Abstract
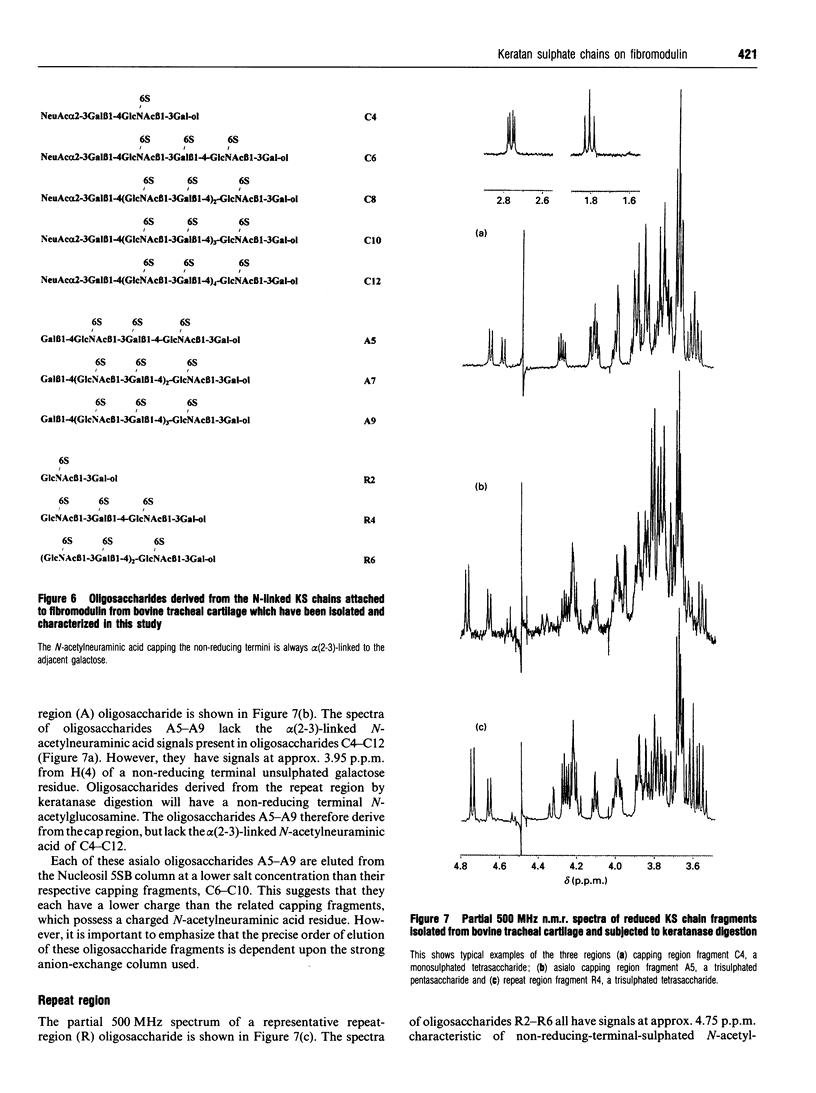
The structure of the repeat region and chain caps of the N-linked keratan sulphate chains attached to bovine tracheal cartilage fibromodulin has been examined. The chains were fragmented by keratanase digestion, the resultant oligosaccharides isolated by strong anion-exchange chromatography, and their structures determined using high-field 1H-n.m.r. spectroscopy. The chains were found to possess the following general structure: [formula: see text] All of the capping oligosaccharides isolated terminate with alpha(2-3)-linked N-acetylneuraminic acid. No alpha(2-6)-linked N-acetylneuraminic acid chain terminators, nor any fucose, alpha (1-3)-linked to N-acetylglucosamine along the repeat region, were detected. This work demonstrates that the structure of the repeat region and chain caps of N-linked keratan sulphate attached to fibromodulin isolated from bovine tracheal cartilage is identical with that of O-linked keratan sulphate chains attached to aggrecan derived from non-articular cartilage.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonsson P., Heinegård D., Oldberg A. Posttranslational modifications of fibromodulin. J Biol Chem. 1991 Sep 5;266(25):16859–16861. [PubMed] [Google Scholar]
- Bhavanandan V. P., Meyer K. Studies on keratosulfates. Methylation, desulfation, and acid hydrolysis studies on old human rib cartilage keratosulfate. J Biol Chem. 1968 Mar 10;243(5):1052–1059. [PubMed] [Google Scholar]
- Bray B. A., Lieberman R., Meyer K. Structure of human skeletal keratosulfate. The linkage region. J Biol Chem. 1967 Jul 25;242(14):3373–3380. [PubMed] [Google Scholar]
- Brown G. M., Huckerby T. N., Morris H. G., Abram B. L., Nieduszynski I. A. Oligosaccharides derived from bovine articular cartilage keratan sulfates after keratanase II digestion: implications for keratan sulfate structural fingerprinting. Biochemistry. 1994 Apr 26;33(16):4836–4846. doi: 10.1021/bi00182a012. [DOI] [PubMed] [Google Scholar]
- Brown G. M., Huckerby T. N., Morris H. G., Nieduszynski I. A. Degradation of articular cartilage keratan sulphates using hydrazinolysis and nitrous acid. Environment of fucose residues. Biochem J. 1992 Aug 15;286(Pt 1):235–241. doi: 10.1042/bj2860235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickenson J. M., Huckerby T. N., Nieduszynski I. A. A non-reducing terminal fragment from tracheal cartilage keratan sulphate chains contains alpha (2-3)-linked N-acetylneuraminic acid. Biochem J. 1991 Sep 15;278(Pt 3):779–785. doi: 10.1042/bj2780779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickenson J. M., Huckerby T. N., Nieduszynski I. A. Skeletal keratan sulphate chains isolated from bovine intervertebral disc may terminate in alpha(2----6)-linked N-acetylneuraminic acid. Biochem J. 1992 Feb 15;282(Pt 1):267–271. doi: 10.1042/bj2820267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickenson J. M., Huckerby T. N., Nieduszynski I. A. Two linkage-region fragments isolated from skeletal keratan sulphate contain a sulphated N-acetylglucosamine residue. Biochem J. 1990 Jul 1;269(1):55–59. doi: 10.1042/bj2690055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farndale R. W., Sayers C. A., Barrett A. J. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9(4):247–248. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- Hedbom E., Heinegård D. Interaction of a 59-kDa connective tissue matrix protein with collagen I and collagen II. J Biol Chem. 1989 Apr 25;264(12):6898–6905. [PubMed] [Google Scholar]
