Our study demonstrates the impact of diet on breast cancer risk, focusing on the interplay between diet, the gut microbiome, and mammary gland inflammation.
Abstract
Several studies indicate a strong link between obesity and the risk of breast cancer. Obesity decreases gut microbial biodiversity and modulates Bacteroidetes-to-Firmicutes phyla proportional abundance, suggesting that increased energy-harvesting capacity from indigestible dietary fibers and elevated lipopolysaccharide bioavailability may promote inflammation. To address the limited evidence linking diet-mediated changes in gut microbiota to breast cancer risk, we aimed to determine how diet affects the microbiome and breast cancer risk. For ten weeks, female 3-week-old BALB/c mice were fed six different diets (control, high-sugar, lard, coconut oil, lard + flaxseed oil, and lard + safflower oil). Fecal 16S sequencing was performed for each group. Diet shifted fecal microbiome populations and modulated mammary gland macrophage infiltration. Fecal-conditioned media shifted macrophage polarity and inflammation. In our DMBA-induced breast cancer model, diet differentially modulated tumor and mammary gland metabolism. We demonstrated how dietary patterns change metabolic outcomes and the gut microbiota, possibly contributing to breast tumor risk. Furthermore, we showed the influence of diet on metabolism, inflammation, and macrophage polarity. This study suggests that dietary–microbiome interactions are key mediators of breast cancer risk.
Prevention Relevance: Our study demonstrates the impact of diet on breast cancer risk, focusing on the interplay between diet, the gut microbiome, and mammary gland inflammation.
Introduction
In 2023, an estimated 297,790 new cases of invasive breast cancer and 55,720 new cases of noninvasive (in situ) breast cancer will be diagnosed in women in the United States despite significant progress in diagnosis and treatment (1). Obesity is a recognized risk factor for both breast cancer development and recurrence, even in patients receiving appropriate treatment. Compared with nonobese women with breast cancer, obese women with breast cancer have worse disease-free and overall survival rates despite appropriate treatment. Furthermore, obese patients are at an increased risk of recurrence compared with normal-weight women, as both systemic chemotherapy and endocrine-targeting therapies are less effective (2).
The prevalence of obesity has nearly tripled in the population since 1975. According to the World Health Organization, more than 1.9 billion adults (39%) were considered overweight and 650 million adults considered obese in 2016 (3). Obesity results from the accumulation of excessive fat mass due to abnormal energy intake and expenditure concomitant with chronic low-grade inflammation (4). Obesity is associated with lipid and glucose metabolism disorders, chronic inflammation, and oxidative stress (5). It is a multifactorial chronic disease associated with other metabolic disorders, including type 2 diabetes and 13 different cancer types (6).
Recent studies identified a link between obesity and the composition and functionality of gut microorganisms (7, 8). The gut microbiome encompasses a dynamic population of microorganisms including bacteria, archaea, fungi, and viruses. Alterations in microbial diversity can disturb host metabolism and gut microbiome homeostasis, contributing to obesity progression. For example, studies have found that the number of Firmicutes increases, whereas the number of Bacteroidetes decreases in obese animals and humans (9). The increased Firmicutes/Bacteroidetes (F/B) ratio plays a role in increasing energy storage in host adipose tissue by facilitating the extraction of energy, which has been linked to obesity development (4).
The gut microbiota regulates energy intake through the production of short-chain fatty acids (SCFA) from nondigestible polysaccharides (10). SCFAs such as acetate, butyrate, and propionate, produced by bacterial fermentation, act as energy substrates and modulators of satiety and food consumption when they bind to G-protein coupled receptor 41 (GPR41) and GPR43 in intestinal epithelial cells (11). Furthermore, gut microbiota diversity, composition, and metabolic activity are closely associated with nutrient intake and dietary patterns; therefore, diet is a crucial component related to the interactions between gut microbiota and obesity progression (12).
Diet is critical for both the progression of obesity and the gut microbial composition (13). Both high-fat diet (HFD) and high-sugar diets (HS) contribute to obesity and alter gut microbiota composition by reducing microbial diversity, particularly the abundance of beneficial Bifidobacterium and Akkermansia. Furthermore, epidemiological studies indicate that women who consume a HFD have a five-fold higher risk of breast cancer than those who consume low-fat diets (14). Although dietary fat is known to play an important role in carcinogenesis, it remains unclear which specific fatty acids contribute to breast cancer development (15). Therefore, we aimed to determine how different dietary fats mediate changes within the gut microbial community to influence breast cancer development.
Materials and Methods
Lipopolysaccharide (LPS) was purchased from (Sigma Aldrich, cat# L2630). Antibodies against Gram-positive bacteria (Santa Cruz; cat# sc-57752), CD68-FITC, CD80-Cy5, F4/80-FITC, CD80-APC, CD206-PE (BioLegend; cat# 137006, 104711, 123108, 104714, 141706), TNFα, iNOS, ARG-1, and β-actin (Cell Signaling Technology; cat# 11948, 13120, 9819, 4967) were used.
Animals
Thirty female 3-week-old BALB/c mice were purchased from Harlan. All Teklad custom diets were purchased from Envigo. Mice were placed on a 10% fat/10% sucrose control diet (Control; TD.08806), a 10% fat/60% sucrose diet [HS; TD.160065], 60% kcal from fat/10% sucrose lard-based diet (Lard diet; TD.06414), 60% kcal from fat/10% sucrose coconut oil-based diet [Coconut oil diet (CO); TD.08500], 60% kcal from fat/10% sucrose lard + flaxseed oil-based diet [Flaxseed + lard diet (FO); TD.160066], or 60% kcal from fat/10% sucrose lard + safflower oil-based diet [Safflower lard diet (SO); 160067] for ten weeks (n = 5 per group). See Table 1 for details on the dietary components. The diets were stored at 4°C in vacuum-sealed packages containing 2 kg of diet per package. Diets were used within one month of opening.
Table 1.
Nutritional data for preclinical murine experimental diets.
| Control diet (TD.08806) | HS diet (TD.160065) | Lard diet (TD.06414) | CO diet (TD.08500) | Lard + flaxseed oil diet (TD.160066) | Lard + safflower oil diet (TD.160067) | |
|---|---|---|---|---|---|---|
| Protein (% kcal) | 20.5% | 19.5% | 18.3% | 18.3% | 18.3% | 18.3% |
| Carbohydrates(% kcal) | 69.1% | 70.6% | 21.4% | 21.4% | 21.4% | 21.4% |
| Fat (% kcal) | 10% | 9.9% | 60.3% | 60.3% | 60.3% | 60.3% |
| Kcal/gram | 3.6 | 3.8 | 5.1 | 5.1 | 5.1 | 5.1 |
| Saturated fat | 27% | 29% | 29% | 92.5% | 22% | 21% |
| Monounsaturated fat | 36.5% | 37% | 37% | 2% | 32% | 28% |
| Polyunsaturated fat | 36.5% | 34% | 34% | 5.5% | 33% | 51% |
| n-6:n-3 ratio | 7.1 | 7.1 | 8.8 | 6.7 | 0.4 | 122 |
| Sucrose | 11.2% | 62.7% | 12.1% | 12.1% | 12.1% | 12.1% |
| Cholesterol (mg/kg) | 60 | 60 | 350 | 53 | 186 | 186 |
| Sodium (g/kg) | 1.0 | 1.0 | 1.4 | 1.4 | 1.4 | 1.4 |
| Potassium (g/kg) | 3.6 | 3.6 | 4.9 | 4.9 | 4.9 | 4.9 |
| Magnesium (mg/kg) | 520 | 520 | 710 | 710 | 710 | 710 |
| Fiber | 3.7% | 3.7% | 6.6% | 6.6% | 6.6% | 6.6% |
| Vitamin A (IU/kg) | 6,000 | 6,000 | 8,400 | 8,400 | 8,400 | 8,400 |
| Vitamin B12 (ug/kg) | 37.5 | 37.5 | 52.5 | 52.5 | 52.5 | 52.5 |
| Vitamin D (IU/kg) | 1,500 | 1,500 | 2,100 | 2,100 | 2,100 | 2,100 |
| Vitamin E (IU/kg) | 112.5 | 112.5 | 157.5 | 157.5 | 157.5 | 157.5 |
Metabolic parameters such as weight were measured weekly and glucose tolerance testing was performed after ten weeks of diet administration. Fecal matter was collected and frozen after 10 weeks of diet administration for 16S sequencing. The 4/5 inguinal mammary glands were frozen or FFPE for analysis. The protocol was approved by the Animal Care and Use Committee of the Wake Forest School of Medicine (protocol # A16-010), and all procedures were performed in accordance with the relevant guidelines and regulations.
