Summary
Background
Synovitis has long been considered a common and modifiable inflammatory feature of osteoarthritis (OA), but current disease-modifying anti-inflammatory treatments appear ineffective in OA clinical trials. Elucidating the temporal relationship between synovitis and OA could provide insight into the role of synovitis in OA.
Methods
We conducted a prospective cohort study based on the baseline and three-year follow-up data from the Xiangya Osteoarthritis (XO) Study. We assessed bidirectional associations between ultrasound-detected synovitis and radiographic and symptomatic OA at knee and hand sites using generalized estimating equations. Additionally, we performed bidirectional Mendelian randomization (MR) analyses to test these hypotheses utilising whole-genome sequencing data in the XO population. Age, sex, body mass index, smoking, alcohol consumption, educational level, physical activity, and joint injury history were adjusted for these analyses.
Findings
A total of 2211, 2420, 2280, and 2600 participants were enrolled for analyses of radiographic knee OA (RKOA), symptomatic knee OA (SKOA), radiographic hand OA (RHOA) and symptomatic hand OA (SHOA), respectively. The baseline synovitis (i.e., with synovitis vs. without synovitis) was associated with the incident RKOA (76/277 vs. 557/3674 knees), SKOA (49/387 vs. 287/4213 knees), RHOA (171/358 vs. 686/3664 hands) and SHOA (35/689 vs. 76/4327 hands), with adjusted odds ratio (aORs) of 2.2 (95% CI 1.7–3.1), 2.0 (1.3–2.9), 3.4 (2.7–4.4), and 2.4 (1.5–3.8), respectively. The baseline RKOA (with OA vs. without OA: 409/1246 vs. 481/3758 knees), SKOA (200/576 vs. 675/4356 knees), RHOA (192/778 vs. 410/3723 hands), and SHOA (41/162 vs. 548/4285 hands) were also associated with the incident synovitis, with aORs of 3.4 (95% CI 2.9–4.1), 2.7 (2.1–3.4), 2.3 (1.8–2.9) and 1.9 (1.2–2.8), respectively. These bidirectional associations were stronger when more active synovitis was compared with the reference group (all P < 0.05). MR analyses further supported bidirectional associations that synovitis significantly increased the odds of incident OA at both sites and vice versa (all ORs ranged from 1.2–1.7).
Interpretation
Our population-based cohort study found novel evidence of a bidirectional association between synovitis and OA, which was further validated through MR analysis and suggested that the bidirectional association is likely causal. Our findings indicated that synovitis is both a risk factor and a consequence of the OA rather than solely a risk factor.
Funding
The National Key Research and Development Plan, the National Natural Science Foundation of China, the Key Research and Development Program of Hunan Province, the Natural Science Foundation of Hunan Province, the Central South University Innovation-Driven Research Programme, and the Fundamental Research Funds for the Central Universities of Central South University.
Keywords: Osteoarthritis, Synovitis, Incidence, General population
Research in context.
Evidence before this study
Synovitis has long been considered a common and modifiable inflammatory feature of osteoarthritis (OA), but current disease-modifying anti-inflammatory treatments appear ineffective in OA clinical trials. Determining the temporal relationship between synovitis and OA may help clarify the role of synovitis in OA. We searched PubMed for studies using terms including (“synovitis” or “synovial hypertrophy” OR “effusion” OR “Power Doppler signal”) AND (“osteoarthritis”) for articles relating to the association between synovitis and OA. We searched the databases from inception to May 9, 2024. However, to date, there is a paucity of evidence about the bidirectional association between synovitis and OA at any joint site.
Added value of this study
To our knowledge, this study is the first to assess the bidirectional association between synovitis and OA. Based on data from a general population-based cohort study, we found a significant temporal association of synovitis, especially in more active synovitis, with knee and hand OA, and vice versa. Mendelian randomization analyses further supported the bidirectional associations between synovitis and OA.
