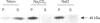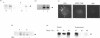Abstract
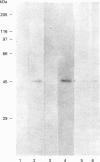
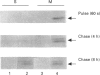
During its asexual life cycle, the human malaria parasite Plasmodium falciparum exports numerous proteins beyond its surface to its host erythrocyte. We have studied the biosynthesis, processing and export of a 45 kDa parasite protein resident in membrane clefts in the erythrocyte cytoplasm. Our results indicate that this cleft protein is made as a single tightly membrane-bound 45 kDa polypeptide in ring- and trophozoite-infected erythrocytes (0-36 h in the life cycle). Using ring/trophozoite parasites released from erythrocytes, the 45 kDa protein is shown to be efficiently transported to the cell surface. This export is specifically blocked by the drug brefeldin A, and at 15 and 20 degrees C. These results indicate that transport blocks seen in the Golgi of mammalian cells are conserved in P. falciparum. Further, the newly synthesized 45 kDa protein passes through parasite Golgi compartments before its export to clefts in the erythrocyte. In mid-to-late-ring-infected erythrocytes, a fraction of the newly synthesized 45 kDa protein is processed to a second membrane-bound phosphorylated 47 kDa protein. The t1/2 of this processing step is about 4 h, suggesting that it occurs subsequent to protein export from the parasite. Evidence is presented that, in later trophozoite stages (24-36 h), the exported 45 and 47 kDa proteins are partially converted into soluble molecules in the intraerythrocytic space. Taken together, the results indicate that the lower eukaryote P. falciparum modulates a classical secretory pathway to support membrane export beyond its plasma membrane to clefts in the erythrocyte. Subsequent to export, phosphorylation and/or conversion into a soluble form may regulate the interactions of the 45 kDa protein with the clefts during parasite development.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnwell J. W. Vesicle-mediated transport of membrane and proteins in malaria-infected erythrocytes. Blood Cells. 1990;16(2-3):379–395. [PubMed] [Google Scholar]
- Boyle W. J., van der Geer P., Hunter T. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 1991;201:110–149. doi: 10.1016/0076-6879(91)01013-r. [DOI] [PubMed] [Google Scholar]
- Chasis J. A., Prenant M., Leung A., Mohandas N. Membrane assembly and remodeling during reticulocyte maturation. Blood. 1989 Aug 15;74(3):1112–1120. [PubMed] [Google Scholar]
- Coppel R. L., Lustigman S., Murray L., Anders R. F. MESA is a Plasmodium falciparum phosphoprotein associated with the erythrocyte membrane skeleton. Mol Biochem Parasitol. 1988 Dec;31(3):223–231. doi: 10.1016/0166-6851(88)90152-1. [DOI] [PubMed] [Google Scholar]
- Crary J. L., Haldar K. Brefeldin A inhibits protein secretion and parasite maturation in the ring stage of Plasmodium falciparum. Mol Biochem Parasitol. 1992 Jul;53(1-2):185–192. doi: 10.1016/0166-6851(92)90020-k. [DOI] [PubMed] [Google Scholar]
- Dieckmann-Schuppert A., Bender S., Odenthal-Schnittler M., Bause E., Schwarz R. T. Apparent lack of N-glycosylation in the asexual intraerythrocytic stage of Plasmodium falciparum. Eur J Biochem. 1992 Apr 15;205(2):815–825. doi: 10.1111/j.1432-1033.1992.tb16846.x. [DOI] [PubMed] [Google Scholar]
- Elford B. C., Ferguson D. J. Secretory processes in Plasmodium. Parasitol Today. 1993 Mar;9(3):80–81. doi: 10.1016/0169-4758(93)90205-t. [DOI] [PubMed] [Google Scholar]