DMBA-mammary carcinogenesis model
Ninety female 3-week-old BALB/c mice were randomized into a 10% fat/10% sucrose control diet (Control; TD.08806), 10% fat/60% sucrose diet [HS; TD.160065], 60% kcal from fat/10% sucrose lard-based diet (Lard diet; TD.06414), 60% kcal from fat/10% sucrose coconut oil-based diet [CO; TD.08500], 60% kcal from fat/10% sucrose lard + flaxseed oil-based diet [Flaxseed lard diet (FO); TD.160066], or 60% kcal from fat/10% sucrose lard + safflower oil-based diet [Safflower lard diet (SO); 160067] for the duration of the study (n = 15 per group). At six weeks of age, mice were treated with a single subcutaneous injection of 15 mg medroxyprogesterone acetate, followed by weekly doses of 1 mg DMBA in peanut oil for three weeks to induce mammary tumorigenesis (16). Mice were palpated weekly for tumor formation, 16 weeks post-DMBA administration. Tumor-free survival, tumor multiplicity, and tumor wet weight were recorded. Plasma, tumors, and tissues from the mammary glands, intestines, and liver were collected at the end of the study period.
16S sequencing and statistics
Fecal bacterial microbiome 16S sequencing was performed by Microbiome Insights Inc. (Vancouver, British Columbia). DNA was isolated from feces using the MoBio PowerSoil Extraction Kit. The 16S rRNA genes were PCR-amplified with dual-barcoded primers targeting the V4 region, as previously described (17,18). Amplicons were sequenced on an Illumina MiSeq using a 250-bp paired-end kit (v.2). Bacterial sequences were denoized, taxonomically classified using Greengenes (v. 13_8), and clustered into 97% similarity operational taxonomic units (OTU) using the Mothur software package (v. 1.38), following the recommended protocol (https://www.mothur.org/wiki/MiSeq_SOP; accessed Sep 09, 2016). MiSeq-generated Fastq files were quality filtered and clustered into 97% similarity OTUs using the Mothur software package (http://www.mothur.org). We obtained 7.92666105 high-quality reads. Our final dataset had 11,222 OTUs (including those occurring once with a count of 1) and per-sample read ranges of 1.4186 × 104 and 4.4213 × 104. High-quality reads were classified using Greengenes v. 13_8 as the reference database. We excluded OTUs occurring in fewer than three samples with a count of less than three and calculated alpha (Shannon) and beta diversity (Bray–Curtis dissimilarity) indices using the phyloseq R package. We visualized community composition, emphasizing differences across sites, using non-metric multidimensional (NMDS) ordination. The significance of differences in diversity was tested using ANOVA. Community structure variations were assessed using permutational multivariate analyses of variance, with the treatment group as the main fixed factor and 4,999 permutations for significance testing. We corrected for the paired nature of the experimental design in the fecal transplant study by restricting the permutations to mouse ID. Finally, we used DESeq2 to examine the OTUs across the treatment groups.
Cell culture
The cells were grown at 37°C in a humidified atmosphere containing 5% CO2 and 95% air. Mouse macrophage cell lines (RAW 264.7) were grown in phenol red-containing RPMI medium containing 10% fetal bovine serum under basal growth conditions. RAW 264.7 cells were treated with a 1:10 dilution of 10% fecal-derived conditioned media (sterile-filtered before use) for 24 hours. Fecal-derived conditioned media were formulated by mixing 1 g of feces from mice on different diets in 10 mL of basal growth RPMI medium. The feces–media mixture was vortexed until uniformly suspended in media and incubated for 1 hour in a 37°C water bath. The media were sterile-filtered to remove bacterial contaminants, leaving fecal-derived bacterial compounds and metabolites.
RT-PCR
RNA was extracted from mammary glands and RAW 264.7 macrophage cells using TRIzol, following the manufacturer’s protocol. cDNA was synthesized from 5 μg of total RNA using Superscript first-strand RT-PCR reagents, as described by the manufacturer. RT-qPCR was performed using the SYBR Green kit. Primers were used for CD68, NOS2, ARG1, TNFα, IL6, IL10, and HPRT.
Western blot
Protein homogenates from RAW 264.7 cells treated with 1 µg/mL LPS or 10% (100 µL in 0.9 mL basal growth media) fecal-derived conditioned media overnight were used to determine the effect of microbiota regulated metabolites on macrophage polarity markers. The membranes were incubated with iNOS-, ARG-1-, and TNF-α-specific antibodies, and the relative protein levels were measured by chemiluminescence densitometry. β-Actin was used to normalize the protein loading.
Immunofluorescence
Mammary gland tissues from mice in each diet group were immunostained for CD68-FITC (5 µg/mL) and CD80-Cy5 (5 µg/mL), and counterstained with DAPI to identify infiltrating M1 macrophage populations. Fluorescent signals were visualized with a 40× objective using a Mantra Quantitative Pathology workstation with inForm imaging analysis software (PerkinElmer).
Flow cytometry
Three-week-old female BALB/c mice were fed a control, HS, lard, CO, lard + safflower oil, or lard + flaxseed oil diet for five weeks (n = 5 per group). Mammary glands were removed, minced, and digested in collagenase medium (5 mg/mL collagenase in RPMI medium) for 1 hour at 37°C. The mixture was passed through a cell strainer to remove fibrous tissue, red blood cells were lysed, and the pellet was centrifuged at 350 rpm for 10 minutes. The pellet was fixed, washed, and stained with F4/80-FITC (1:30), CD80-APC (1:30), and CD206-PE (1:50). The mammary gland macrophage populations were determined by flow cytometry using a BD FACSCalibur instrument.
Immunohistochemistry
Sections of paraffin-embedded mammary gland tissues from mice on different diets and DMBA-induced breast tumors were stained for Gram-positive bacteria (Firmicutes are the main phyla that, for the most part, are Gram-positive) to identify the presence of a tumor/gland-specific microbial population, and staining was visualized by the Mantra Quantitative Pathology Image System with a 40× objective.
Metabolomics
Untargeted metabolomics was performed on snap-frozen mammary glands and tumors from the diet-DMBA-mammary carcinogenesis model (Metabolon®, Raleigh, NC), as previously described (19, 20). Briefly, samples were prepared using the automated MicroLab Star system from the Hamilton Company. Several recovery standards were added before the first step of the extraction process for quality control (QC) purposes. The extract was divided into five fractions: two for analysis by two separate reverse phase (RP)/UPLC-MS/MS with positive-ion mode electrospray ionization (ESI), one for analysis by RP/UPLC-MS/MS with negative-ion mode ESI, one for analysis by HILIC/UPLC-MS/MS with negative-mode ESI, and one for backup. A Waters ACQUITY ultra-performance liquid chromatography (UPLC) system, Thermo Scientific Q-Exactive mass spectrometer interfaced with a heated electrospray ionization (HESI-II) source, and Orbitrap mass analyzer were used. The compounds were compared with the library entries of purified standards or recurrent unknown entities for identification. Peaks were quantified using the area under the curve. The informatics system consisted of a Laboratory Information Management System, data extraction and peak-identification software, data processing tools for QC and compound identification, and a collection of information interpretation and visualization tools. The LAN backbone and a database server running Oracle 10.2.1.1 Enterprise Edition were the hardware and software foundations for these informatics components, respectively. The log transformation and imputation of missing values were performed with the minimum observed value for each compound. Welch’s two-sample t-test was used to identify biochemicals that differed significantly between the experimental groups. A total of 4,722 known biochemical compounds were identified. Differences were considered statistically significant at P < 0.05.
Statistics
Data are presented as mean ± standard deviation. Statistical differences were evaluated by one-way ANOVA followed by Bonferroni or Tukey post-hoc tests, or survival data using the log-rank Mantel-Cox test. Statistical significance was P < 0.05.
Data availability
Genus-level 16S sequencing OTU proportional abundance data are available (Supplementary Table S1. Genus OTU proportional abundance in feces from mice fed varying diets). A complete untargeted metabolomic dataset is available (Supplementary Material). The data generated in this study are available within the article and its supplementary data files.
