Abstract
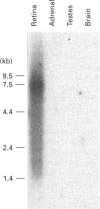
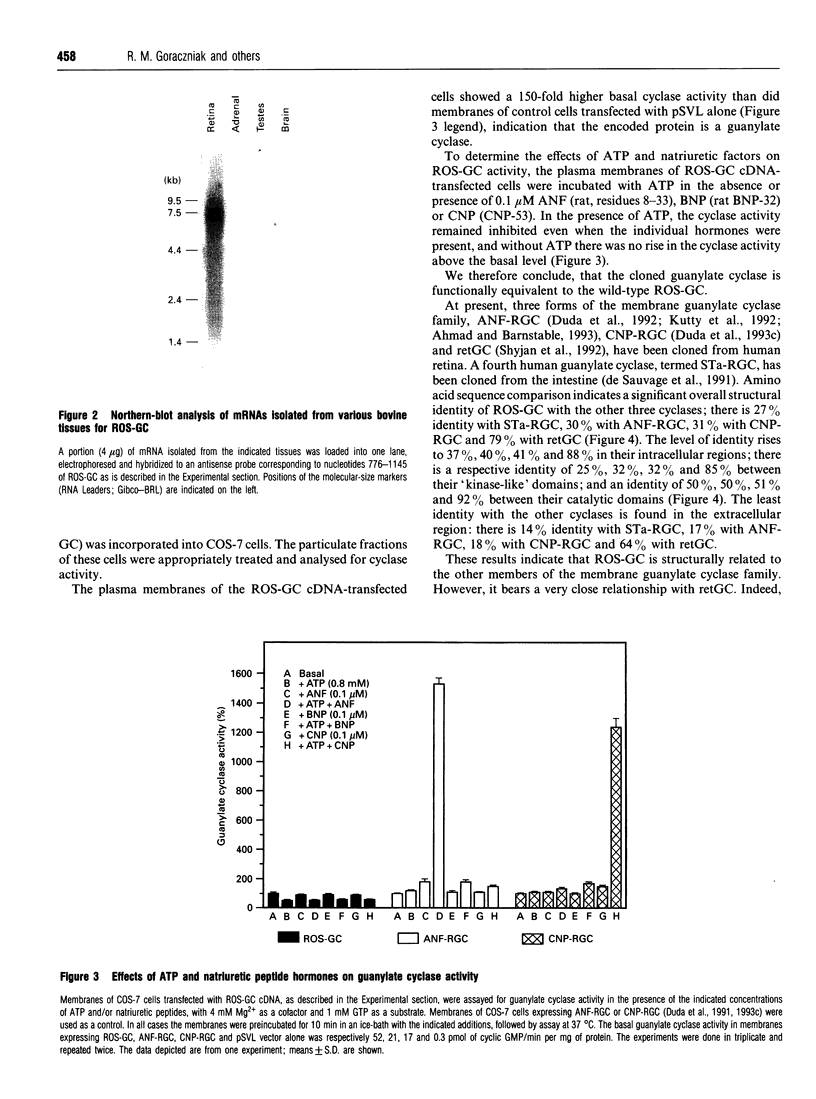
In the vertebrate photoreceptor cell, rod outer segment (ROS) is the site of visual signal-transduction process, and a pivotal molecule that regulates this process is cyclic GMP. Cyclic GMP controls the cationic conductance into the ROS, and light causes a decrease in the conductance by activating hydrolysis of the cyclic nucleotide. The identity of the granylate cyclase (ROS-GC) that synthesizes this pool of cyclic GMP is unknown. We now report the cloning, expression and functional characterization of a DNA from bovine retina that encodes ROS-GC.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad I., Barnstable C. J. Differential laminar expression of particulate and soluble guanylate cyclase genes in rat retina. Exp Eye Res. 1993 Jan;56(1):51–62. doi: 10.1006/exer.1993.1008. [DOI] [PubMed] [Google Scholar]
- Chinkers M., Garbers D. L., Chang M. S., Lowe D. G., Chin H. M., Goeddel D. V., Schulz S. A membrane form of guanylate cyclase is an atrial natriuretic peptide receptor. Nature. 1989 Mar 2;338(6210):78–83. doi: 10.1038/338078a0. [DOI] [PubMed] [Google Scholar]
- Duda T., Goraczniak R. M., Sharma R. K. Core sequence of ATP regulatory module in receptor guanylate cyclases. FEBS Lett. 1993 Jan 4;315(2):143–148. doi: 10.1016/0014-5793(93)81151-o. [DOI] [PubMed] [Google Scholar]
- Duda T., Goraczniak R. M., Sharma R. K. Site-directed mutational analysis of a membrane guanylate cyclase cDNA reveals the atrial natriuretic factor signaling site. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7882–7886. doi: 10.1073/pnas.88.17.7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duda T., Goraczniak R. M., Sharma R. K. The glycine residue of ATP regulatory module in receptor guanylate cyclases that is essential in natriuretic factor signaling. FEBS Lett. 1993 Dec 13;335(3):309–314. doi: 10.1016/0014-5793(93)80408-m. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Hakki S., Sitaramayya A. Guanylate cyclase from bovine rod outer segments: solubilization, partial purification, and regulation by inorganic pyrophosphate. Biochemistry. 1990 Jan 30;29(4):1088–1094. doi: 10.1021/bi00456a035. [DOI] [PubMed] [Google Scholar]
- Hayashi F., Yamazaki A. Polymorphism in purified guanylate cyclase from vertebrate rod photoreceptors. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4746–4750. doi: 10.1073/pnas.88.11.4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch K. W. Purification and identification of photoreceptor guanylate cyclase. J Biol Chem. 1991 May 5;266(13):8634–8637. [PubMed] [Google Scholar]
