Abstract
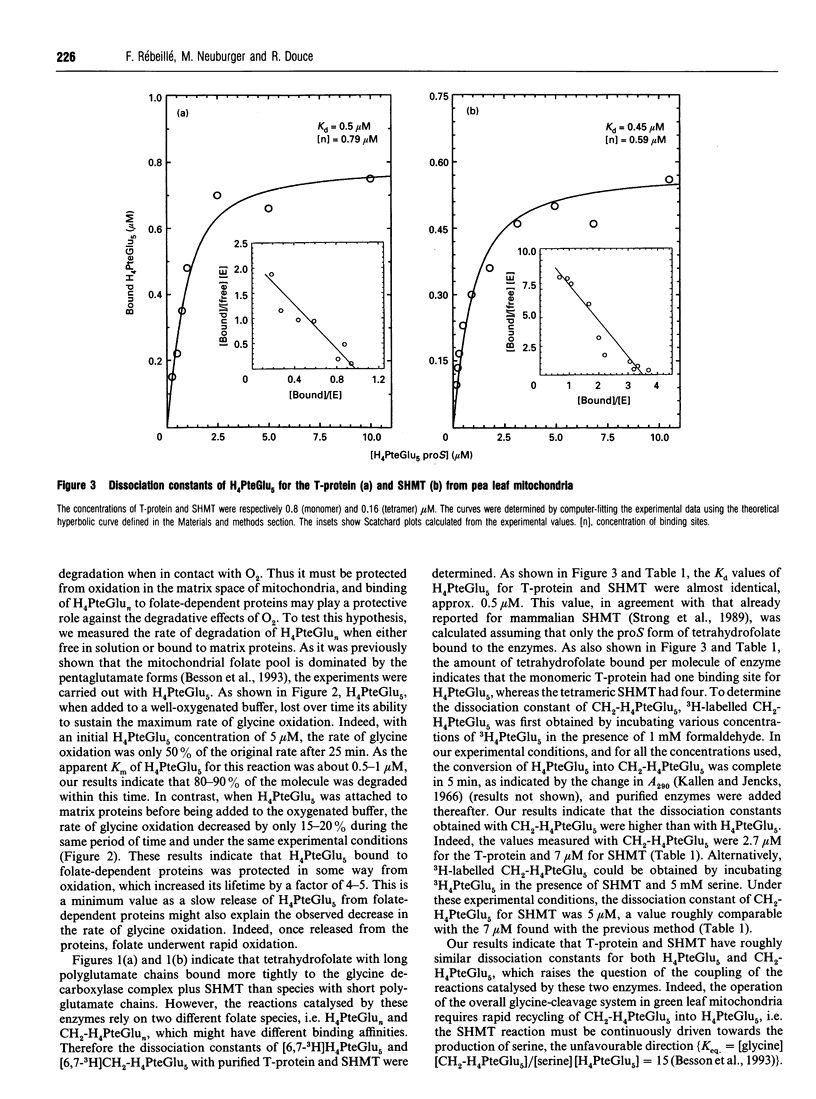
The aim of the present work was to further determine how the T-protein of the glycine-cleavage system and serine hydroxy-methyltransferase (SHMT), two folate-dependent enzymes from pea leaf mitochondria, interact through a common pool of tetrahydrofolate polyglutamates (H4PteGlun). It was observed that the binding affinity of tetrahydrofolate polyglutamates for these proteins continuously increased with increasing number of glutamates up to six residues. It was also established that, once bound to the proteins, tetrahydrofolate, a very O2-sensitive molecule, was protected from oxidative degradation. The dissociation constants (Kd) of H4PteGlu5, the most predominant form of polyglutamate in the mitochondria, were approximately 0.5 microM for both T-protein and SHMT, whereas the Kd values of CH2-H4PteGlu5 were higher, 2.7 and 7 microM respectively. In a matrix extract from pea leaf mitochondria, the maximal activity of the glycine-cleavage system was about 2.5 times higher than the maximal activity of SHMT. This resulted in a permanent disequilibrium of the SHMT-catalysed reaction which was therefore driven toward the production of serine and H4PteGlun, the thermodynamically unfavourable direction. Indeed, measurements of the steady-state ratio of CH2-H4PteGlun/H4PteGlun (n = 1 or n = 5) during the course of glycine oxidation demonstrated that the methylene form accounted for 65-80% of the folate pool. This indicates that, in our in vitro experiments, CH2-H4PteGlun with long polyglutamate chains accumulated in the bulk medium. This observation suggests that, in these in vitro experiments at least, there was no channelling of CH2-H4PteGlu5 between the T-protein and SHMT.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Besson V., Rebeille F., Neuburger M., Douce R., Cossins E. A. Effects of tetrahydrofolate polyglutamates on the kinetic parameters of serine hydroxymethyltransferase and glycine decarboxylase from pea leaf mitochondria. Biochem J. 1993 Jun 1;292(Pt 2):425–430. doi: 10.1042/bj2920425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguignon J., Neuburger M., Douce R. Resolution and characterization of the glycine-cleavage reaction in pea leaf mitochondria. Properties of the forward reaction catalysed by glycine decarboxylase and serine hydroxymethyltransferase. Biochem J. 1988 Oct 1;255(1):169–178. doi: 10.1042/bj2550169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguignon J., Vauclare P., Merand V., Forest E., Neuburger M., Douce R. Glycine decarboxylase complex from higher plants. Molecular cloning, tissue distribution and mass spectrometry analyses of the T protein. Eur J Biochem. 1993 Oct 1;217(1):377–386. doi: 10.1111/j.1432-1033.1993.tb18256.x. [DOI] [PubMed] [Google Scholar]