- Elmendorf H. G., Haldar K. Identification and localization of ERD2 in the malaria parasite Plasmodium falciparum: separation from sites of sphingomyelin synthesis and implications for organization of the Golgi. EMBO J. 1993 Dec;12(12):4763–4773. doi: 10.1002/j.1460-2075.1993.tb06165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmendorf H. G., Haldar K. Plasmodium falciparum exports the Golgi marker sphingomyelin synthase into a tubovesicular network in the cytoplasm of mature erythrocytes. J Cell Biol. 1994 Feb;124(4):449–462. doi: 10.1083/jcb.124.4.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmendorf H. G., Haldar K. Secretory transport in Plasmodium. Parasitol Today. 1993 Mar;9(3):98–102. doi: 10.1016/0169-4758(93)90216-3. [DOI] [PubMed] [Google Scholar]
- Etzion Z., Perkins M. E. Localization of a parasite encoded protein to erythrocyte cytoplasmic vesicles of Plasmodium falciparum-infected cells. Eur J Cell Biol. 1989 Apr;48(2):174–179. [PubMed] [Google Scholar]
- Gormley J. A., Howard R. J., Taraschi T. F. Trafficking of malarial proteins to the host cell cytoplasm and erythrocyte surface membrane involves multiple pathways. J Cell Biol. 1992 Dec;119(6):1481–1495. doi: 10.1083/jcb.119.6.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldar K., Ferguson M. A., Cross G. A. Acylation of a Plasmodium falciparum merozoite surface antigen via sn-1,2-diacyl glycerol. J Biol Chem. 1985 Apr 25;260(8):4969–4974. [PubMed] [Google Scholar]
- Haldar K., Holder A. A. Export of parasite proteins to the erythrocyte in Plasmodium falciparum-infected cells. Semin Cell Biol. 1993 Oct;4(5):345–353. doi: 10.1006/scel.1993.1041. [DOI] [PubMed] [Google Scholar]
- Haldar K. Lipid transport in Plasmodium. Infect Agents Dis. 1992 Oct;1(5):254–262. [PubMed] [Google Scholar]
- Haldar K., Uyetake L., Ghori N., Elmendorf H. G., Li W. L. The accumulation and metabolism of a fluorescent ceramide derivative in Plasmodium falciparum-infected erythrocytes. Mol Biochem Parasitol. 1991 Nov;49(1):143–156. doi: 10.1016/0166-6851(91)90137-u. [DOI] [PubMed] [Google Scholar]
- Howard R. J., Lyon J. A., Uni S., Saul A. J., Aley S. B., Klotz F., Panton L. J., Sherwood J. A., Marsh K., Aikawa M. Transport of an Mr approximately 300,000 Plasmodium falciparum protein (Pf EMP 2) from the intraerythrocytic asexual parasite to the cytoplasmic face of the host cell membrane. J Cell Biol. 1987 May;104(5):1269–1280. doi: 10.1083/jcb.104.5.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard R. J., Uni S., Aikawa M., Aley S. B., Leech J. H., Lew A. M., Wellems T. E., Rener J., Taylor D. W. Secretion of a malarial histidine-rich protein (Pf HRP II) from Plasmodium falciparum-infected erythrocytes. J Cell Biol. 1986 Oct;103(4):1269–1277. doi: 10.1083/jcb.103.4.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui G. S., Siddiqui W. A. Characterization of a Plasmodium falciparum polypeptide associated with membrane vesicles in the infected erythrocytes. Mol Biochem Parasitol. 1988 Jun;29(2-3):283–293. doi: 10.1016/0166-6851(88)90083-7. [DOI] [PubMed] [Google Scholar]
- Kara U. A., Stenzel D. J., Ingram L. T., Kidson C. The parasitophorous vacuole membrane of Plasmodium falciparum: demonstration of vesicle formation using an immunoprobe. Eur J Cell Biol. 1988 Apr;46(1):9–17. [PubMed] [Google Scholar]