- Koller K. J., Lowe D. G., Bennett G. L., Minamino N., Kangawa K., Matsuo H., Goeddel D. V. Selective activation of the B natriuretic peptide receptor by C-type natriuretic peptide (CNP). Science. 1991 Apr 5;252(5002):120–123. doi: 10.1126/science.1672777. [DOI] [PubMed] [Google Scholar]
- Kutty R. K., Fletcher R. T., Chader G. J., Krishna G. Expression of guanylate cyclase-A mRNA in the rat retina: detection using polymerase chain reaction. Biochem Biophys Res Commun. 1992 Jan 31;182(2):851–857. doi: 10.1016/0006-291x(92)91810-d. [DOI] [PubMed] [Google Scholar]
- Lowe D. G., Chang M. S., Hellmiss R., Chen E., Singh S., Garbers D. L., Goeddel D. V. Human atrial natriuretic peptide receptor defines a new paradigm for second messenger signal transduction. EMBO J. 1989 May;8(5):1377–1384. doi: 10.1002/j.1460-2075.1989.tb03518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulis A., Goraczniak R. M., Duda T., Sharma R. K., Sitaramayya A. Structural and biochemical identity of retinal rod outer segment membrane guanylate cyclase. Biochem Biophys Res Commun. 1993 Jul 30;194(2):855–861. doi: 10.1006/bbrc.1993.1900. [DOI] [PubMed] [Google Scholar]
- Paul A. K., Marala R. B., Jaiswal R. K., Sharma R. K. Coexistence of guanylate cyclase and atrial natriuretic factor receptor in a 180-kD protein. Science. 1987 Mar 6;235(4793):1224–1226. doi: 10.1126/science.2881352. [DOI] [PubMed] [Google Scholar]
- Pugh E. N., Jr, Lamb T. D. Amplification and kinetics of the activation steps in phototransduction. Biochim Biophys Acta. 1993 Mar 1;1141(2-3):111–149. doi: 10.1016/0005-2728(93)90038-h. [DOI] [PubMed] [Google Scholar]
- Sabatini D. D., Kreibich G., Morimoto T., Adesnik M. Mechanisms for the incorporation of proteins in membranes and organelles. J Cell Biol. 1982 Jan;92(1):1–22. doi: 10.1083/jcb.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyjan A. W., de Sauvage F. J., Gillett N. A., Goeddel D. V., Lowe D. G. Molecular cloning of a retina-specific membrane guanylyl cyclase. Neuron. 1992 Oct;9(4):727–737. doi: 10.1016/0896-6273(92)90035-c. [DOI] [PubMed] [Google Scholar]
- Sitaramayya A., Marala R. B., Hakki S., Sharma R. K. Interactions of nucleotide analogues with rod outer segment guanylate cyclase. Biochemistry. 1991 Jul 9;30(27):6742–6747. doi: 10.1021/bi00241a016. [DOI] [PubMed] [Google Scholar]
- Wong S. K., Garbers D. L. Receptor guanylyl cyclases. J Clin Invest. 1992 Aug;90(2):299–305. doi: 10.1172/JCI115862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Sauvage F. J., Camerato T. R., Goeddel D. V. Primary structure and functional expression of the human receptor for Escherichia coli heat-stable enterotoxin. J Biol Chem. 1991 Sep 25;266(27):17912–17918. [PubMed] [Google Scholar]