Results
Dietary-induced metabolic changes
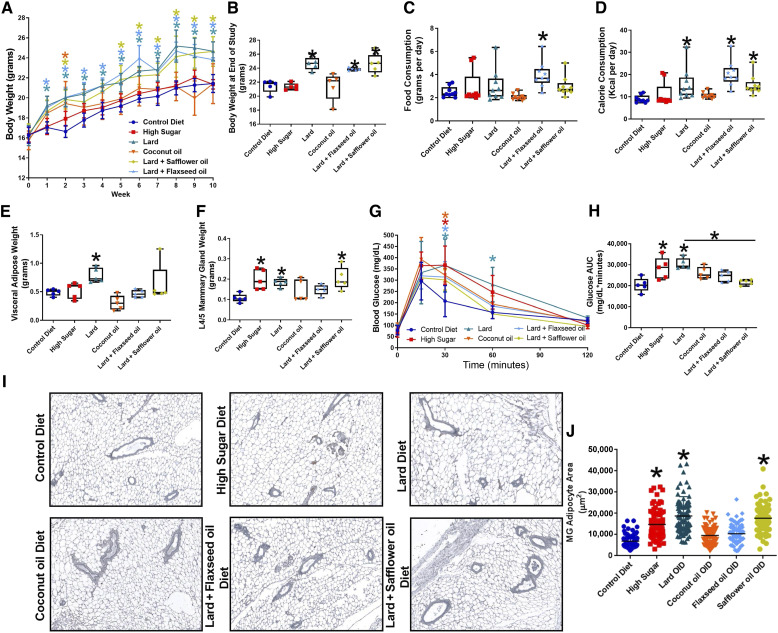
Mice consuming lard, flaxseed oil + lard (FO), and safflower oil + lard (SO) diets gained significantly more weight than mice fed the control diet (Fig. 1A and B). Consumption of the HS diet did not affect mouse weight. CO-fed mice initially gained weight; however, by week 5, their weights were similar to those of mice fed the control diet. In line with these data, mice fed the FO diet consumed significantly more (Fig. 1C), and mice fed lard, FO, and SO diets consumed more calories than mice fed the control diet (Fig. 1D). At the end of the 10-week study, mice fed the lard diet had elevated visceral adipose weight (gonadal fat pad) compared with control diet-fed mice (Fig. 1E). Mammary glands were approximately 2-fold heavier in mice that consumed the HS, lard, or SO diets than in mice fed the control diet (Fig. 1F). After ten weeks, glucose tolerance tests were performed on mice fed different diets; the consumption of lard and HS decreased blood glucose clearance (Fig. 1G and H). FO diet-fed mice had a significantly decreased glucose area under the curve than lard diet-fed mice. Changes in MG adipocytes were also observed, with significant increases in MG adipocyte area observed in the HS, lard, and SO diet-fed mice (Fig. 1I and J).
Figure 1.
Diet affects the metabolic parameters. Three-week-old female BALB/c mice were placed on either a control, high-sucrose, lard diet, coconut oil (CO) diet, flaxseed oil + lard diet, or a safflower oil + lard diet for ten weeks. A, Mouse weight. B, Body weight at the end of the study. C, Food consumption. D, Calorie consumption. E, Visceral adipose weight (gonadal fat pad). F, Mammary gland weight (L4/5 mammary gland). G, Blood glucose curves. H, Glucose area under the curve. I, Mammary gland representative images. J, Mammary gland adipocyte area. 100 adipocytes measured per image; *, P < 0.05; n = 5 per group.
Diet shifts fecal microbiome composition
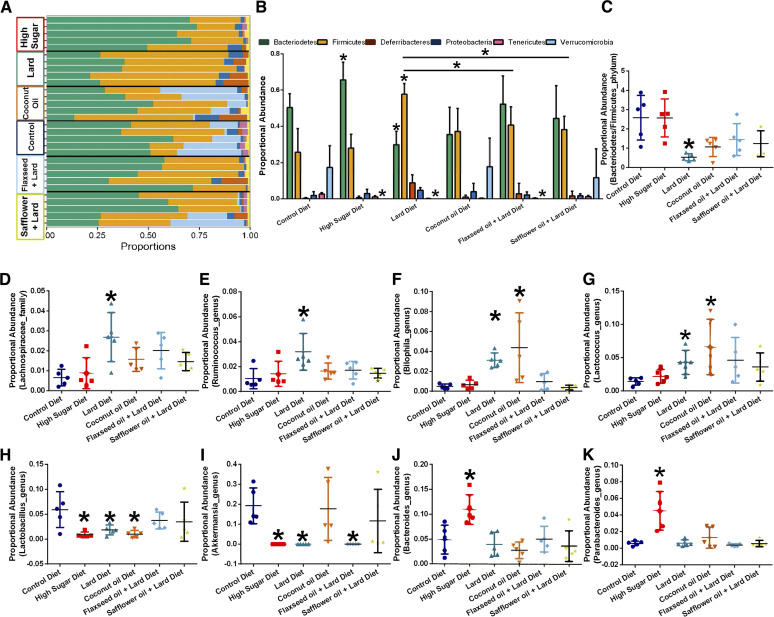
We sequenced the 16S rRNA V4 amplicons generated from mouse fecal samples (collected 10 weeks after diet administration) on a MiSeq. We summarized OTU abundances into Bray–Curtis dissimilarities and performed non-metric multidimensional scaling (NMDS) ordination (Supplementary Fig. S1A). We found a strong overall effect of diet on community-level differences between diet types (permutational multivariate analysis of variance: P < 0.001; R2 = 0.44). However, the microbial diversity (Shannon index) was not significantly affected by diet type (Supplementary Fig. S1B). Mice that consumed a HS diet had a higher relative abundance of Bacteroidetes, whereas the opposite was true for mice that consumed a lard diet (Fig. 2A and B). Mice that consumed high-fat diets with increased polyunsaturated fatty acids (FO and SO diets) had a significantly reduced relative abundance of Firmicutes compared with lard diet-fed mice (Fig. 2A and B). A comparison of Bacteroidetes-to-Firmicutes ratios (Fig. 2C) shows that consumption of a lard diet resulted in a reduced fecal Bacteroidetes-to-Firmicutes ratio, similar to that observed in human obesity. Moreover, HS, lard, and FO diet-fed mice displayed reduced fecal Verrucomicrobia compared to the control diet-fed mice fecal population (Fig. 2A and B).
Figure 2.
Dietary consumption affects the gut microbiome populations. Fecal samples were collected from each mouse after ten weeks of diet administration. 16S profiling was performed on each sample. A, Phylum-level proportional abundance. Each line represents the fecal phylum proportion of one mouse. n = 5 per diet. B, Quantification of proportional abundance of fecal phylum segregated by diet consumption. n = 5; *, P < 0.05; two-way ANOVA with Bonferroni post-hoc analysis. C, Fecal bacterial proportional abundance of the Bacteroidetes-to-Firmicutes ratio. n = 5 per group; *, P < 0.05; one-way ANOVA with Bonferroni post-hoc analysis. General linear models to test for differences in operational taxonomic units indicate diet-regulated changes in Lachnospiraceae (D), Ruminococcus (E), Bilophila (F), Lactococcus (G), Lactobacillales (H), Akkermansia (I), Bacteroides (J), and Parabacteroides (K) microbiota.
We aggregated OTUs into family-level taxa (Supplementary Fig. S1C) and plotted the relative abundance of the most abundant microbes. General linear models were run to test for differences in OTU abundance by examining the overall community. After adjusting for P-values (α-value threshold = 0.01), we identified 106 differentially abundant OTUs. Notably, similar to other studies utilizing high-fat diets, at the family level, we observed that the consumption of a lard diet increased fecal Lachnospiraceae proportional abundance when compared with feces from control diet-fed mice (Fig. 2D). At the genus level, the lard diet consumption increased Ruminococcus (Fig. 2E). We also observed that a lard diet and CO diet elevated the fecal abundance of Bilophila (Fig. 2F), a major producer of hydrogen sulfide, which is a genotoxic compound that may promote tumorigenesis. A lard or CO diet increased fecal Lactococcus abundance (Fig. 2G). The HS, lard diet, and CO diet groups also displayed a significantly decreased relative proportional abundance of commensal Lactobacillus populations (Fig. 2H). HS, lard diet, and FO consumption significantly reduced fecal Akkermansia abundance (Fig. 2I); however, the CO and SO diets had no significant effect on the proportional abundance of Akkermansia. Consumption of the HS diet increased fecal Bacteroides (Fig. 2J) and Parabacteroides (Fig. 2K) abundances. Overall, dietary consumption drove robust changes in the gut microbiome; consumption of HS, lard, and CO diets reduced commensal bacterial populations (Akkermansia or Lactobacillus) and increased genotoxic proinflammatory populations (Bilophila).