- Kuchler K., Sterne R. E., Thorner J. Saccharomyces cerevisiae STE6 gene product: a novel pathway for protein export in eukaryotic cells. EMBO J. 1989 Dec 20;8(13):3973–3984. doi: 10.1002/j.1460-2075.1989.tb08580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li W. L., Das A., Song J. Y., Crary J. L., Haldar K. Stage-specific expression of plasmodial proteins containing an antigenic marker of the intraerythrocytic cisternae. Mol Biochem Parasitol. 1991 Nov;49(1):157–168. doi: 10.1016/0166-6851(91)90138-v. [DOI] [PubMed] [Google Scholar]
- Lingelbach K. R. Plasmodium falciparum: a molecular view of protein transport from the parasite into the host erythrocyte. Exp Parasitol. 1993 May;76(3):318–327. doi: 10.1006/expr.1993.1039. [DOI] [PubMed] [Google Scholar]
- Perkins M. Stage-dependent processing and localization of a Plasmodium falciparum protein of 130,000 molecular weight. Exp Parasitol. 1988 Feb;65(1):61–68. doi: 10.1016/0014-4894(88)90107-5. [DOI] [PubMed] [Google Scholar]
- Rothman J. E., Orci L. Molecular dissection of the secretory pathway. Nature. 1992 Jan 30;355(6359):409–415. doi: 10.1038/355409a0. [DOI] [PubMed] [Google Scholar]
- Rubartelli A., Cozzolino F., Talio M., Sitia R. A novel secretory pathway for interleukin-1 beta, a protein lacking a signal sequence. EMBO J. 1990 May;9(5):1503–1510. doi: 10.1002/j.1460-2075.1990.tb08268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz R. T., Riveros-Moreno V., Lockyer M. J., Nicholls S. C., Davey L. S., Hillman Y., Sandhu J. S., Freeman R. R., Holder A. A. Structural diversity of the major surface antigen of Plasmodium falciparum merozoites. Mol Cell Biol. 1986 Mar;6(3):964–968. doi: 10.1128/mcb.6.3.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D., Woollett G., Bergin-Cartwright M., Kay D., Scaife J. A malaria protein exported into a new compartment within the host erythrocyte. EMBO J. 1987 Feb;6(2):485–491. doi: 10.1002/j.1460-2075.1987.tb04779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley H. A., Langreth S. G., Reese R. T. Plasmodium falciparum antigens associated with membrane structures in the host erythrocyte cytoplasm. Mol Biochem Parasitol. 1989 Sep;36(2):139–149. doi: 10.1016/0166-6851(89)90186-2. [DOI] [PubMed] [Google Scholar]
- Taylor D. W., Parra M., Chapman G. B., Stearns M. E., Rener J., Aikawa M., Uni S., Aley S. B., Panton L. J., Howard R. J. Localization of Plasmodium falciparum histidine-rich protein 1 in the erythrocyte skeleton under knobs. Mol Biochem Parasitol. 1987 Sep;25(2):165–174. doi: 10.1016/0166-6851(87)90005-3. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Vernot-Hernandez J. P., Heidrich H. G. Time-course of synthesis, transport and incorporation of a protein identified in purified membranes of host erythrocytes infected with a knob-forming strain of Plasmodium falciparum. Mol Biochem Parasitol. 1984 Jul;12(3):337–350. doi: 10.1016/0166-6851(84)90090-2. [DOI] [PubMed] [Google Scholar]
- Vial H. J., Ancelin M. L., Philippot J. R., Thuet M. J. Biosynthesis and dynamics of lipids in Plasmodium-infected mature mammalian erythrocytes. Blood Cells. 1990;16(2-3):531–561. [PubMed] [Google Scholar]
- Wiser M. F., Lanners H. N. Rapid transport of the acidic phosphoproteins of Plasmodium berghei and P. chabaudi from the intraerythrocytic parasite to the host membrane using a miniaturized fractionation procedure. Parasitol Res. 1992;78(3):193–200. doi: 10.1007/BF00931726. [DOI] [PubMed] [Google Scholar]