Abstract
BACKGROUND
The oral cavity harbors more than 700 species of bacteria, which play crucial roles in the development of various oral diseases including caries, endodontic infection, periodontal infection, and diverse oral diseases.
AIM
To investigate the antimicrobial action of Cymbopogon Schoenanthus and Pelargonium graveolens essential oils against Streptococcus mutans, Staphylococcus aureus, Candida albicans, Ca. dubliniensis, and Ca. krusei.
METHODS
Minimum microbicidal concentration was determined following Clinical and Laboratory Standards Institute documents. The synergistic antimicrobial activity was evaluated using the Broth microdilution checkerboard method, and the antibiofilm activity was evaluated with the 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide assay. Data were analyzed by one-way analysis of variance followed by the Tukey post-hoc test (P ≤ 0.05).
RESULTS
C. schoenanthus and P. graveolens essential oils were as effective as 0.12% chlorhexidine against S. mutans and St. aureus monotypic biofilms after 24 h. After 24 h P. graveolens essential oil at 0.25% was more effective than the nystatin group, and C. schoenanthus essential oil at 0.25% was as effective as the nystatin group.
CONCLUSION
C. schoenanthus and P. graveolens essential oils are effective against S. mutans, St. aureus, Ca. albicans, Ca. dubliniensis, and Ca. krusei at different concentrations after 5 min and 24 h.
Keywords: Antifungal effect, Lemongrass, Geranium, Candida albicans, Candida dubliniensis, Candida krusei, Staphylococcus aureus, Streptococcus mutans
Core Tip: To the best of our knowledge, no study has evaluated the antimicrobial action of the essential oils of herbal plants, Cymbopogon Schoenanthus and Pelargonium graveolens, against different Candida spp. Therefore, the aim of this study was to investigate the antimicrobial action of C. schoenanthus and P. graveolens essential oils against Streptococcus mutans, Staphylococcus aureus, Candida albicans, Ca. dubliniensis, and Ca. krusei monotopic biofilms. The results will facilitate the future development of novel antimicrobials and antifungals capable of combating these multiresistant microorganisms present in the hospital environment, acting both prophylactically and to combat already colonized microorganisms.
INTRODUCTION
The oral cavity harbors more than 700 species of bacteria, which play crucial roles in the development of various oral diseases including caries, endodontic infection, periodontal infection, and diverse oral diseases[1]. Biofilm formation is the main virulence factor that leads to the development of these diseases[2]. Moreover, the hospital environment hosts a multitude of microorganisms, exposing patients in the intensive care unit (ICU) to diverse types of bacteria, fungi, and viruses[3,4]. Hospital-acquired pneumonia and ventilator-associated pneumonia often caused by Staphylococcus aureus contamination, represent significant threats to ICU patients[5]. Additionally, an increase in the incidence of Candida spp. infection in the hospital setting, with Candida being the most frequently isolated pathogen, underscores the urgent need for effective antimicrobial agents throughout patients' hospital stays[6].
This requires the utilization of an antimicrobial agent effective in tackling these microorganisms throughout the patient’s hospital stay[5]. At the same time, it is worth noting that both fungi and bacteria have become increasingly resistant to currently used antibiotics and antifungals, leading to the need for new compounds for treatment and prevention, with herbal therapy being a potential avenue for this purpose[7].
Streptococcus mutans is a facultatively anaerobic, Gram-positive bacterium. It has a critical role in caries development because of its ability to metabolize a large variety of carbohydrates for acid production[8], and is involved in endodontic and periodontal infections[9,10]. S. aureus, which is a Gram-positive spherically shaped bacterium, is involved in diverse oral cavity infections and diseases[11], and causes diverse fatal infections such as pneumonia and septicemia[12]. Its control is becoming challenging due to the emergence of antimicrobial-resistant strains[13]. Likewise, Candida spp. are capable of causing infection ranging from superficial to invasive such as candidemia and candidiasis[14,15]. Seven different Candida spp. have been identified in necrotic pulp with chronic periapical processes including C. albicans, C. dubliniensis, C. guilliermondii, C. krusei, C. parapsilopsis, C. tropicalis, and C. glabrata[16].
In this context, the use of phytotherapy is gaining a greater space in dentistry to combat these microorganisms and others[17-25]. Lemongrass or Cymbopogon Schoenanthus, the herbal plant of Southern Asia and Northern Africa, has effective antimicrobial activity against St. aureus, Escherichia coli, and Klebsiella pneumoniae[26,27]. In addition, Pelargonium graveolens, a Pelargonium species native to South African countries, is widely used as a flavoring agent[28]. Geranium (P. graveolens) essential oil, has antimicrobial action against multidrug-resistant clinical strains of Mycobacterium tuberculosis, M. kansasii, and M. fortuitum[29].
To the best of our knowledge, no study has assessed the antimicrobial action of these herbal plants (Cy. Schoenanthus and P. graveolens) essential oils against different Candida spp. Therefore, the aim of this study was to investigate the antimicrobial action of Cy. schoenanthus and P. graveolens essential oils against St. mutans, S. aureus, C. albicans, C. dubliniensis, and C. krusei monotopic biofilms enabling the future development of a new antimicrobial and antifungal capable of combating these multiresistant microorganisms present in the hospital environment, acting both prophylactically and in combating already colonized microorganisms. The null hypothesis is that Cy. schoenanthus and P. graveolens essential oils have no antimicrobial action against these five pathogens.
MATERIALS AND METHODS
Microbial strains and plant extracts
In this study, five different inocula of St. mutans (ATCC 35688), S. aureus (ATCC 6538), C. albicans (ATCC 18804), C. dubliniensis (ATCC MYA 646), and C. krusei (ATCC 6258) were used. The strains were kept frozen at -80 ºC in brain heart infusion broth (BHI; Kasvi, Roseto degli Abruzzi, Italy) with 20% glycerol.
Cy. schoenanthus and P. graveolens essential oils were supplied commercially (WNF Óleos Essenciais, São Paulo, Brazil) and the chemical composition was verified by gas chromatography-mass spectrometry (GC-MS). The use of plant parts in the present study complied with international, national, and/or institutional guidelines. According to data provided by the manufacturer, the main components of geranium essential oil were geraniol (30.5%), citronellol (14.21%), and lianool (10.09%); and the main components of lemongrass essential oil were nerol (56.20%), nerol acetate (13.36%), and geranial (10.42%).
First, the microorganisms were seeded on the respective culture medium, namely, St. mutans on Mitis Salivarius Bacitracin Saccharose agar supplemented with 2 IU/mL bacitracin and 150 g/L sucrose (Difco Laboratories, Inc., Detroit, MI, United States); S. aureus on BHI agar (HiMedia Laboratories Private Ltd., Mumbai, India) supplemented with 0.75% sterile saline solution (NaCl) (Kasvi); and Candida species including C. albicans, C. dubliniensis, and C. krusei on Sabouraud-Dextrose (SD) agar (HiMedia). All were incubated for 24 h at 37 ºC (with 5% CO2 for St. mutans). Then a standardized inoculum of each strain was prepared, standardized in saline solution (NaCl 0.9%) (Eurofarma Laboratórios S.A., São Paulo, Brazil) and at 1 × 106 colony forming units (CFU)/mL in a spectrophotometer (Micronal S. A., São Paulo, Brazil).