Diet impacts mammary gland inflammation
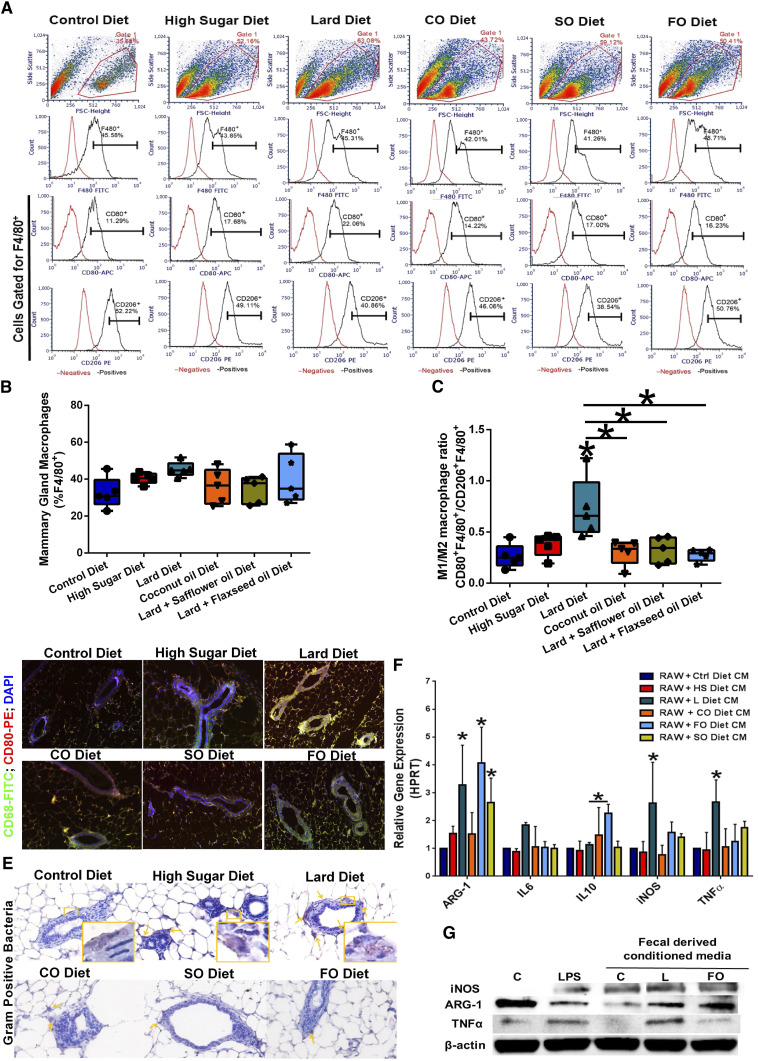
We determined the effect of diet on the infiltrating macrophage populations in the mammary glands using flow cytometry. The unstained and single-antibody control data are shown in Supplementary Fig. S2. HS and lard diet consumption elevated F4/80+CD80+ M1-like macrophages in mammary gland tissue (Fig. 3A and B). HS and SO diet-fed mice also displayed elevated F4/80+CD206+ M2-like macrophage populations in mammary gland tissue. Calculation of the M1/M2 macrophage ratio in the mammary glands indicated that mice consuming the lard diet had a significantly higher M1/M2 ratio (Fig. 3C), suggesting an increased proinflammatory macrophage population in the mammary gland tissue of the lard diet-fed mice. We also stained mammary gland tissues from mice in all diet groups with CD68-FITC (pan macrophage marker) and CD80-PE (monocyte and M1 macrophage polarity markers) to determine the mammary gland-infiltrating macrophage populations. Tissues from lard diet-fed mice showed elevated CD68+CD80+ co-expression, supporting the flow data and indicating increased proinflammatory M1-like macrophage infiltration (Fig. 3D). The individual filter components of the mammary gland fluorescence images are shown in Supplementary Fig. S3A. We also stained the mammary glands with a Gram-positive lipoteichoic acid (LTA) bacterial antibody to confirm the presence of a gland-specific microbiota (Fig. 3E). Although mice from all diet groups had Gram-positive bacteria-laden periductal cells in the mammary gland tissue, lard diet-fed mice had more Gram-positive bacteria-containing cells than the other diet groups (quantified in Supplementary Fig. S3B), suggesting that diet can affect mammary gland bacterial content. To determine the specific cellular localization of Gram-positive bacteria in the mammary gland, we co-stained mammary gland tissue from control diet-fed and lard diet-fed mice with the Gram-positive bacteria FITC and CD68-PE (Supplementary Fig. S3C). In mammary glands from control diet-fed mice, bacteria and macrophage markers colocalize, suggesting that the bacterial content in the mammary gland is restricted to macrophages. In mammary glands from lard diet-fed mice, bacteria and macrophage markers co-label, but also single-label, suggesting bacterial content in other non-macrophage cell types in the mammary gland.
Figure 3.
Diet modulates mammary gland inflammation and bacterial localization. A, Mammary glands were dissociated, and cell suspension stained with F4/80-FITC, CD80-APC, and CD206-PE antibodies. M1- and M2-like macrophage populations were determined by flow cytometry. Representative density plots for each diet are shown. B, Total macrophage (F4/80+) mammary gland populations were quantified. n = 5 per group. C, M1/M2 macrophage ratio in mammary gland populations, as determined by flow cytometry. Lard diet consumption significantly increased the mammary gland M1/M2 ratio. n = 5; *, P < 0.05. D, Mammary gland tissue sections from mice fed different diets were stained with fluorescent-labeled CD68 (green) and CD80 (red) to assess M1-like macrophage infiltration. E, Mammary gland tissue sections from mice fed different diets were stained with LTA bacteria-recognizing antibodies. F, RAW 264.7 mouse macrophage cells were treated with different diet-derived fecal CM overnight and macrophage polarity markers (ARG-1, IL6, IL-10, iNOS, and TNFα) gene expression was determined by RT-PCR. n = 4–6; *, P < 0.05. G, Representative Western blot hybridization images of RAW 264.7 mouse macrophage cells were treated with different diet-derived fecal CM overnight and macrophage polarity protein markers (iNOS, ARG-1, and TNFα) were determined.
To determine the effects of dietary-derived bacteria on macrophage-mediated inflammation, we used ex-vivo fecal-derived conditioned media (CM) to assess macrophage polarity. The impact of fecal-derived conditioned media on macrophage polarity and inflammation was determined by incubating RAW 264.7 mouse macrophage cells with fecal-derived CM for 24 hours (Fig. 3F). The expression of the macrophage M2 polarity marker ARG-1 was upregulated in lard diet CM, FO diet CM, and SO diet CM-treated RAW 264.7 cells. The expression of the M1 macrophage polarity markers NOS2 (iNOS) and tumor necrosis factor (TNF)-α was markedly upregulated by lard diet CM treatment. FO diet CM treatment of macrophages elevated antiinflammatory M2-like IL10 cytokine expression. We also determined the effect of control, lard, and FO-derived conditions on protein markers of macrophage polarity (Fig. 3G). FO diet CM had elevated ARG-1 and decreased TNFα protein levels when compared with macrophages incubated with lard diet CM. Taken together, these data suggest that lard diet fecal-derived CM elevated M1 and M2 macrophage markers, while FO diet fecal-derived CM selectively induced anti-inflammatory M2 macrophage polarization (Fig. 3).
Diet influences breast cancer risk in a carcinogen-mediated murine model
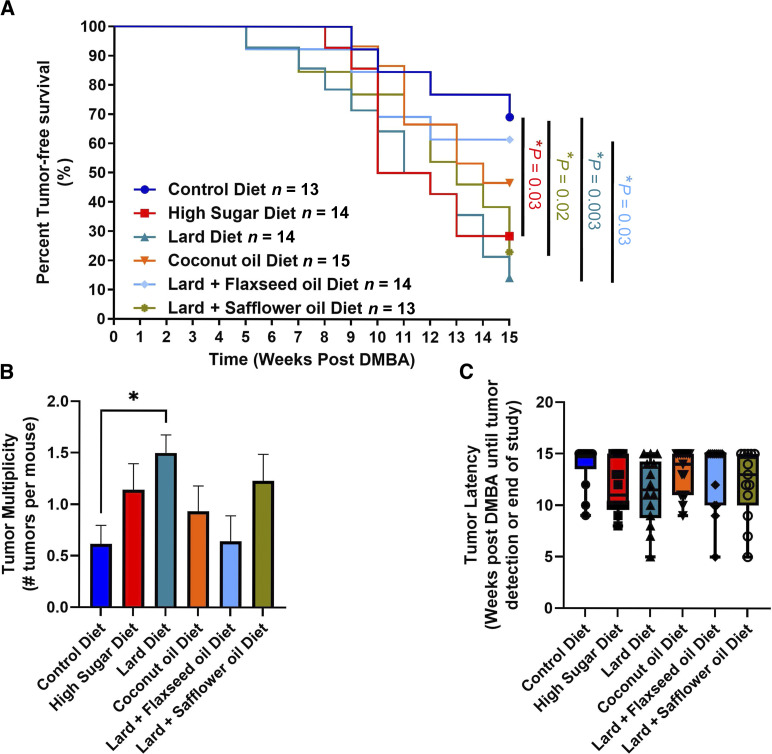
At weaning, female mice were fed diets and tumors were induced using our MPA/DMBA-mammary carcinogenesis protocol (see the “Materials and Methods” section for details). The HS, SO, and Lard diets significantly reduced tumor-free survival compared with the control diet (Fig. 4A). Although not significant, CO- and FO-fed mice also displayed reduced tumor-free survival compared with the control diet-fed mice (Fig. 4A). FO-fed mice displayed a significantly improved tumor-free survival compared to lard diet-fed mice. Changes in tumor multiplicity were also observed, with lard diet-fed mice displaying a significantly increased number of tumors compared with controls (Fig. 4B). However, no differences in tumor latency were observed (Fig. 4C).