- Kallen R. G., Jencks W. P. The mechanism of the condensation of formaldehyde with tetrahydrofolic acid. J Biol Chem. 1966 Dec 25;241(24):5851–5863. [PubMed] [Google Scholar]
- Kisliuk R. L. Pteroylpolyglutamates. Mol Cell Biochem. 1981 Sep 25;39:331–345. doi: 10.1007/BF00232583. [DOI] [PubMed] [Google Scholar]
- Maley G. F., Maley F., Baugh C. M. Studies on identifying the folylpolyglutamate binding sites of Lactobacillus casei thymidylate synthetase. Arch Biochem Biophys. 1982 Jul;216(2):551–558. doi: 10.1016/0003-9861(82)90244-2. [DOI] [PubMed] [Google Scholar]
- McGuire J. J., Bertino J. R. Enzymatic synthesis and function of folylpolyglutamates. Mol Cell Biochem. 1981 Aug 11;38(Spec No)(Pt 1):19–48. doi: 10.1007/BF00235686. [DOI] [PubMed] [Google Scholar]
- Oliver D. J., Neuburger M., Bourguignon J., Douce R. Interaction between the Component Enzymes of the Glycine Decarboxylase Multienzyme Complex. Plant Physiol. 1990 Oct;94(2):833–839. doi: 10.1104/pp.94.2.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquin J., Baugh C. M., MacKenzie R. E. Channeling between the active sites of formiminotransferase-cyclodeaminase. Binding and kinetic studies. J Biol Chem. 1985 Dec 5;260(28):14925–14931. [PubMed] [Google Scholar]
- Schirch V., Strong W. B. Interaction of folylpolyglutamates with enzymes in one-carbon metabolism. Arch Biochem Biophys. 1989 Mar;269(2):371–380. doi: 10.1016/0003-9861(89)90120-3. [DOI] [PubMed] [Google Scholar]
- Scrimgeour K. G., Vitols K. S. The reduction of folate by borohydride. Biochemistry. 1966 Apr;5(4):1438–1443. doi: 10.1021/bi00868a042. [DOI] [PubMed] [Google Scholar]
- Strong W. B., Cook R., Schirch V. Interaction of tetrahydropteroylpolyglutamates with two enzymes from mitochondria. Biochemistry. 1989 Jan 10;28(1):106–114. doi: 10.1021/bi00427a016. [DOI] [PubMed] [Google Scholar]
- Strong W. B., Tendler S. J., Seither R. L., Goldman I. D., Schirch V. Purification and properties of serine hydroxymethyltransferase and C1-tetrahydrofolate synthase from L1210 cells. J Biol Chem. 1990 Jul 25;265(21):12149–12155. [PubMed] [Google Scholar]
- Turner S. R., Ireland R., Morgan C., Rawsthorne S. Identification and localization of multiple forms of serine hydroxymethyltransferase in pea (Pisum sativum) and characterization of a cDNA encoding a mitochondrial isoform. J Biol Chem. 1992 Jul 5;267(19):13528–13534. [PubMed] [Google Scholar]
- Usha R., Savithri H. S., Rao N. A. Arginine residues involved in binding of tetrahydrofolate to sheep liver serine hydroxymethyltransferase. J Biol Chem. 1992 May 5;267(13):9289–9293. [PubMed] [Google Scholar]