In different 96-well microplates (Techno Plastic Products AG, Trasadingen, Switzerland) for each microbial strain, 100 µL/well Mueller Hinton (MH) broth (HiMedia) for bacterial strains and RPMI 1640 (INLAB, São Paulo, Brazil) for yeast strains were added. Then, 200 µL Cy. schoenanthus and P. graveolens essential oils were added to the first well, where serial dilutions were carried out. As an essential oil emulsifier, 0.5% (v/v) Tween 20 (Computational Biology Laboratory [LBC], São Paulo, Brazil) was used[30]. Lastly, 100 µL/well of the respective microbial inoculum was added. The microplates were incubated for 24 h at 37 ºC. The final concentrations of the essential oils ranged between 16% and 0.03% (v/v). Sole broth (MH or RPMI 1640) was used as the negative control group. Conversely, 0.12% chlorhexidine (CHX) (Periotrat; Kley Hertz S, Porto Alegre, Brazil) served as the positive control for St. mutans and S. aureus plates, and nystatin 100.000 IU/mL (Teuto, Anápolis - GO, Brazil) for C. albicans, C. dubliniensis, and C. krusei plates.
To determine the minimum microbicidal concentration (MMC) of Cy. schoenanthus and P. graveolens essential oils, the broth microdilution method was used according to Clinical and Laboratory Standards Institute documents M27-A2 and M7-A6. Aliquots of 5 µL/well were transferred to BHI agar for bacterial strains or SD agar for yeast strains, and then incubated for 48 h at 37 ºC. The MMC was defined as the lowest concentration of the essential oil that inhibited total microorganism growth.
Synergistic antimicrobial activity
The broth microdilution checkerboard method was used to evaluate the interactions between Cy. schoenanthus and P. graveolens essential oils. Previously obtained MMC values were considered to determine the concentrations of essential oil. The final concentration of the essential oils ranged from MMC to MMC/64.
A total of 200 µL BHI (for S. aureus and St. mutans) or RPMI 1640 (for Candida species) was added to each well of a 96-well plate. Then, 50 µL essential oil of P. graveolens was diluted along the x-axis and 50 µL essential oil of Cy. schoenanthus was diluted along the y-axis. Standardized inoculum at 1 × 106 CFU/mL (planktonic culture) or 1 × 107 CFU/mL (biofilm culture) of each strain was prepared, and 100 µL was inoculated into the 96-well plate. The microplates were incubated at 37 ºC for 24 h (planktonic) or 48 h (biofilm). Next, for the planktonic culture, aliquots of 5 µL/well were transferred to BHI (for bacterial strains) or SD agar (for yeast strains) and incubated for 48 h at 37 ºC. The combined MMC was defined as the lowest concentration of essential oil that inhibited total microorganism growth. For biofilm culture, the metabolic activity of each microorganism was evaluated using the 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) assay. The fractional inhibitory concentration (FIC) was calculated as well using the formula: FIC index = FICA + FICB = (MMC of extract A in combination/MMC of extract A alone) + (MMC of extract B in combination/MMC of extract B alone).
Antimicrobial activity against biofilm formation
Microbial inoculum of each microorganism was prepared again as described above and standardized in saline solution (NaCl 0.9%) at 1 × 107 CFU/mL in a spectrophotometer. Biofilms of each microorganism were formed in a 96-well plate (Kasvi) by adding 100 mL/well of BHI or RPMI 1640 and 100 mL/well of the respective microbial inoculum. The plates were incubated for 48 h at 37 ºC with replacement of culture medium after 24 h. The biofilms were exposed to 100 µL Cy. schoenanthus and P. graveolens essential oils at the concentrations of MMC, MMC × 2, and MMC × 4 for 5 min and 24 h. BHI broth was used as a negative control; 0.12% CHX (Periotrat; Kley Hertz) for St. mutans and S. aureus plates; and nystatin 100.000 IU/mL (Laboratorio Teuto Brasileiro, Anápolis - GO, Brazil) for C. albicans, C. dubliniensis, and C. krusei plates. Subsequently, the biofilms were washed with 0.9% NaCl (saline solution) to remove affected cells from exposure to the essential oil of each experimental group, with two replicates of each group (n = 10).
The metabolic activity of each microorganism was evaluated with the MTT assay using 0.5 mg/mL MTT solution (Sigma Aldrich, St. Louis, MO, United States) prepared with BHI broth. In each well, 100 mL prepared MTT solution was added and the plates were incubated for 1 h in the dark. Then the supernatant was discarded and 100 µL/well of dimethyl sulfoxide was added for 10 min. The plates were shaken for 10 min. Lastly, the absorbance of the wells was measured with a spectrophotometer at 570 nm and generated data were converted to cell viability percentage using the formula: % viability = (optical density [OD] Treated Group × 100)/Mean OD Control Group).
Statistical analyses
All experiments were performed in duplicate. Data were submitted to normality tests, and then were analyzed by one-way analysis of variance followed by the Tukey post-hoc test using GraphPad Prism 5.0. P ≤ 0.05 was considered statistically significant.
RESULTS
The MMC of Cy. schoenanthus and P. graveolens essential oils ranged from 0.03% to 16% against all of the tested pathogens (Table 1).
Table 1.
Minimum microbicidal concentration values of Cymbopogon schoenanthus (lemongrass) and Pelargonium graveolens (geranium) essential oils against Streptococcus mutans, Staphylococcus aureus, Candida albicans, C. krusei, and C. dubliniensis
|
Microorganism
|
Cymbopogon schoenanthus
|
Pelargonium graveolens
|
| Streptococcus mutans | 16 | 16 |
| Staphylococcus aureus | 4 | 4 |
| Candida albicans | 0.06 | 0.06 |
| Candida krusei | 0.06 | 0.03 |
| Candida dubliniensis | 0.03 | 0.06 |
Data are minimum microbicidal concentration (%).
Synergistic antimicrobial activity
The combination of Cy. schoenanthus and P. graveolens essential oils resulted in one synergistic effect and three additive effects against S. aureus (Table 2). Conversely, this combination did not show synergistic or additive effects against St. mutans, C. albicans, C. krusei and C. dubliniensis.
Table 2.
Minimum microbicidal concentration values of isolated essential oils, and synergistic and additive combinations of Cymbopogon schoenanthus and Pelargonium graveolens essential oils against Staphylococcus aureus
| Combination |
Individual MMC values (%)
|
Combined concentrations (%)
|
FICI |
MMC reduction
|
Effect | |||||
|
LE
|
GE
|
LE
|
GE
|
LE
|
GE
|
|||||
| C. + P. | 4 | 4 | 2 | 0.5 | 0.62 | 2 × | 8 × | Additive | ||
| C. + P. | 2 | 0.25 | 0.56 | 2 × | 16 × | Additive | ||||
| C. + P. | 2 | 0.12 | 0.53 | 2 × | 33 × | Additive | ||||
| C. + P. | 1 | 0.06 | 0.26 | 4 × | 66 × | Synergistic | ||||
C.: Cymbopogon schoenanthus; FICI: Fractional inhibitory concentration index; GE: Pelargonium graveolens; LE: Cymbopogon schoenanthus; MMC: Minimum microbicidal concentration; P.: Pelargonium graveolens.