Figure 4.
Diet influences breast cancer risk in a carcinogen-mediated murine tumorigenesis model. A, Significant differences in tumor-free survival were observed. B, Differences in tumor multiplicity were observed across the diets, with lard diet-fed mice displaying a significant increase in tumor multiplicity. C, No differences in tumor latency were observed. *, P < 0.05; n = 13–15 per group.
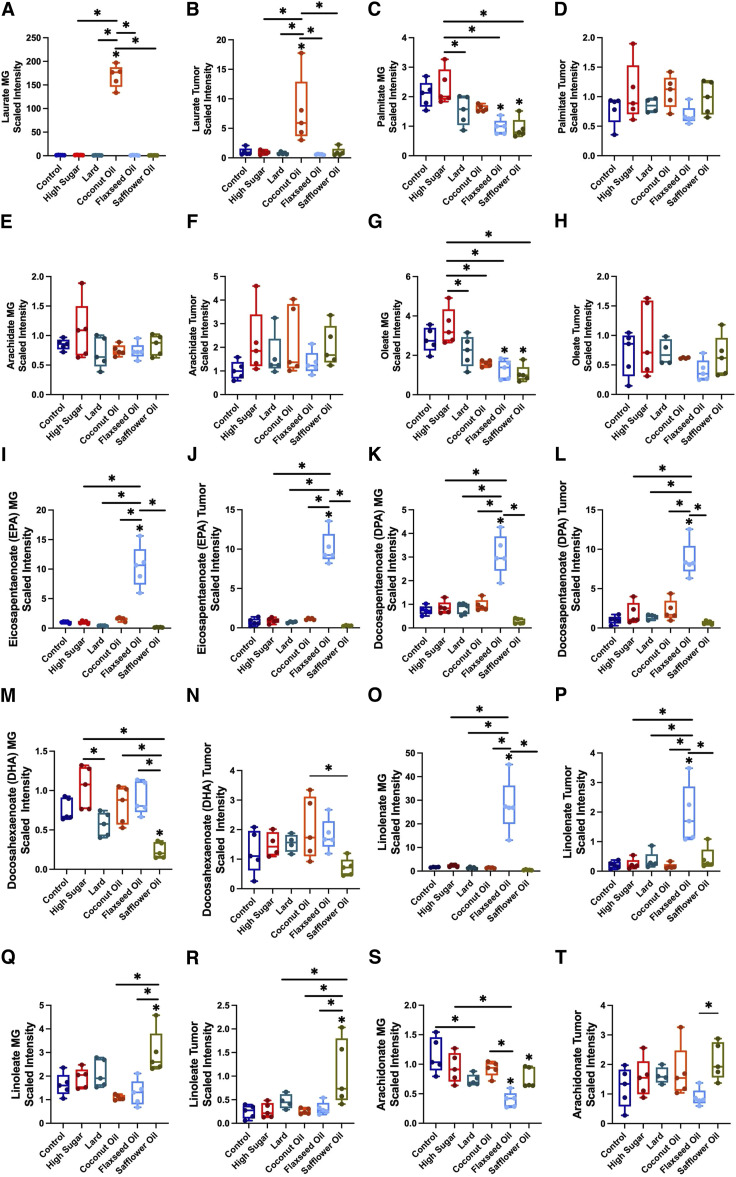
Diet differentially modulates tumor and mammary gland metabolism
At the end of the study, nontumor-bearing mammary glands and tumor tissues were snap-frozen, and untargeted metabolomics was performed on these tissues. Metabolomic analysis revealed differences in lipid metabolites associated with different diets in the mammary glands and tumor tissues (Fig. 5). Laurate or lauric acid (LA), which is the primary medium-chain fatty acid in CO, was significantly increased in mammary glands (Fig. 5A) and tumors (Fig. 5B) in the CO diet-fed mice compared with those in the other diets.
Figure 5.
Dietary patterns differentially shift tumor and mammary gland (MG) metabolism. A, Laurate significantly increased in MG from coconut oil (CO)-fed mice. B, Laurate significantly increased in tumors from the CO-fed mice. C, MG palmitate levels displayed differential shifts with diet. D, Tumor palmitate levels displayed differential shifts with diet. Arachidate levels in (E) MG and (F) tumor tissue. G, oleate was significantly elevated in high sugar (HS)-fed mice and significantly decreased in flaxseed oil (FO) and safflower oil (SO)-fed mice (H). No significant differences in oleate were observed in tumor tissue. Eicosapentaenoate (EPA) was significantly elevated in (I). MG and (J) tumor tissue in FO-fed mice. K, Docosapentaenoate (DPA) was significantly elevated in MG from FO-fed mice. L, Tumor levels of DPA were significantly elevated with the FO diet. Differential shifts in docosahexaenoate (DHA) were observed in (M) MG and (N) tumor tissue. Linolenate significantly increased with FO consumption in both (O) MG and (P) tumor tissue. Linoleate levels significantly increased with SO consumption in both (Q) MG and (R) tumor tissue. S, Significant differences were observed across diets in MG arachidonate levels. T, Minimal shifts were observed in tumor arachidonate levels across the different diets. *, P < 0.05; n = 5 per group. One-way ANOVA with Tukey post-hoc analysis.
Diets high in saturated fats, such as palmitate and arachidate, are a major cause of obesity and potential risk factor for breast cancer (14, 21). Palmitic acid (PA) is the most common saturated fatty acid, accounting for 20% to 30% of the total fatty acids in the body. Interestingly, palmitate levels were significantly elevated in the mammary gland tissues of the HS mice (Fig. 5C). Although not significant, a trending increase was observed in HS tumors (Fig. 5D). Accumulation of arachidonic acid in adipose tissue is associated with low-grade inflammation in overweight/obese women, which may promote tumor emergence or progression (21). Similar to palmitate, arachidate was elevated in the HS mammary glands (Fig. 5E); however, no significant trends were observed in the HS tumors (Fig. 5F).
Oleate, the ester of oleic acid, a monounsaturated fatty acid, was also significantly increased in the HS mammary glands (Fig. 5G); however, no significant differences were observed in tumors from the HS diet-fed mice (Fig. 5H). A significant decrease in oleate levels was also observed in the FO and SO mammary glands, but not in the tumors (Fig. 5G and H).
There are two types of dietary polyunsaturated fatty acids (PUFA): n-3 and n-6 PUFAs. n-3 PUFAs, including eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA), and docosahexaenoic acid (DHA), which are commonly derived from fish oil, vegetable oil, nuts, and flaxseeds, are generally thought to decrease cancer risk via anti-inflammatory effects (22). EPA and DPA levels were significantly increased in the mammary glands (Fig. 5I and K) and tumor tissues (Fig. 5J and L) of flaxseed oil-fed mice, consistent with the increased tumor-free survival compared with the other diets. DHA levels in mammary glands and tumors displayed different diet trends (Fig. 5M and N). Linolenate, an n-3 PUFA derived from flaxseed oil, was also significantly increased in the mammary glands (Fig. 5O) and tumor tissues (Fig. 5P) of FO mice.
Unlike n-3 PUFAs, diets high in n-6 PUFAs such as linoleate and arachidonate, promote proinflammatory immune responses and are associated with tumor progression (15). Linoleate, which accounts for ∼75% of safflower oil, was significantly increased in the mammary gland (Fig. 5Q) and tumor tissue (Fig. 5R) of SO-fed mice. Arachidonate was significantly decreased in the SO mammary glands than in the control (Fig. 5S). In tumor tissue, arachidonate was only significantly increased in SO diet-fed mice compared with FO diet-fed mice (Fig. 5T).
Discussion
Despite significant progress in breast cancer diagnosis and treatment, more than 40,000 deaths occur annually. Moreover, genetics accounts for only 10% of breast cancers, and as many as 70% of breast cancers occur in women at an average risk, suggesting the presence of other risk factors. Recent studies have implicated the gut microbiome as a potential risk factor for breast cancer as well as an explanation for different responses to therapy (23, 24). Gut dysbiosis contributes to the development of breast cancer by altering the production of beneficial anticancer metabolites and disrupting estrogen metabolism in the gut. Specifically, diet-induced dysbiosis has been linked to many key changes in gut populations, suggesting that diet-induced microbial alterations may transform healthy gut microbiota into a disease-inducing state. However, few studies have linked diet-specific changes in the gut microbiota to breast cancer risk. We now report that different dietary patterns shift the gut microbiota to influence inflammatory and metabolic parameters that impact breast tumor risk.