Implications of all the available evidence
Our novel findings that synovitis may occur before and after the occurrence of OA imply that synovitis is either a risk factor of the OA or a sequel of the disease. This bidirectional association may supplement the prevailing view that synovitis is merely a ‘risk factor’ for OA and partially explain the lack of efficacy of disease-modifying anti-inflammatory treatments in OA.
Introduction
Osteoarthritis (OA) is the most common joint disease worldwide and affects more than 595 million people (approximately 7.6% of the world's population), with a particularly high prevalence among individuals over 65 years.1,2 OA is a leading cause of chronic pain and disability. The risk of OA continues to increase, with a growing tendency to affect younger individuals.3 This condition significantly impacts both individuals and society, leading to reduced quality of life, the necessity for arthroplasty, and substantial economic burdens.2,3 Despite its formidable impact, there are currently no established disease-modifying OA drug (DMOAD) treatments capable of improving both structural and patient-centred outcomes.
Synovitis has long been recognized as an inflammatory feature common in OA and considered modifiable. It has been associated with clinical symptoms and involvement in tissue degradation in OA.4 There is an expectation that targeting synovitis alleviates symptoms and prevents the structural progression of the disease.5 However, disease-modifying anti-inflammatory treatments (e.g., methotrexate, hydroxychloroquine, etanercept, and adalimumab) have not provided clinically significant pain relief or subsequent structural remodeling above placebo in OA randomised controlled trial (RCTs).5,6 To date, it remains unclear whether OA increases the risk of incident synovitis. Therefore, it is crucial to reevaluate the nature of synovitis in OA and reconsider its suitability as a potential treatment target. Elucidating the temporal bidirectional relationship between synovitis and OA (i.e., synovitis increases the risk of OA, and vice versa) may shed light on the natural history of OA and provide insights into the failure of current disease-modifying anti-inflammatory drugs in OA treatment. However, to date, there is a paucity of evidence about the bidirectional association between synovitis and OA at any joint site.
To address this knowledge gap, we conducted prospective cohort studies investigating the associations between baseline synovitis status and incident OA over three years of follow-up, as well as baseline OA status and incident synovitis over the same period. Additionally, we verified this bidirectional association using a Mendelian randomization (MR) approach.
Methods
Study population
Participants were recruited from the Xiangya Osteoarthritis (XO) Study, a population-based prospective cohort study of the natural history and associated risk factors for the development of OA.7,8 All individuals aged 50 years or older were randomly selected from rural mountainous villages in Longshan County, Hunan Province, China (NCT04033757). Specifically, to select the initial 14 communities, we first adopted a sampling method of probability proportionate to population size. All the villages in the selected communities were then listed randomly. Village-to-village recruitment began in the first village in the first community until the number of participants in that community met the predetermined quota. Eventually, 25 rural mountainous villages in Longshan County were included in the XO Study. Among 4742 randomly selected individuals, 4080 (86.04%) consented to participate at baseline. This study consists of three sub-cohorts (i.e., sub-cohorts I, II, and III), initiated in 2015 (n = 1469), 2018 (n = 1271) and 2019 (n = 1340), respectively. The diagram of examination cycles shows the baseline and follow-ups for each sub-cohort of the XO Study (Appendix p 12). Included in the current study are participants who underwent baseline examinations (i.e., radiographs, ultrasound, and symptoms) and completed the three-year follow-up assessment.
The Ethics Committee of Xiangya Hospital, Central South University (201510506) reviewed and approved the XO Study, and written informed consent was obtained from all participants.
Assessment of knee and hand synovitis
Knee and hand ultrasound examinations were performed in the XO Study starting from 2017 (the second-year follow-up of sub-cohort I), 2018 (the baseline of sub-cohort II), and 2019 (the baseline of sub-cohort III), which constitute the baseline of our current study (Appendix p 12). An experienced ultrasonographer (TJ; over ten years of experience in musculoskeletal ultrasonography) conducted ultrasound examinations according to the protocol recommended by the Outcome Measures in Rheumatology (OMERACT) group.9 Details of the ultrasound examination are provided in the Appendix (pp 3–4). Bilateral knees and hands were evaluated using ultrasound.