- Heinegård D., Larsson T., Sommarin Y., Franzén A., Paulsson M., Hedbom E. Two novel matrix proteins isolated from articular cartilage show wide distributions among connective tissues. J Biol Chem. 1986 Oct 15;261(29):13866–13872. [PubMed] [Google Scholar]
- Hounsell E. F., Feeney J., Scudder P., Tang P. W., Feizi T. 1H-NMR studies at 500 MHz of a neutral disaccharide and sulphated di-, tetra-, hexa- and larger oligosaccharides obtained by endo-beta-galactosidase treatment of keratan sulphate. Eur J Biochem. 1986 Jun 2;157(2):375–384. doi: 10.1111/j.1432-1033.1986.tb09679.x. [DOI] [PubMed] [Google Scholar]
- Krusius T., Finne J., Margolis R. K., Margolis R. U. Identification of an O-glycosidic mannose-linked sialylated tetrasaccharide and keratan sulfate oligosaccharides in the chondroitin sulfate proteoglycan of brain. J Biol Chem. 1986 Jun 25;261(18):8237–8242. [PubMed] [Google Scholar]
- Nakazawa K., Suzuki S. Purification of Keratan Sulfate-endogalactosidase and its action on keratan sulfates of different origin. J Biol Chem. 1975 Feb 10;250(3):912–917. [PubMed] [Google Scholar]
- Nieduszynski I. A., Huckerby T. N., Dickenson J. M., Brown G. M., Tai G. H., Morris H. G., Eady S. There are two major types of skeletal keratan sulphates. Biochem J. 1990 Oct 1;271(1):243–245. doi: 10.1042/bj2710243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeben M., Keller R., Stuhlsatz H. W., Greiling H. Constant and variable domains of different disaccharide structure in corneal keratan sulphate chains. Biochem J. 1987 Nov 15;248(1):85–93. doi: 10.1042/bj2480085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldberg A., Antonsson P., Lindblom K., Heinegård D. A collagen-binding 59-kd protein (fibromodulin) is structurally related to the small interstitial proteoglycans PG-S1 and PG-S2 (decorin). EMBO J. 1989 Sep;8(9):2601–2604. doi: 10.1002/j.1460-2075.1989.tb08399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaas A. H., Ison A. L., Ackland J. Synthesis of small proteoglycans substituted with keratan sulfate by rabbit articular chondrocytes. J Biol Chem. 1989 Aug 25;264(24):14447–14454. [PubMed] [Google Scholar]
- Plaas A. H., Neame P. J., Nivens C. M., Reiss L. Identification of the keratan sulfate attachment sites on bovine fibromodulin. J Biol Chem. 1990 Nov 25;265(33):20634–20640. [PubMed] [Google Scholar]
- Tai G. H., Huckerby T. N., Nieduszynski I. A. N.m.r. spectroscopic studies of fucose-containing oligosaccharides derived from keratanase digestion of articular cartilage keratan sulphates. Influence of fucose residues on keratanase cleavage. Biochem J. 1993 May 1;291(Pt 3):889–894. doi: 10.1042/bj2910889. [DOI] [PMC free article] [PubMed] [Google Scholar]