Antimicrobial activity against biofilm
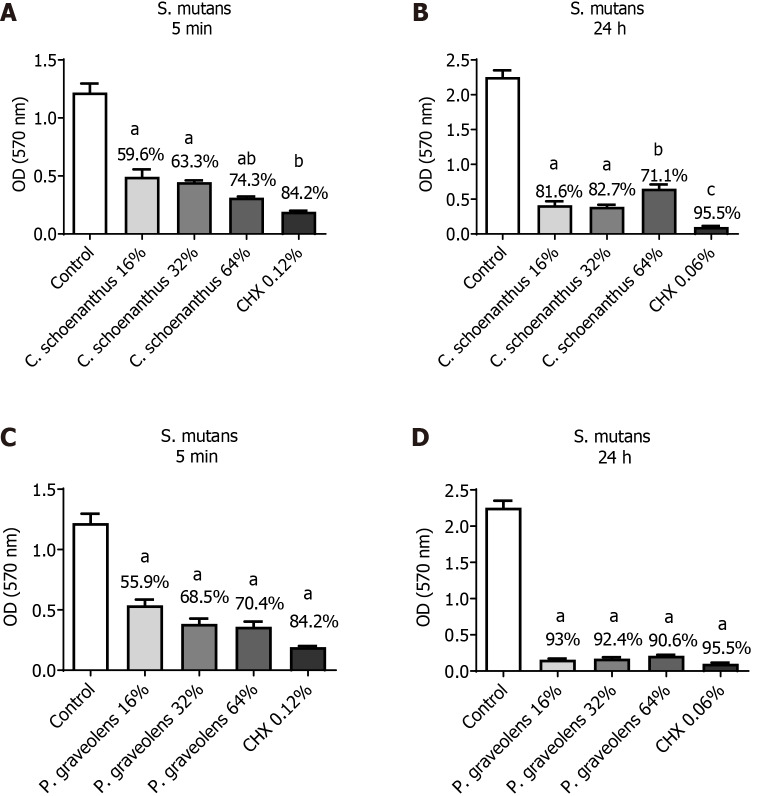
St. mutans monotypic biofilm: As mentioned above, the experiments were performed with two replicates of each group (n = 10). After 5 min of treatment, Cy. schoenanthus essential oil (LE) at 16%, 32%, and 64% reduced the St. mutans biofilm formation in 59.6%, 63.3%, and 74.3%, respectively. P. graveolens essential oil (GE) at 16%, 32%, and 64% reduced St. mutans biofilm formation in 55.9%, 68.5%, and 70.4%, respectively. These reductions had a statistically significant difference with the control group for both essential oils. Besides, both essential oils at 64% were as effective as 0.12% CHX (Figure 1). After 24 h, a greater reduction was observed at all concentrations except Cy. schoenanthus essential oil (LE) at 64%. However, all of the tested concentrations of P. graveolens essential oil (GE) were as effective as 0.12% CHX (Figure 1).
Figure 1.
Reduction of Streptococcus mutans monotipic biofilm (in %) calculated using the MTT test after treatment with experimental groups. Data are presented as the mean ± standard deviation. Statistical analysis was performed using one-way analysis of variance with Tukey’s post-hoc test (n = 10, α = 0.05). Statistical significance is indicated as follows: aP ≤ 0.0001 vs the control group, bP < 0.0025 vs Cymbopogon schoenanthus 32%, and cP < 0.0001 vs C. schoenanthus 64%. A: Streptococcus mutans biofilm treated for 5 min with C. schoenanthus at various concentrations; B: S. mutans biofilm treated for 24 h with C. schoenanthus at various concentrations; C: S. mutans biofilm treated for 5 min with Pelargonium graveolens at various concentrations; D: S. mutans biofilm treated for 24 h with P. graveolens at various concentrations.
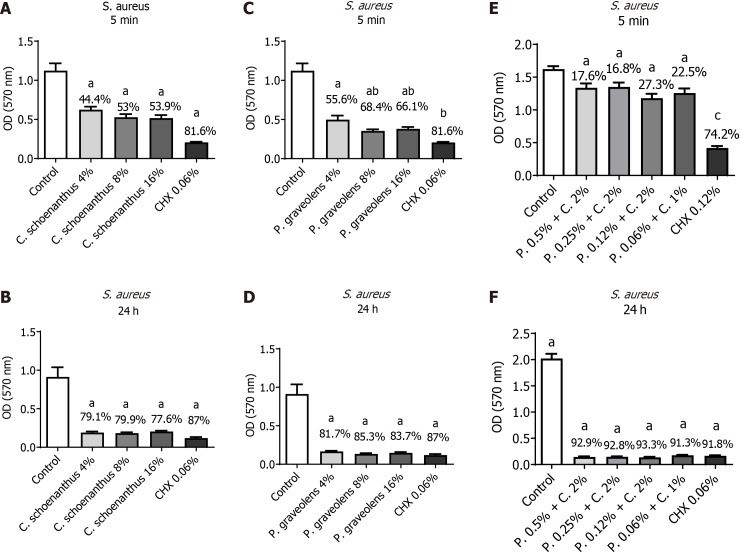
S. aureus monotypic biofilm: After 5 min of treatment, Cy. schoenanthus essential oil (LE) at 4%, 8%, and 16% reduced S. aureus biofilm formation in 44.4%, 53%, and 53.9%, respectively. P. graveolens essential oil (GE) at 4%, 8%, and 16% reduced S. aureus biofilm formation in 55.6%, 68.4%, and 66.1%, respectively. These reductions were statistically significantly different from the control group for both essential oils. Besides, all of the tested concentrations of both essential oils, except P. graveolens at 4%, were as effective as 0.12% CHX (Figure 2). After 24 h, a greater reduction was observed, in which all of the tested concentrations were as effective as 0.12% CHX and were statistically significantly different from the control group (Figure 2). The combined effect of both essential oils resulted in reductions of S. aureus biofilm formation with a statistically significant difference from the control group after 5 min and 24 h; however, it was as effective as CHX 0.12% only after 24 h (Figure 2).
Figure 2.
Reduction of Staphylococcus aureus monotipic biofilm (in %) calculated using the MTT test after treatment with experimental groups. Data are presented as the mean ± standard deviation. Statistical analysis was performed using one-way analysis of variance with Tukey’s post-hoc test (n = 10, α = 0.05). Statistical significance is indicated as follows: aP ≤ 0.0001 vs the control group, bP < 0.0025 vs Pelargonium graveolens 4%, and cP < 0.0001 vs all synergistic groups. A: Staphylococcus aureus biofilm treated for 5 min with Cymbopogon schoenanthus at various concentrations; B: S. aureus biofilm treated for 24 h with C. schoenanthus at various concentrations; C: S. aureus biofilm treated for 5 min with P. graveolens at various concentrations; D: S. aureus biofilm treated for 24 h with P. graveolens at various concentrations; E: S. aureus biofilm treated for 5 min with C. schoenanthus (C) and P. graveolens (P), combined, at various concentrations; F: S. aureus biofilm treated for 24 h with C. schoenanthus (C) and P. graveolens (P) combined, at various concentrations.