For knee synovitis, the maximal synovial thickness was measured in millimeters along the longitudinal axis. The presence of synovitis was defined as synovial thickness ≥4 mm, following criteria of the European Alliance of Associations for Rheumatology (EULAR) study.10 Hand synovitis was estimated by gray-scale synovitis, which combines synovial hypertrophy and effusion. It was assessed using a validated semiquantitative grading scale ranging from 0 to 3.9 Hand synovitis was defined as the presence of gray-scale synovitis (score ≥2) in at least one joint of each hand.8 Power Doppler signal (PDS) indicated the presence of flow signals within synovial areas, reflecting the inflammatory activity of synovitis. We divided the activity of synovitis into three categories for both the knee and hand joints: control (no synovitis), synovitis without PDS, and synovitis with PDS. Incident knee or hand synovitis was defined as meeting the above definitions at the three-year follow-up but not at baseline.
The intra- and inter-rater reliability were moderate to excellent for ultrasound-detected knee synovitis (intra-class correlation coefficients [ICC] of 0.99, 95% CI 0.98–1.00; ICC of 0.94, 95% CI 0.87–0.97), PDS within knee synovitis (Kappa of 1.00, 95% CI 1.00–1.00; Kappa of 0.82, 95% CI 0.66–0.97), and substantial for hand grey-scale synovitis (Kappa of 0.67, 95% CI 0.63–0.72; Kappa of 0.61, 95% CI 0.55–0.67).8,11 The intra- and inter-rater reliability for PDS within hand synovitis was not evaluated because only one subject had PDS in the reliability sample. Details of the reliability of ultrasound assessment are presented in the Appendix (pp 3–4).
Assessment of knee OA or hand OA
Participants underwent bilateral knee (weight-bearing semi-flexed postero-anterior tibiofemoral and supine skyline patellofemoral radiographs) and hand (posteroanterior view) radiographs at baseline and follow-up visits. Using a modified Kellgren/Lawrence (KL) scoring atlas (scale ranging from 0 to 4) for radiographic knee OA (RKOA) and radiographic hand OA (RHOA),12 one reader (TY; a radiologist with over ten years of experience) evaluated and graded the radiographs of bilateral knee (i.e., tibiofemoral and patellofemoral joints) and hand joints (i.e., carpometacarpal 1, metacarpophalangeal 1–5, proximal interphalangeal 1–5 and distal interphalangeal 2–5 joints). The definition of RKOA was the presence of a KL grade of ≥2 in either the tibiofemoral or patellofemoral joint, both of which are key components of the knee joint and can develop OA.12 RHOA was defined as the presence of a KL grade of ≥2 in any hand joint.12 The intra- and inter-rater reliability were moderate to excellent for the identification of knee KL grade (Kappa of 0.91, 95% CI 0.88–0.95; Kappa of 0.76, 95% CI 0.62–0.91) and for the diagnosis of RHOA (Kappa of 0.91, 95% CI 0.83–0.99; Kappa of 0.71, 95% CI 0.45–0.96).13,14 Details of the reliability of radiograph assessment are presented in the Appendix (p 5).
The presence of knee symptoms was determined by participants responding ‘yes’ to the question, “On most days, do you have pain, aching, or stiffness in your knee?” for each knee at both baseline and follow-up visits.15 Symptomatic knee OA (SKOA) was defined as having RKOA plus self-reported symptoms in the same knee.15 Similarly, hand symptoms were ascertained by noting the participant's response to the question, “On most days, do you have pain, aching, or stiffness in your left/right hand?” If the participants answered ‘yes’ to the question, they were shown a homunculus and asked to indicate which joint(s) were symptomatic. Symptomatic hand OA (SHOA) was defined as having both RHOA and self-reported symptoms in the same joint.16 Incident radiographic or symptomatic OA of the knee and hand joints was defined as meeting the above definitions at the three-year follow-up but not at baseline.