Several studies have demonstrated that gut microbiota plays a crucial role in obesity and related metabolic disorders, suggesting that obesity is strongly correlated with altered gut microbiota (25). Gut microbial shifts occurred across different diets. Lard diet-fed mice displayed a significantly reduced Bacteroidetes/Firmicutes ratio, which is indicative of obesity-induced dysbiosis. This finding is consistent with the observed increase in body weight, calorie consumption, and visceral adipose weight (26). Changes in the Firmicutes phylum members of the Lachnospiraceae family and Ruminococcus genus were significantly elevated by the Lard diet. Bilophila genus enrichment, significantly elevated with both the Lard diet and CO, was increased in breast cancer patients compared with healthy controls, suggesting a potential tumorigenic role for these bacteria (27). HFD consumption is associated with Bilophila wadsworthia outgrowth, which potentially promotes higher inflammation, intestinal barrier dysfunction, bile acid dysmetabolism, and glucose dysmetabolism. Consistently, lard diet consumption dysregulated glucose homeostasis and led to elevated levels of proinflammatory M1-like mammary gland macrophages and an increased M1/M2 macrophage ratio. These results suggest that diet-induced changes in the gut microbial composition may affect breast cancer development through metabolic and immune pathways; however, further studies are needed to determine the exact mechanism.
Studies have shown that HFD intake is associated with reduced anti-inflammatory Lactobacillus species and disproportionately increased proinflammatory species, including Clostridiales and Enterobacteriales (28, 29). This imbalance of anti- and proinflammatory bacteria contributes to obesity-mediated metabolic disorders and endotoxemia-induced inflammation (30). Probiotic Lactobacillus reduces inflammation in the gut and systemically through several mechanisms, including products that directly modulate the NF-B inflammatory program of mucosal, epithelial, and hematopoietic cell types or indirectly by changing the composition or functional activity of the gut microbial community (31). Lactobacillus also promotes the host innate immune function by influencing the activity of phagocytic cells and modulating pathogen-induced inflammatory responses (32). Therefore, the decreased abundance of Lactobacillus observed in HS-, Lard-, and CO-diet-fed mice may contribute to the increased number of M1-like macrophages observed in the mammary glands. Furthermore, RAW 264.7 macrophages treated with Lard diet-derived fecal CM significantly increased proinflammatory iNOS and TNF-α gene expression. The abundance of Lactobacillus intestinalis negatively correlates with fat mass and body weight, consistent with the increased visceral adiposity and body weight in lard diet mice (29).
Lactococcus, another genus of Gram-positive bacteria, was significantly increased in the lard- and CO-fed animals. Breast tissue from patients with breast cancer displays reduced Lactococcus abundance compared with that in healthy individuals (33). Similar to Lactobacillus species, Lactococcus species possess a broad range of immunomodulatory capabilities, including enhancement of phagocytic cell activity, promoting proinflammatory cytokine production, and modulating immune and inflammatory signaling pathways. For example, Lactococcus lactis stimulates the production of the proinflammatory cytokines interferon-γ and tumor necrosis factor (TNF)-α by immune cells (34). L. lactis also modulates various immune and inflammatory signaling pathways, including the NF-κB and mitogen-activated protein kinase pathways, essential for initiating and regulating immune responses (35). Therefore, the elevated M1 and M1/M2 macrophage ratios observed in mammary glands from lard diet-fed animals may be due to the elevated Lactococcus genus proportional abundance.
The Akkermansia genus, which was also decreased in the lard diet, HS, and FO, is negatively correlated with numerous diseases, including obesity (36). A. muciniphila, contributes to the maintenance of gut health and glucose homeostasis by lowering body fat mass, improving glucose homeostasis, decreasing adipose tissue inflammation, and increasing gut integrity by increasing mucin layer thickness, decreasing metabolic endotoxemia, and increasing the number of goblet cells (36). The decreased abundance of the Akkermansia genus is consistent with changes in metabolic parameters, particularly glucose homeostasis and body weight. Gut Akkermansia populations are associated with responsiveness to chemotherapy and immune checkpoint therapy blockade therapy in preclinical TNBC models (37), implicating diet in mediating gut microbiome interactions on breast cancer therapeutic outcomes.
Obesity is associated with a higher risk of estrogen receptor (ER)–positive breast cancer in postmenopausal women and ER-negative and triple-negative breast cancers in premenopausal women (38). The proposed mechanisms include alterations in the gut microbiome and chronic systemic inflammation (39). HFD-induced changes in gut microbiota and the resulting metabolic perturbations appear to be dependent on the fat content of milk fat-based, lard-based (saturated fatty acid sources), or safflower oil (polyunsaturated fatty acid)-based HFDs, which induced dramatic and specific 16S rRNA phylogenic profiles that were associated with different inflammatory and lipogenic mediator profiles of mesenteric and gonadal fat depots. Indeed, increased amounts of free fatty acids (FFA) present in HFDs led to elevated production of proinflammatory cytokines such as interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α in the gut, increasing the delivery of intestinal LPS, proinflammatory cytokines, and FFAs into the systemic circulation and portal circulation, thus leading to systemic low-grade inflammation. The connection between inflammation and cancer is well established, as inflammation is associated with all stages of tumor development and progression in many types of cancer, resulting in a unique immune microenvironment (40). We show that, irrespective of weight change, diets high in different fat sources influence cancer development in a spontaneously induced carcinogen mouse model. Additionally, regardless of the fat source, all mice displayed reduced tumor-free survival compared to the controls.
Obesity is characterized by increased levels of circulating FFAs (41); and has been linked to breast cancer development (42, 43). Metabolomics of mammary gland and tumor tissues consistently revealed alterations in fatty acids. Accordingly, dietary fatty acids and their metabolites are crucial for regulating energy metabolism, structural cell integrity, cellular signaling, and immune functions (44, 45). However, disruptions in fatty acid metabolism are involved in several hallmarks of cancer, including proliferation, migration, angiogenesis, antitumor immunity, and tumor-promoting inflammation (45, 46). For example, saturated fatty acid (SFA) intake is negatively associated with survival in patients with breast cancer (47). Unsurprisingly, SFA and PA concentrations enhance the tumor formation capacity of breast cancer cells (48). Additionally, a recent case–cohort analysis found an elevated breast cancer risk in women with greater PA incorporation into plasma phospholipids (49). In our study, HS diet-fed mice displayed elevated mammary gland and tumor palmitate levels, consistent with the significantly decreased tumor-free survival.
Polyunsaturated fatty acids (PUFA) are fatty acids with two or more double bonds that play important roles in human health and disease. Most PUFAs cannot be synthesized endogenously and must be obtained from the diet. Overconsumption of n-6 PUFAs with low intake of n-3 PUFAs is highly associated with the pathogenesis of many diet-related chronic diseases, such as obesity (50). The most common dietary sources of n-3 PUFAs are fish oil, vegetable oil, nuts, and flaxseeds. In contrast, the most common dietary sources of n-6 PUFAs are meat, poultry, eggs, sunflower oil, and soybean oil (43). PUFAs from both families regulate inflammation, immunity, and cellular growth in an antagonistic manner.
Flaxseed oil is one of the richest sources of n-3 PUFAs from plant sources, particularly α-linolenic acid (ALA; ref. 51). L-linolenic acid, as well as its metabolites eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), exhibit anti-inflammatory effects by decreasing the production of inflammatory cytokines, lipids, and lipoproteins (52). Furthermore, ALA has cytotoxic effects on breast cancer cell growth (53). As expected, the elevated linoleate, EPA, and docosapentaenoate (DPA) levels in mammary gland and tumor tissues from FO-fed mice, in conjunction with the increased tumor-free survival compared to other diets, are consistent with the anticancer effects associated with flaxseed oil.
Conversely, high n-6 PUFA-rich diets are known to increase mammary tumorigenesis in rat models of breast cancer and lead to worse prognosis in a murine model of HER2+ breast cancer (54, 55). Safflower oil is rich in linoleic acid (LA), which negatively affects health in large amounts (56). LA contributes to excess inflammation through the formation of arachidonic acid and subsequent synthesis of proinflammatory eicosanoids [e.g., as prostaglandin E2 (PGE2), leukotriene B4 (LTB4), and thromboxane A4 (TXA2; 56, 57)]. Elevated proinflammatory eicosanoid generation could increase other biomarkers of inflammation [e.g., interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), C-reactive protein (CRP)], which are associated with an increased incidence of cancer and other chronic diseases (58). Linoleate was significantly elevated in the mammary glands and tumors of SO-fed mice, and was associated with decreased tumor-free survival.