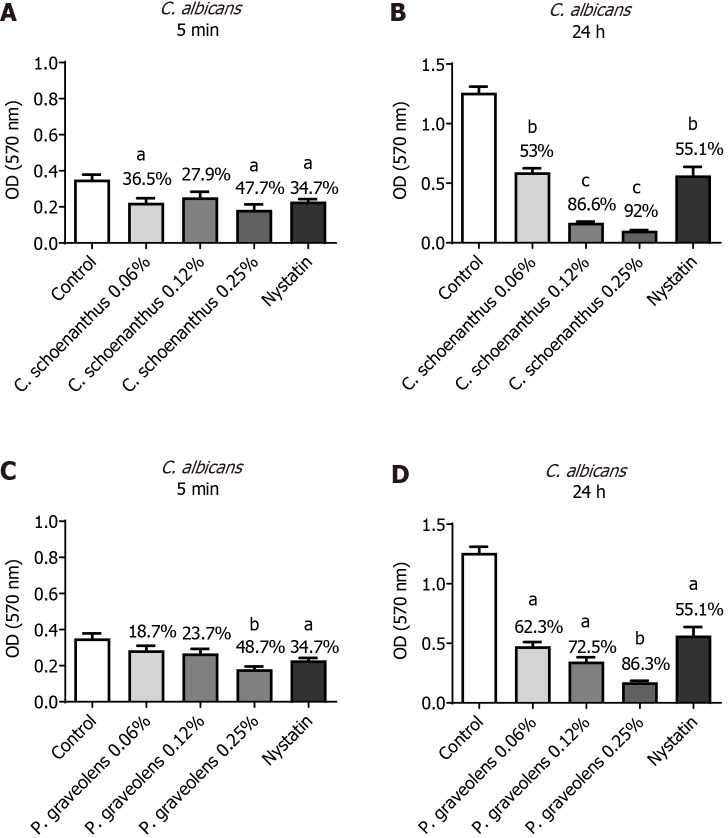
C. albicans monotypic biofilm: After 5 min of treatment, Cy. schoenanthus essential oil (LE) at 0.06%, 0.12%, and 0.25% reduced C. albicans biofilm formation in 36.5%, 27.9%, and 47.7%, respectively. P. graveolens essential oil (GE) at 0.06%, 0.12%, and 0.25% reduced C. albicans biofilm formation in 18.7%, 23.7%, and 48.7%, respectively (Figure 3). After 24 h, a greater reduction was observed at all of the tested concentrations with a statistically significant difference from the control group. Furthermore, P. graveolens essential oil (GE) at 0.25% was more effective than the nystatin group with a statistically significant difference (Figure 3).
Figure 3.
Reduction of Candida albicans monotipic biofilm (in %) calculated using the MTT test after treatment with experimental groups. Data are presented as the mean ± standard deviation. Statistical analysis was performed using one-way analysis of variance with Tukey’s post-hoc test (n = 10, α = 0.05). Statistical significance is indicated as follows: aP ≤ 0.0004 vs the control group, bP ≤ 0.0001 vs the control group and cP ≤ 0.0001 vs nystatin. A: Candida albicans biofilm treated for 5 min with Cymbopogon schoenanthus at various concentrations; B: C. albicans biofilm treated for 24 h with Cy. schoenanthus at various concentrations; C: C. albicans biofilm treated for 5 min with Pelargonium graveolens at various concentrations; D: C. albicans biofilm treated for 24 h with P. graveolens at various concentrations.
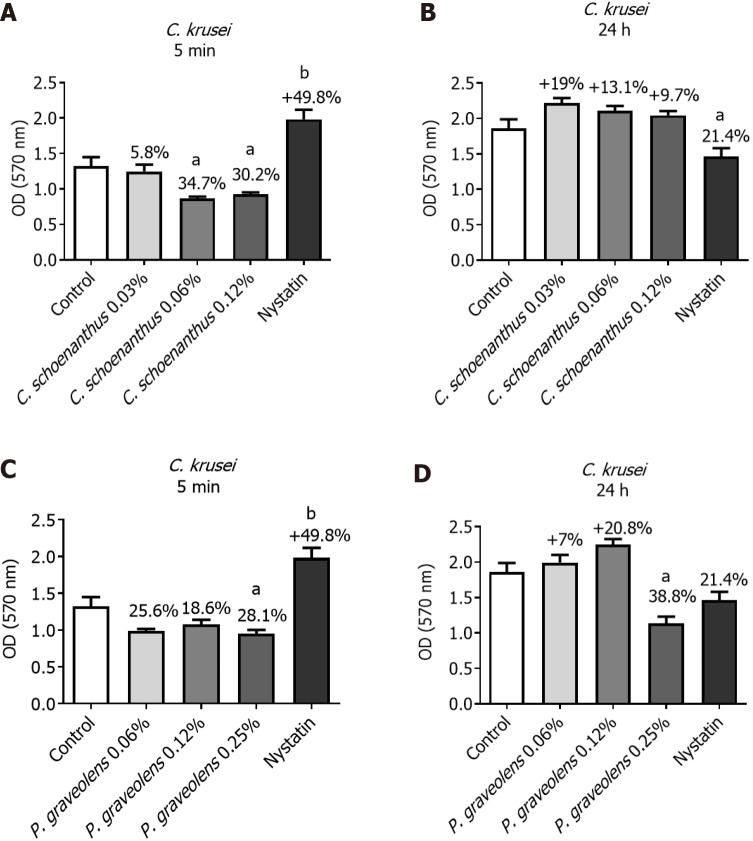
C. krusei monotypic biofilm: After 5 min of treatment, Cy. schoenanthus essential oil (LE) at 0.06 and 0.12% reduced the C. krusei biofilm formation in 34.7% and 30.2%%, respectively. P. graveolens essential oil (GE) at 0.06%, 0.12% and 0.25% reduced the C. krusei biofilm formation in 25.6%, 18.6% and 28.1%, respectively, however, it seems like this efficacy tends to reduce after 24 h except for P. graveolens essential oil (GE) at 0.25% (Figure 4).
Figure 4.
Reduction of Candida krusei monotipic biofilm (in %) calculated using the MTT test after treatment with experimental groups. Data are presented as the mean ± standard deviation. Statistical analysis was performed using one-way analysis of variance with Tukey’s post-hoc test (n = 10, α = 0.05). Statistical significance is indicated as follows: aP ≤ 0.04 vs the control group and bP ≤ 0.0001 vs the control group. A: Candida krusei biofilm treated for 5 min with Cymbopogon schoenanthus at various concentrations; B: C. krusei biofilm treated for 24 h with Cy. schoenanthus at various concentrations; C: C. krusei biofilm treated for 5 min with Pelargonium graveolens at various concentrations; D: C. krusei biofilm treated for 24 h with P. graveolens at various concentrations.
