the TGF-beta family. Cell. 1992 Jun 26;69(7):1067–1070. doi: 10.1016/0092-8674(92)90627-o. [DOI] [PubMed] [Google Scholar]
- Massagué J. The transforming growth factor-beta family. Annu Rev Cell Biol. 1990;6:597–641. doi: 10.1146/annurev.cb.06.110190.003121. [DOI] [PubMed] [Google Scholar]
- Morén A., Ichijo H., Miyazono K. Molecular cloning and characterization of the human and porcine transforming growth factor-beta type III receptors. Biochem Biophys Res Commun. 1992 Nov 30;189(1):356–362. doi: 10.1016/0006-291x(92)91566-9. [DOI] [PubMed] [Google Scholar]
- Moustakas A., Lin H. Y., Henis Y. I., Plamondon J., O'Connor-McCourt M. D., Lodish H. F. The transforming growth factor beta receptors types I, II, and III form hetero-oligomeric complexes in the presence of ligand. J Biol Chem. 1993 Oct 25;268(30):22215–22218. [PubMed] [Google Scholar]
- Saksela O., Rifkin D. B. Release of basic fibroblast growth factor-heparan sulfate complexes from endothelial cells by plasminogen activator-mediated proteolytic activity. J Cell Biol. 1990 Mar;110(3):767–775. doi: 10.1083/jcb.110.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y., Rifkin D. B. Inhibition of endothelial cell movement by pericytes and smooth muscle cells: activation of a latent transforming growth factor-beta 1-like molecule by plasmin during co-culture. J Cell Biol. 1989 Jul;109(1):309–315. doi: 10.1083/jcb.109.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segarini P. R., Seyedin S. M. The high molecular weight receptor to transforming growth factor-beta contains glycosaminoglycan chains. J Biol Chem. 1988 Jun 15;263(17):8366–8370. [PubMed] [Google Scholar]
- Shipley G. D., Tucker R. F., Moses H. L. Type beta transforming growth factor/growth inhibitor stimulates entry of monolayer cultures of AKR-2B cells into S phase after a prolonged prereplicative interval. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4147–4151. doi: 10.1073/pnas.82.12.4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Transforming growth factor-beta: recent progress and new challenges. J Cell Biol. 1992 Dec;119(5):1017–1021. doi: 10.1083/jcb.119.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taipale J., Koli K., Keski-Oja J. Release of transforming growth factor-beta 1 from the pericellular matrix of cultured fibroblasts and fibrosarcoma cells by plasmin and thrombin. J Biol Chem. 1992 Dec 15;267(35):25378–25384. [PubMed] [Google Scholar]
- Wang X. F., Lin H. Y., Ng-Eaton E., Downward J., Lodish H. F., Weinberg R. A. Expression cloning and characterization of the TGF-beta type III receptor. Cell. 1991 Nov 15;67(4):797–805. doi: 10.1016/0092-8674(91)90074-9. [DOI] [PubMed] [Google Scholar]
- Wrana J. L., Attisano L., Cárcamo J., Zentella A., Doody J., Laiho M., Wang X. F., Massagué J. TGF beta signals through a heteromeric protein kinase receptor complex. Cell. 1992 Dec 11;71(6):1003–1014. doi: 10.1016/0092-8674(92)90395-s. [DOI] [PubMed] [Google Scholar]