HFDs have been identified as a major cause of obesity and are, therefore, potential risk factors for breast cancer. Furthermore, complex interactions between HFDs and altered gut microbiota have been implicated in disease progression. However, the impact of high-fat diet-induced obesity on gut microbial changes during breast cancer development remains unknown. The results of our study indicate that changes in the fat source, not the amount of fat influence gut microbes to potentially impact cancer development. Specifically, while most diets altered gut microbiota somehow, only HS, lard, and SO altered macrophage populations.
Furthermore, only the HS and lard diets increased tumorigenesis, whereas addition of FO reduced tumorigenesis compared to lard diet-fed mice. The results from the CO and SO diets were inconclusive and further studies are needed. Our study indicates that dietary patterns change metabolic outcomes, gut microbiota, and breast tumor risk. Furthermore, dietary patterns influence metabolism, inflammation, and macrophage polarity, suggesting that diet-microbiome interactions are key mediators of breast cancer risk.
Identifying the association between dietary intake, the gut microbiome, and its impact on breast carcinogenesis is crucial as it could inform dietary and lifestyle changes that could potentially reduce breast cancer incidence and improve outcomes.
Supplementary Material
Supplemental Figure 1: Diet regulates fecal microbiota populations. A. Summarized OTU abundances into Bray–Curtis dissimilarities and performed a non-metric multidimensional scaling (NMDS) ordination. There is a strong overall effect of diet on community-level differences between diet types (permutational multivariate analysis of variance: P < 0.001, R-squared = 0.44, n = 5). B. Microbial diversity, measured by the Shannon index, was not significantly affected by diet type. C. OTUs were aggregated into family-level taxa, and the relative abundance of the most abundant microbes was plotted. General linear models were used to test for differences in OTU abundances by examining the overall community alone. After adjusting for P-values (α value threshold = 0.01), we found 106 differentially abundant OTUs.
Supplemental Figure 2: A. Representative unstained flow plot controls. B. Representative single-antibody controls.
Supplemental Figure 3: A. Individual filter components of mammary gland fluorescent images DAPI, CD68-FITC, CD80-PE, and overlay. B. Quantification of periductal Gram-positive bacteria-laden cells in the mammary glands of mice fed differing diets. C. Gram-positive bacteria in mammary gland tissue do not colocalize with macrophages in lard diet-fed animals.
Supplemental Material: Effect of diet on mammary gland and tumor metabolism. Heat map of untargeted metabolomic results from mammary glands and mammary tumors from mice fed a control, HS, lard, CO, lard + flaxseed oil, or lard + safflower oil diet
Supplemental Table 1: Fecal 16S Genus-Level abundance from non-tumor bearing mice fed different diets
Acknowledgments
A.A. Arnone was previously supported by a T32 training grant (5T32AI007401). D.R. Soto-Pantoja was supported by an American Cancer Society Research Scholar grant (133727-RSG-19-150-01-LIB). K.L. Cook was supported by a Prevent Cancer Foundation Grant, an American Cancer Society Research Scholar grant (RSG-16-204-01-NEC), and grants from the Department of Defense Breast Cancer Research Program (BC190271 and BC210715). Shared Resource services were provided by Wake Forest Baptist Comprehensive Cancer Center’s NCI Cancer Center Support Grant P30CA012197.
Footnotes
Note: Supplementary data for this article are available at Cancer Prevention Research Online (http://cancerprevres.aacrjournals.org/).
Authors’ Disclosures
K.L. Cook reports grants from the National Institutes of Health, the American Cancer Society, the Prevent Cancer Foundation, and the Department of Defense Breast Cancer Research Program during the study. D.R. Soto-Pantoja reports grants from the American Cancer Society. No disclosures were reported by the other authors.
Authors’ Contributions
A.A. Arnone: Formal analysis, investigation, writing–original draft, writing–review and editing. A.S. Wilson: Formal analysis, investigation, methodology. D.R. Soto-Pantoja: Resources, formal analysis, supervision, methodology, writing–review and editing. K.L. Cook: Conceptualization, resources, supervision, funding acquisition, writing–original draft, project administration, writing–review and editing.
References
- 1. American Cancer Society . Cancer Facts and Figures 2023. Atlanta (GA): American Cancer Society; 2023[cited 2024 Apr 25]. Available from:https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/2023-cancer-facts-figures.html. [Google Scholar]
- 2. Lee K, Kruper L, Dieli-Conwright CM, Mortimer JE. The impact of obesity on breast cancer diagnosis and treatment. Curr Oncol Rep 2019;21:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. NCD Risk Factor Collaboration (NCD-RisC) . Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet 2016;387:1377–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hill JO, Wyatt HR, Peters JC. Energy balance and obesity. Circulation 2012;126:126–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu B-N, Liu X-T, Liang Z-H, Wang J-H. Gut microbiota in obesity. World J Gastroenterol 2021;27:3837–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brown KA. Metabolic pathways in obesity-related breast cancer. Nat Rev Endocrinol 2021;17:350–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hossain F, Majumder S, David J, Bunnell BA, Miele L. Obesity modulates the gut microbiome in triple-negative breast cancer. Nutrients 2021;13:3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Castaner O, Goday A, Park Y-M, Lee S-H, Magkos F, Toh Ee Shiow S-A, et al. The gut microbiome profile in obesity: a systematic review. Int J Endocrinol 2018;2018:4095789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Koliada A, Syzenko G, Moseiko V, Budovska L, Puchkov K, Perederiy V, et al. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol 2017;17:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chambers ES, Preston T, Frost G, Morrison DJ. Role of gut microbiota-generated short-chain fatty acids in metabolic and cardiovascular health. Curr Nutr Rep 2018;7:198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ang Z, Ding JL. GPR41 and GPR43 in obesity and inflammation - protective or causative? Front Immunol 2016;7:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim B, Choi H-N, Yim J-E. Effect of diet on the gut microbiota associated with obesity. J Obes Metab Syndr 2019;28:216–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lazar V, Ditu L-M, Pircalabioru GG, Picu A, Petcu L, Cucu N, et al. Gut microbiota, host organism, and diet trialogue in diabetes and obesity. Front Nutr 2019;6:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Uhomoibhi TO, Okobi TJ, Okobi OE, Koko JO, Uhomoibhi O, Igbinosun OE, et al. High-fat diet as a risk factor for breast cancer: a meta-analysis. Cureus 2022;14:e32309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Westheim AJF, Stoffels LM, Dubois LJ, van Bergenhenegouwen J, van Helvoort A, Langen RCJ, et al. The modulatory effects of fatty acids on cancer progression. Biomedicines 2023;11:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sumis A, Cook KL, Andrade FO, Hu R, Kidney E, Zhang X, et al. Social isolation induces autophagy in the mouse mammary gland: link to increased mammary cancer risk. Endocr Relat Cancer 2016;23:839–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl Environ Microbiol 2013;79:5112–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 2009;75:7537–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arnone AA, Cline JM, Soto-Pantoja DR, Cook KL. Investigating the role of endogenous estrogens, hormone replacement therapy, and blockade of estrogen receptor-α activity on breast metabolic signaling. Breast Cancer Res Treat 2021;190:53–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shively CA, Register TC, Appt SE, Clarkson TB, Uberseder B, Clear KYJ, et al. Consumption of mediterranean versus western diet leads to distinct mammary gland microbiome populations. Cell Rep 2018;25:47–56.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ouldamer L, Jourdan M-L, Pinault M, Arbion F, Goupille C. Accumulation of arachidonic acid, precursor of proinflammatory eicosanoids, in adipose tissue of obese women: association with breast cancer aggressiveness indicators. Biomedicines 2022;10:995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu J, Ma DWL. The role of n-3 polyunsaturated fatty acids in the prevention and treatment of breast cancer. Nutrients 2014;6:5184–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Soto-Pantoja DR, Gaber M, Arnone AA, Bronson SM, Cruz-Diaz N, Wilson AS, et al. Diet alters entero-mammary signaling to regulate the breast microbiome and tumorigenesis. Cancer Res 2021;81:3890–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Arnone AA, Cook KL. Gut and breast microbiota as endocrine regulators of hormone receptor-positive breast cancer risk and therapy response. Endocrinology 2022;164:bqac177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Régnier M, Van Hul M, Knauf C, Cani PD. Gut microbiome, endocrine control of gut barrier function and metabolic diseases. J Endocrinol 2021;248:R67–82. [DOI] [PubMed] [Google Scholar]