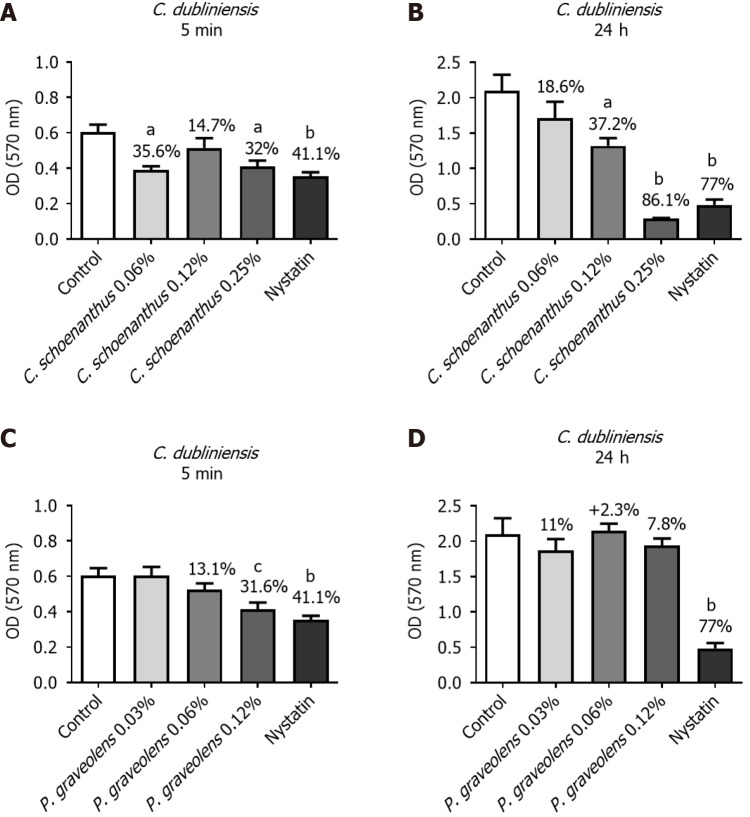
C. dubliniensis monotypic biofilm: After 5 min of treatment, Cy. schoenanthus essential oil (LE) at 0.06%, 0.12%, and 0.25% reduced C. dubliniensis biofilm formation in 35.6%, 14.7%, and 32.0%%, respectively. P. graveolens essential oil (GE) at 0.06% and 0.12% reduced C. dubliniensis biofilm formation in 13.1% and 31.6%, respectively (Figure 5). After 24 h, a greater reduction was observed at all of tested concentrations of Cy. schoenanthus essential oil (LE), with at 0.25% as effective as the nystatin group. However, the efficacy of P. graveolens essential oil (GE) at 0.06% and 0.12% tended to decrease after 24 h (Figure 5).
Figure 5.
Reduction of Candida dubliniensis monotipic biofilm (in %) calculated using the MTT test after treatment with experimental groups. Data are presented as the mean ± standard deviation. Statistical analysis was performed using one-way analysis of variance with Tukey’s post-hoc test (n = 10, α = 0.05). Statistical significance is indicated as follows: aP ≤ 0.007 vs the control group, bP ≤ 0.0005 vs the control group, and cP ≤ 0.01 vs the control group. A: Candida dubliniensis biofilm treated for 5 min with Cymbopogon schoenanthus at various concentrations; B: C. dubliniensis biofilm treated for 24 h with Cy. schoenanthus at various concentrations; C: C. dubliniensis biofilm treated for 5 min with Pelargonium graveolens at various concentrations; D: C. dubliniensis biofilm treated for 24 h with P. graveolens at various concentrations.
DISCUSSION
The integration of medicinal plants and herbs into contemporary dentistry is gaining momentum due to their antimicrobial action and biocompatibility[17-22]. The null hypothesis of this study was that Cy. schoenanthus and P. graveolens essential oils have no antimicrobial action against these tested pathogens. It was found that Cy. schoenanthus and P. graveolens essential oils had antimicrobial action against the tested pathogens after 5 min and/or 24 h at different concentrations; thus the null hypothesis was rejected. In this study, the MMC of Cy. schoenanthus and P. graveolens essential oils ranged from 0.03% to 16% against the planktonic forms of the pathogens St. mutans, S. aureus, C. albicans, C. dubliniensis, and C. krusei.
In the literature, Cy. schoenanthus essential oil has effective antimicrobial action against a planktonic culture of S. aureus proved by broth microdilution method[27]. The water extracts of Cy. schoenanthus essential oils antimicrobial action was also evaluated against 10 different strains of Gram-positive and Gram-negative bacteria including S. aureus, where they were effective at different concentrations[26]. In another study, the aqueous extract of Cy. schoenanthus presented selective activities against different tested pathogens including S. aureus[31]. In a study by Malti et al[32], the antimicrobial activity of Cy. schoenanthus essential oil was tested against S. aureus and C. albicans, and it was found to be effective. In addition, in a more recent study proved its effective antifungal activity against some fungi[5].
In the present study, Cy. Schoenanthus essential oil was effective against St. mutans, S. aureus, C. albicans, C. dubliniensis, and C. krusei at different concentrations after 5 min and 24 h, except for its antifungal activity against C. krusei which tended to decrease after 24 h. To the best of our knowledge, this is the first study to evaluate Cy. schoenanthus essential oil antimicrobial action against St. mutans, C. dubliniensis, and C. krusei.
According to the manufacturer, the main components of Cy. schoenanthus essential oil are nerol (56.20%), nerol acetate (13.36%), and geranial (10.42%). Nerol is a monoterpene with effective antibacterial and antifungal activity[33,34], which may explain the antimicrobial action of Cy. schoenanthus essential oil found in this study.
In this study, P. graveolens essential oil (GE) at 16%, 32%, and 64% reduced St. mutans biofilm formation in 55.9%, 68.5%, and 70.4%, respectively. These reductions were statistically significantly different from the control group for both essential oils. Besides, both essential oils at 64% were as effective as CHX 0.12%. In the literature, using the agar diffusion method, the antibacterial properties of the methanolic extracts of P. pelargonium against St. mutans and S. sanguinis[35] were proven. Furthermore, the addition of geranium essential oil to modified resin composites improves its antimicrobial action against St. mutans and C. albicans[36]. In this study as well. P. graveolens essential oil (GE) at 64% was as effective as CHX 0.12% against St. mutans biofilms. More interestingly, all of the tested concentrations were as effective as CHX 0.12% after 24 h.
P. graveolens essential oil is effective against three strains of S. aureus[37]. The addition of ciprofloxacin improved its antimicrobial action against different uropathogens including S. aureus[38]. Another paper tested P. graveolens essential oil against 1 standard S. aureus strain ATCC 433000 and 70 clinical strains, and found that P. graveolens essential oil had strong activity against all of the clinical S. aureus isolates, including multidrug resistant strains, with a relatively low minimum inhibitory ranging from 0.25 to 2.50 μL/mL[39]. More recently, it presented considerable antimicrobial activity against S. aureus[40]. In the present study, P. graveolens essential oil (GE) at 4%, 8%, and 16% reduced S. aureus biofilm formation with a statistically significant difference from the control group. In addition, 8% and 16% were as effective as CHX 0.12%; this efficacy tended to increase after 24 h.
In this investigation, the essential oil of P. graveolens demonstrated a notable reduction in biofilm formation at concentrations of 0.06%, 0.12%, and 0.25% within both 5 min and 24 h. It was also effective against C. krusei and C. dubliniensis; however, the efficacy tended to decreased after 24 h except for P. graveolens essential oil (GE) at 0.25%. Gucwa et al[41] verified the antifungal activity of 37 essential oils, including P. graveolens, which was one of the most effective against clinical strains of C. albicans. A study by Szweda et al[42] evaluated the antifungal activity of essential oils, propolis, silver nanoparticles, and ethanolic extracts against azole-resistant C. albicans, C. glabrata, and C. krusei clinical strains. Among the different formulations studied, the essential oils stood out for their remarkable effectiveness against fungi, mainly the essential oils of Cinnamomum cassia (cinnamon), Citrus limonum (lemon), Ocimum basilicum (basil), Thymus vulgaris (thyme), P. graveolens (geranium), and Eugenia caryophyllus (clove Additionally, literature suggests that P. graveolens holds promise as a supplementary agent to enhance the efficacy of fluconazole-based therapy for C. albicans infections[43].
A study by Giongo et al[44] revealed that P. graveolens essential oil had no significant effect on biofilm formation except for C. krusei. In our study, we found that after a 5-min treatment, P. graveolens essential oil at concentrations of 0.06%, 0.12%, and 0.25% reduced C. krusei biofilm formation by 25.6%, 18.6%, and 28.1%, respectively. However, this efficacy appeared to diminish after 24 h, except for the concentration of 0.25%.
In a recent study, using a GC-MS analysis, a total of 70 chemicals were found in P. graveolens essential oil[3], highlighting, according to the information provided by the manufacturer, the main components of P. graveolens essential oil including geraniol (30.5%), citronellol (14.21%), and lianool (10.09%). Thus the antimicrobial action of P. graveolens essential oil is explained as it contains an important antimicrobial agent, namely, lianool[45,46].
To the best of our knowledge, this is the first study to evaluate the synergistic effect of the combination of Cy. schoenanthus and P. graveolens essential oils, which resulted in one synergistic effect and three additive effects against S. aureus. Conversely, this combination did not show synergistic or additive effects against St. mutans, C. albicans, C. krusei, or C. dubliniensis. In the literature, it has been suggested that the combination of geranium, rosemary, and peppermint essential oils has antibiofilm activity against colistin-resistant Acinetobacter baumannii[47], and that the combination of geranium with antibiotics improves their antibacterial action[30,48].
Finally, the antibacterial and antifungal potential of geranium and lemongrass against the tested pathogens was evident. Nonetheless, it is essential to evaluate the cytotoxicity of these agents on human and animal cells, in order to use them safely and effectively. The outcomes of this study, and others using phytotherapy[17-22], confirm the efficacy of the herbal plants extracts and essential oils as alternative treatments to antibiotics and antifungals. Additional research is needed to propose alternative treatments and complementary techniques with antimicrobial properties.
CONCLUSION
Cy. Schoenanthus and P. graveolens essential oils have antimicrobial action against planktonic and biofilms culture of S. aureus, St. mutans, C. albicans, C. dubliniensis, and C. krusei after 5 min and 24 h at different concentrations.
Footnotes
Institutional review board statement: Since our research was conducted in vitro and did not involve human participants, it was not necessary to obtain approval by the local Institutional Review Board.
Conflict-of-interest statement: The authors have no conflicts of interest to declare.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Microbiology
Country of origin: Brazil
Peer-review report’s classification
Scientific Quality: Grade C, Grade C
Novelty: Grade B, Grade B
Creativity or Innovation: Grade B, Grade C
Scientific Significance: Grade B, Grade B
P-Reviewer: Yu YB, China S-Editor: Liu JH L-Editor: Filipodia P-Editor: Zhao S
Contributor Information
Patrícia Michelle Nagai de Lima, Department of Biosciences and Oral Diagnosis, Institute of Science and Technology, São Paulo State University, São José dos Campos 12245000, SP, Brazil.
Thaís Cristine Pereira, Department of Biosciences and Oral Diagnosis, Institute of Science and Technology, São Paulo State University, São José dos Campos 12245000, SP, Brazil.
Lara Steffany de Carvalho, Department of Biosciences and Oral Diagnosis, Institute of Science and Technology, São Paulo State University, São José dos Campos 12245000, SP, Brazil.
Letícia Ferreira dos Santos, Department of Biosciences and Oral Diagnosis, Institute of Science and Technology, São Paulo State University, São José dos Campos 12245000, SP, Brazil.
Carlos Eduardo Rocha Oliveira, Anhembi Morumbi University, School of Medicine, São José dos Campos 12230-002, SP, Brazil.
Lucas de Paula Ramos, Department of Biosciences and Oral Diagnosis, Institute of Science and Technology, São Paulo State University, São José dos Campos 12245000, SP, Brazil.
Maria Cristina Marcucci, Department of Biosciences and Oral Diagnosis, Institute of Science and Technology, São Paulo State University, São José dos Campos 12245000, SP, Brazil.
Amjad Abu Hasna, Department of Restorative Dentistry, Endodontics Division, Institute of Science and Technology, São Paulo State University, São José dos Campos 12245000, São Paulo, Brazil. d.d.s.amjad@gmail.com.
Luciane Dias de Oliveira, Department of Biosciences and Oral Diagnosis, Institute of Science and Technology, São Paulo State University, São José dos Campos 12245000, SP, Brazil.
Data sharing statement
The data used to support the findings of this study are available upon reasonable request from the corresponding author at d.d.s.amjad@gmail.com.