- 26. Stojanov S, Berlec A, Štrukelj B. The influence of probiotics on the Firmicutes/Bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms 2020;8:1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ma Z, Qu M, Wang X. Analysis of gut microbiota in patients with breast cancer and benign breast lesions. Pol J Microbiol 2022;71:217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sun J, Qiao Y, Qi C, Jiang W, Xiao H, Shi Y, et al. High-fat-diet–induced obesity is associated with decreased anti-inflammatory Lactobacillus reuteri sensitive to oxidative stress in mouse Peyer's patches. Nutrition 2016;32:265–72. [DOI] [PubMed] [Google Scholar]
- 29. Lecomte V, Kaakoush NO, Maloney CA, Raipuria M, Huinao KD, Mitchell HM, et al. Changes in gut microbiota in rats fed a high fat diet correlate with obesity-associated metabolic parameters. PLoS One 2015;10:e0126931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim K-A, Gu W, Lee I-A, Joh E-H, Kim D-H. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS One 2012;7:e47713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yamamoto ML, Maier I, Dang AT, Berry D, Liu J, Ruegger PM, et al. Intestinal bacteria modify lymphoma incidence and latency by affecting systemic inflammatory state, oxidative stress, and leukocyte genotoxicity. Cancer Res 2013;73:4222–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nanjundaiah YS, Wright DA, Baydoun AR, Khaled Z, Ali Z, Dean P, et al. Modulation of macrophage function by Lactobacillus-conditioned medium. Front Cell Dev Biol 2020;8:723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Urbaniak C, Gloor GB, Brackstone M, Scott L, Tangney M, Reid G. The microbiota of breast tissue and its association with breast cancer. Appl Environ Microbiol 2016;82:5039–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jin SW, Lee GH, Jang MJ, Hong GE, Kim JY, Park GD, et al. Immunomodulatory activity of Lactococcus lactis GCWB1176 in cyclophosphamide-induced immunosuppression model. Microorganisms 2020;8:1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jeong H, Kim S, Hwang U-S, Choi H, Park Y-S. Immunostimulatory activity of Lactococcus lactis subsp. lactis CAB701 isolated from Jeju Cabbage. Microorganisms 2023;11:1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dao MC, Everard A, Aron-Wisnewsky J, Sokolovska N, Prifti E, Verger EO, et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut 2016;65:426–36. [DOI] [PubMed] [Google Scholar]
- 37. Bawaneh A, Wilson AS, Levi N, Howard-McNatt MM, Chiba A, Soto-Pantoja DR, et al. Intestinal microbiota influence doxorubicin responsiveness in triple-negative breast cancer. Cancers (Basel) 2022;14:4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ, Friedman ER, Slingerland JM. Obesity and adverse breast cancer risk and outcome: mechanistic insights and strategies for intervention. CA Cancer J Clin 2017;67:378–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Argolo DF, Hudis CA, Iyengar NM. The impact of obesity on breast cancer. Curr Oncol Rep 2018;20:47. [DOI] [PubMed] [Google Scholar]
- 40. Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Boden G, Shulman GI. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. Eur J Clin Invest 2002;32(Suppl 3):14–23. [DOI] [PubMed] [Google Scholar]
- 42. Guo F, Wang M, Guo X, Pu L, Sun M, Li S, et al. The association between fatty acid intake and breast cancer based on the NHANES and Mendelian randomization study. Cancer Epidemiol 2021;73:101966. [DOI] [PubMed] [Google Scholar]
- 43. Westheim AJF, Stoffels LM, Dubois LJ, van Bergenhenegouwen J, van Helvoort A, Langen RCJ, et al. The modulatory effects of fatty acids on cancer progression. Biomedicines 2023;11:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yoon H, Shaw JL, Haigis MC, Greka A. Lipid metabolism in sickness and in health: emerging regulators of lipotoxicity. Mol Cell 2021;81:3708–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nagarajan SR, Butler LM, Hoy AJ. The diversity and breadth of cancer cell fatty acid metabolism. Cancer Metab 2021;9:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Molendijk J, Robinson H, Djuric Z, Hill MM. Lipid mechanisms in hallmarks of cancer. Mol Omics 2020;16:6–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Brennan SF, Woodside JV, Lunny PM, Cardwell CR, Cantwell MM. Dietary fat and breast cancer mortality: a systematic review and meta-analysis. Crit Rev Food Sci Nutr 2017;57:1999–2008. [DOI] [PubMed] [Google Scholar]
- 48. Liu X-Z, Rulina A, Choi MH, Pedersen L, Lepland J, Takle ST, et al. C/EBPB-dependent adaptation to palmitic acid promotes tumor formation in hormone receptor negative breast cancer. Nat Commun 2022;13:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bassett JK, Hodge AM, English DR, MacInnis RJ, Giles GG. Plasma phospholipids fatty acids, dietary fatty acids, and breast cancer risk. Cancer Causes Control 2016;27:759–73. [DOI] [PubMed] [Google Scholar]
- 50. Mariamenatu AH, Abdu EM. Overconsumption of omega-6 polyunsaturated fatty acids (PUFAs) versus deficiency of omega-3 PUFAs in modern-day diets: the disturbing factor for their “balanced antagonistic metabolic functions” in the human body. J Lipids 2021;2021:8848161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Al-Madhagy S, Ashmawy NS, Mamdouh A, Eldahshan OA, Farag MA. A comprehensive review of the health benefits of flaxseed oil in relation to its chemical composition and comparison with other omega-3-rich oils. Eur J Med Res 2023;28:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Purushothaman D, Brown WY, Vanselow BA, Quinn K, Wu S-B. Flaxseed oil supplementation alters the expression of inflammatory-related genes in dogs. Genet Mol Res 2014;13:5322–32. [DOI] [PubMed] [Google Scholar]
- 53. LeMay-Nedjelski L, Mason-Ennis JK, Taibi A, Comelli EM, Thompson LU. Omega-3 polyunsaturated fatty acids time-dependently reduce cell viability and oncogenic microRNA-21 expression in estrogen receptor-positive breast cancer cells (MCF-7). Int J Mol Sci 2018;19:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hillyer LM, Hucik B, Baracuhy EM, Lin Z, Muller WJ, Robinson LE, et al. Her-2 breast cancer outcomes are mitigated by consuming n-3 polyunsaturated, saturated, and monounsaturated fatty acids compared to n-6 polyunsaturated fatty acids. Nutrients 2020;12:3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Moral R, Escrich R, Solanas M, Vela E, Ruiz de Villa MC, Escrich E. Diets high in corn oil or extra-virgin olive oil differentially modify the gene expression profile of the mammary gland and influence experimental breast cancer susceptibility. Eur J Nutr 2016;55:1397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother 2002;56:365–79. [DOI] [PubMed] [Google Scholar]
- 57. Das UN. Essential fatty acids - a review. Curr Pharm Biotechnol 2006;7:467–82. [DOI] [PubMed] [Google Scholar]
- 58. Fritsche KL. Linoleic acid, vegetable oils & inflammation. Mo Med 2014;111:41–3. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Diet regulates fecal microbiota populations. A. Summarized OTU abundances into Bray–Curtis dissimilarities and performed a non-metric multidimensional scaling (NMDS) ordination. There is a strong overall effect of diet on community-level differences between diet types (permutational multivariate analysis of variance: P < 0.001, R-squared = 0.44, n = 5). B. Microbial diversity, measured by the Shannon index, was not significantly affected by diet type. C. OTUs were aggregated into family-level taxa, and the relative abundance of the most abundant microbes was plotted. General linear models were used to test for differences in OTU abundances by examining the overall community alone. After adjusting for P-values (α value threshold = 0.01), we found 106 differentially abundant OTUs.
Supplemental Figure 2: A. Representative unstained flow plot controls. B. Representative single-antibody controls.
Supplemental Figure 3: A. Individual filter components of mammary gland fluorescent images DAPI, CD68-FITC, CD80-PE, and overlay. B. Quantification of periductal Gram-positive bacteria-laden cells in the mammary glands of mice fed differing diets. C. Gram-positive bacteria in mammary gland tissue do not colocalize with macrophages in lard diet-fed animals.
Supplemental Material: Effect of diet on mammary gland and tumor metabolism. Heat map of untargeted metabolomic results from mammary glands and mammary tumors from mice fed a control, HS, lard, CO, lard + flaxseed oil, or lard + safflower oil diet
Supplemental Table 1: Fecal 16S Genus-Level abundance from non-tumor bearing mice fed different diets
Data Availability Statement
Genus-level 16S sequencing OTU proportional abundance data are available (Supplementary Table S1. Genus OTU proportional abundance in feces from mice fed varying diets). A complete untargeted metabolomic dataset is available (Supplementary Material). The data generated in this study are available within the article and its supplementary data files.