References
- 1.Deo PN, Deshmukh R. Oral microbiome: Unveiling the fundamentals. J Oral Maxillofac Pathol. 2019;23:122–128. doi: 10.4103/jomfp.JOMFP_304_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Radaic A, Kapila YL. The oralome and its dysbiosis: New insights into oral microbiome-host interactions. Comput Struct Biotechnol J. 2021;19:1335–1360. doi: 10.1016/j.csbj.2021.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaradat N, Hawash M, Qadi M, Abualhasan M, Odetallah A, Qasim G, Awayssa R, Akkawi A, Abdullah I, Al-Maharik N. Chemical Markers and Pharmacological Characters of Pelargonium graveolens Essential Oil from Palestine. Molecules. 2022;27 doi: 10.3390/molecules27175721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.da Rocha Santos LMB, de Paula Ramos L, Santos CER, Miranda DG, Gimenez MG, Meccatti VM, Abu Hasna A, Dos Santos Oliveira M, Neto MB, Dias de Oliveira L. Saliva culture as a predictive indicator for current blood infections and antimicrobial resistance in the ICU setting. Sci Rep. 2023;13:20317. doi: 10.1038/s41598-023-47143-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sawadogo I, Paré A, Kaboré D, Montet D, Durand N, Bouajila J, Zida EP, Sawadogo-Lingani H, Nikiéma PA, Nebié RHC, Bassolé IHN. Antifungal and Antiaflatoxinogenic Effects of Cymbopogon citratus, Cymbopogon nardus, and Cymbopogon schoenanthus Essential Oils Alone and in Combination. J Fungi (Basel) 2022;8 doi: 10.3390/jof8020117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu JY, Dickter JK. Nosocomial Infections: A History of Hospital-Acquired Infections. Gastrointest Endosc Clin N Am. 2020;30:637–652. doi: 10.1016/j.giec.2020.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Pickens CI, Wunderink RG. Methicillin-Resistant Staphylococcus aureus Hospital-Acquired Pneumonia/Ventilator-Associated Pneumonia. Semin Respir Crit Care Med. 2022;43:304–309. doi: 10.1055/s-0041-1740583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lemos JA, Palmer SR, Zeng L, Wen ZT, Kajfasz JK, Freires IA, Abranches J, Brady LJ. The Biology of Streptococcus mutans. Microbiol Spectr. 2019;7 doi: 10.1128/microbiolspec.GPP3-0051-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Contardo M, Díaz N, Lobos O, Padilla C, Giacaman R. Oral colonization by Streptococcus mutans and its association with the severity of periodontal disease in adults. Revista Clínica de Periodoncia, Implantología y Rehabilitación Oral. 2011;4:9–12. [Google Scholar]
- 10.Lima AR, Herrera DR, Francisco PA, Pereira AC, Lemos J, Abranches J, Gomes BPFA. Detection of Streptococcus mutans in symptomatic and asymptomatic infected root canals. Clin Oral Investig. 2021;25:3535–3542. doi: 10.1007/s00784-020-03676-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCormack MG, Smith AJ, Akram AN, Jackson M, Robertson D, Edwards G. Staphylococcus aureus and the oral cavity: an overlooked source of carriage and infection? Am J Infect Control. 2015;43:35–37. doi: 10.1016/j.ajic.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 12.Cheung GYC, Bae JS, Otto M. Pathogenicity and virulence of Staphylococcus aureus. Virulence. 2021;12:547–569. doi: 10.1080/21505594.2021.1878688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, Zhang Y, Chen C, Cheng H, Deng X, Li D, Bai B, Yu Z, Deng Q, Guo J, Wen Z. Antibacterial activities and action mode of anti-hyperlipidemic lomitapide against Staphylococcus aureus. BMC Microbiol. 2022;22:114. doi: 10.1186/s12866-022-02535-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Talapko J, Juzbašić M, Matijević T, Pustijanac E, Bekić S, Kotris I, Škrlec I. Candida albicans-The Virulence Factors and Clinical Manifestations of Infection. J Fungi (Basel) 2021;7 doi: 10.3390/jof7020079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCarty TP, White CM, Pappas PG. Candidemia and Invasive Candidiasis. Infect Dis Clin North Am. 2021;35:389–413. doi: 10.1016/j.idc.2021.03.007. [DOI] [PubMed] [Google Scholar]
- 16.Nastri N, Nastri M, Jewtuchowicz V, Mujica M, Lovanniti C, Gualtieri A, Ponton J, Rosa A. Prevalence of Candida species in necrotic pulp with chronic periapical processes. Acta Odontol Latinoam. 2011;24:183–187. [PubMed] [Google Scholar]
- 17.Dos Santos Liberato SF, da Cruz Vegian MR, Abu Hasna A, de Alvarenga JA, Dos Santos JG, Tini ÍRP, Amêndola I, Junqueira JC, de Oliveira LD. Antibiofilm action of Persea americana glycolic extract over Acinetobacter baumannii and absence of toxicity in Galleria mellonella. J Complement Integr Med. 2022;19:905–911. doi: 10.1515/jcim-2021-0051. [DOI] [PubMed] [Google Scholar]
- 18.Ferreira ECC, Gonçalves TM, Pereira TC, Hasna AA, Oliveira FED, Jorjão AL, Camargo SEA, Oliveira LDD, Spalding M. The biocompatibility of Achyrocline satureioides plant extract over human gingival fibroblasts. RSD. 2021;10:e37610111902. [Google Scholar]
- 19.Marques Meccatti V, de Souza Moura L, Guerra Pinto J, Ferreira-Strixino J, Abu Hasna A, Alves Figueiredo-Godoi LM, Campos Junqueira J, Marcucci MC, de Paula Ramos L, Carvalho CAT, Pucci CR, de Oliveira LD. Curcuma longa L. Extract and Photodynamic Therapy are Effective against Candida spp. and Do Not Show Toxicity In Vivo. Int J Dent. 2022;2022:5837864. doi: 10.1155/2022/5837864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Abdullah A, Edris S, Abu Hasna A, de Carvalho LS, Al-Nahlawi T. The Effect of Aloe vera and Chlorhexidine as Disinfectants on the Success of Selective Caries Removal Technique: A Randomized Controlled Trial. Int J Dent. 2022;2022:9474677. doi: 10.1155/2022/9474677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meccatti VM, Figueiredo-Godoi LMA, Pereira TC, de Lima PMN, Abu Hasna A, Senna LB, Marcucci MC, Junqueira JC, de Oliveira LD. The biocompatibility and antifungal effect of Rosmarinus officinalis against Candida albicans in Galleria mellonella model. Sci Rep. 2022;12:15611. doi: 10.1038/s41598-022-19425-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Domingues N, Ramos LP, Pereira LM, do Rosário Estevam Dos Santos PB, Scorzoni L, Pereira TC, Abu Hasna A, Carvalho CAT, de Oliveira LD. Antimicrobial action of four herbal plants over mixed-species biofilms of Candida albicans with four different microorganisms. Aust Endod J. 2023;49:262–271. doi: 10.1111/aej.12681. [DOI] [PubMed] [Google Scholar]
- 23.Meccatti VM, Santos LF, de Carvalho LS, Souza CB, Carvalho CAT, Marcucci MC, Abu Hasna A, de Oliveira LD. Antifungal Action of Herbal Plants' Glycolic Extracts against Candida Species. Molecules. 2023;28 doi: 10.3390/molecules28062857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Sá Assis MA, de Paula Ramos L, Abu Hasna A, de Queiroz TS, Pereira TC, Nagai de Lima PM, Berretta AA, Marcucci MC, Talge Carvalho CA, de Oliveira LD. Antimicrobial and Antibiofilm Effect of Brazilian Green Propolis Aqueous Extract against Dental Anaerobic Bacteria. Molecules. 2022;27 doi: 10.3390/molecules27238128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu AR, de Paula Ramos L, de Lima PMN, Abu Hasna A, da Rocha Santos CE, Theotonio dos Santos JM, Pereira TC, de Oliveira LD. Extract Antibiofilm Action against Acinetobacter baumannii Carbapenem-Resistant and Biocompatibility over Human Keratinocytes. J Health Sci. 2022;24:215–219. [Google Scholar]
- 26.Hashim GM, Almasaudi SB, Azhar E, Al Jaouni SK, Harakeh S. Biological activity of Cymbopogon schoenanthus essential oil. Saudi J Biol Sci. 2017;24:1458–1464. doi: 10.1016/j.sjbs.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yagi S, Babiker R, Tzanova T, Schohn H. Chemical composition, antiproliferative, antioxidant and antibacterial activities of essential oils from aromatic plants growing in Sudan. Asian Pac J Trop Med. 2016;9:763–770. doi: 10.1016/j.apjtm.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 28.Narnoliya LK, Jadaun JS, Singh SP. The Phytochemical Composition, Biological Effects and Biotechnological Approaches to the Production of High-Value Essential Oil from Geranium. Essential Oil Research. 2019 [Google Scholar]
- 29.Kardan-Yamchi J, Mahboubi M, Kazemian H, Hamzelou G, Feizabadi MM. The Chemical Composition and Anti-mycobacterial Activities of Trachyspermum copticum and Pelargonium graveolens Essential Oils. Recent Pat Antiinfect Drug Discov. 2020;15:68–74. doi: 10.2174/1574891X14666191028113321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosato A, Vitali C, De Laurentis N, Armenise D, Antonietta Milillo M. Antibacterial effect of some essential oils administered alone or in combination with Norfloxacin. Phytomedicine. 2007;14:727–732. doi: 10.1016/j.phymed.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 31.Rocchetti G, Alcántara C, Bäuerl C, García-Pérez JV, Lorenzo JM, Lucini L, Collado MC, Barba FJ. Bacterial growth and biological properties of Cymbopogon schoenanthus and Ziziphus lotus are modulated by extraction conditions. Food Res Int. 2020;136:109534. doi: 10.1016/j.foodres.2020.109534. [DOI] [PubMed] [Google Scholar]
- 32.Malti CEW, El Haci IA, Hassani F, Paoli M, Gibernau M, Tomi F, Casanova J, Bekhechi C. Composition, Chemical Variability and Biological Activity of Cymbopogon schoenanthus Essential Oil from Central Algeria. Chem Biodivers. 2020;17:e2000138. doi: 10.1002/cbdv.202000138. [DOI] [PubMed] [Google Scholar]
- 33.Kasthuri T, Swetha TK, Bhaskar JP, Pandian SK. Rapid-killing efficacy substantiates the antiseptic property of the synergistic combination of carvacrol and nerol against nosocomial pathogens. Arch Microbiol. 2022;204:590. doi: 10.1007/s00203-022-03197-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Z, Yang K, Chen L, Yan R, Qu S, Li YX, Liu M, Zeng H, Tian J. Activities of Nerol, a natural plant active ingredient, against Candida albicans in vitro and in vivo. Appl Microbiol Biotechnol. 2020;104:5039–5052. doi: 10.1007/s00253-020-10559-2. [DOI] [PubMed] [Google Scholar]
- 35.Coronado-López S, Caballero-García S, Aguilar-Luis MA, Mazulis F, Del Valle-Mendoza J. Antibacterial Activity and Cytotoxic Effect of Pelargonium peltatum (Geranium) against Streptococcus mutans and Streptococcus sanguinis. Int J Dent. 2018;2018:2714350. doi: 10.1155/2018/2714350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lapinska B, Szram A, Zarzycka B, Grzegorczyk J, Hardan L, Sokolowski J, Lukomska-Szymanska M. An In Vitro Study on the Antimicrobial Properties of Essential Oil Modified Resin Composite against Oral Pathogens. Materials (Basel) 2020;13 doi: 10.3390/ma13194383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nogueira JC, Diniz Mde F, Lima EO. In vitro antimicrobial activity of plants in Acute Otitis Externa. Braz J Otorhinolaryngol. 2008;74:118–124. doi: 10.1016/S1808-8694(15)30761-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malik T, Singh P, Pant S, Chauhan N, Lohani H. Potentiation of antimicrobial activity of ciprofloxacin by Pelargonium graveolens essential oil against selected uropathogens. Phytother Res. 2011;25:1225–1228. doi: 10.1002/ptr.3479. [DOI] [PubMed] [Google Scholar]
- 39.Bigos M, Wasiela M, Kalemba D, Sienkiewicz M. Antimicrobial activity of geranium oil against clinical strains of Staphylococcus aureus. Molecules. 2012;17:10276–10291. doi: 10.3390/molecules170910276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ennaifer M, Bouzaiene T, Messaoud C, Hamdi M. Phytochemicals, antioxidant, anti-acetyl-cholinesterase, and antimicrobial activities of decoction and infusion of Pelargonium graveolens. Nat Prod Res. 2020;34:2634–2638. doi: 10.1080/14786419.2018.1547299. [DOI] [PubMed] [Google Scholar]
- 41.Gucwa K, Milewski S, Dymerski T, Szweda P. Investigation of the Antifungal Activity and Mode of Action of Thymus vulgaris, Citrus limonum, Pelargonium graveolens, Cinnamomum cassia, Ocimum basilicum, and Eugenia caryophyllus Essential Oils. Molecules. 2018;23 doi: 10.3390/molecules23051116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Szweda P, Gucwa K, Kurzyk E, Romanowska E, Dzierżanowska-Fangrat K, Zielińska Jurek A, Kuś PM, Milewski S. Essential Oils, Silver Nanoparticles and Propolis as Alternative Agents Against Fluconazole Resistant Candida albicans, Candida glabrata and Candida krusei Clinical Isolates. Indian J Microbiol. 2015;55:175–183. doi: 10.1007/s12088-014-0508-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Essid R, Hammami M, Gharbi D, Karkouch I, Hamouda TB, Elkahoui S, Limam F, Tabbene O. Antifungal mechanism of the combination of Cinnamomum verum and Pelargonium graveolens essential oils with fluconazole against pathogenic Candida strains. Appl Microbiol Biotechnol. 2017;101:6993–7006. doi: 10.1007/s00253-017-8442-y. [DOI] [PubMed] [Google Scholar]
- 44.Giongo JL, de Almeida Vaucher R, Fausto VP, Quatrin PM, Lopes LQS, Santos RCV, Gündel A, Gomes P, Steppe M. Anti-Candida activity assessment of Pelargonium graveolens oil free and nanoemulsion in biofilm formation in hospital medical supplies. Microb Pathog. 2016;100:170–178. doi: 10.1016/j.micpath.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 45.Ben Hsouna A, Hamdi N. Phytochemical composition and antimicrobial activities of the essential oils and organic extracts from Pelargonium graveolens growing in Tunisia. Lipids Health Dis. 2012;11:167. doi: 10.1186/1476-511X-11-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herman A, Tambor K, Herman A. Linalool Affects the Antimicrobial Efficacy of Essential Oils. Curr Microbiol. 2016;72:165–172. doi: 10.1007/s00284-015-0933-4. [DOI] [PubMed] [Google Scholar]
- 47.Kafa AHT, Aslan R, Celik C, Hasbek M. Antimicrobial synergism and antibiofilm activities of Pelargonium graveolens, Rosemary officinalis, and Mentha piperita essential oils against extreme drug-resistant Acinetobacter baumannii clinical isolates. Z Naturforsch C J Biosci. 2022;77:95–104. doi: 10.1515/znc-2021-0079. [DOI] [PubMed] [Google Scholar]
- 48.Kwiatkowski P, Pruss A, Grygorcewicz B, Wojciuk B, Dołęgowska B, Giedrys-Kalemba S, Kochan E, Sienkiewicz M. Preliminary Study on the Antibacterial Activity of Essential Oils Alone and in Combination with Gentamicin Against Extended-Spectrum β-Lactamase-Producing and New Delhi Metallo-β-Lactamase-1-Producing Klebsiella pneumoniae Isolates. Microb Drug Resist. 2018;24:1368–1375. doi: 10.1089/mdr.2018.0051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available upon reasonable request from the corresponding author at d.d.s.amjad@gmail.com.