Whole-genome sequencing (WGS)
A total of 2980 samples that passed quality control (including concentration, sample integrity, and purity) were included in the subsequent whole-genome sequencing. DNA was purified from blood samples obtained during the initial assessment. The DNA samples were sequenced to an average depth of 38.4 × (with a minimum depth of 21.8 × ) and an average coverage of 99.2% (with a minimum coverage of 98.7%). This resulted in identifying 54 916 001 high-quality single-nucleotide polymorphisms (SNPs) and insertion-deletion markers. Details of the WGS procedures are provided in the Appendix (pp 6–8).
Covariates assessment
Demographic and lifestyle information, including age, sex, smoking habits, alcohol consumption, education level and joint injury history, was collected through face-to-face interviews by trained professionals. A history of knee or hand injury was considered present if it resulted in limitation of knee or hand function for at least one week. Participant's height and weight were measured by trained personnel, and their body mass index (BMI) was calculated as weight (kg) divided by the square of height (m2).
Statistical analysis
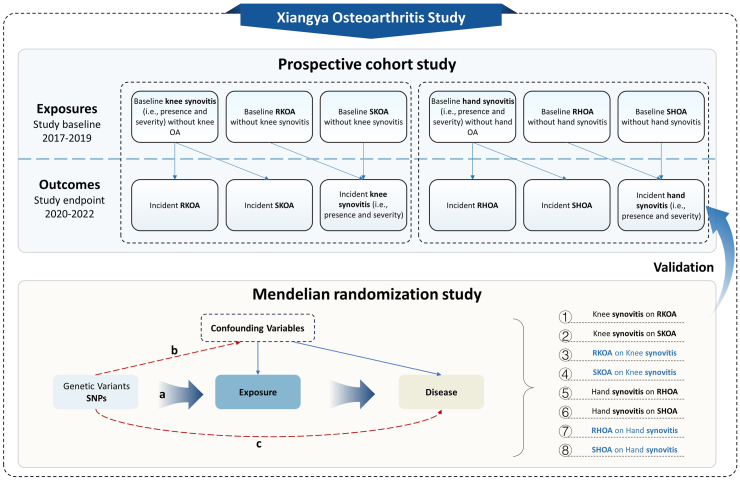
The overview of study design is depicted in Fig. 1. Categorical variables were presented as percentages, and continuous variables were expressed as means with standard deviation (SD). We conducted logistic regressions to examine the associations of age (50–59 years, 60–69 years, ≥70 years) and sex with radiographic and symptomatic OA. Then, we examined the relations of the presence of synovitis at baseline (compared with the group without synovitis) to the incident radiographic and symptomatic OA of the knee (or hand) joints over three years of follow-up, respectively. Both crude odds ratio (OR) and multivariable-adjusted OR (aOR) with their 95% confidence interval (CI) were obtained using generalized estimating equations (GEE). The GEE method was used to account for the correlation between two knees (or hands) within the same individuals as they are not independent observational units. In the GEE models, we used a logit link and an exchangeable working correlation matrix structure. We used the same approach to examine the relation of the presence of the knee (or hand) OA at baseline (compared with the group without OA) to the risk of incident synovitis over three years of follow-up. In these analyses, we excluded individuals with baseline synovitis or OA. The variables in the multivariable-adjusted model included age (continuous variables), sex (male, female), BMI (continuous variables), smoking status (non-smokers, ex-smokers, current smokers), alcohol consumption (non-drinkers, ex-drinkers, current drinkers), educational level (educated or non-educated), physical activity (low or moderate, high) and knee or hand injury history (yes or no).
Fig. 1.
Overview of study design. The genetic variants (a) are associated with the exposure, (b) are independent of the confounders, and (c) influence the outcome only through the exposure. RKOA, radiographic knee osteoarthritis; SKOA, symptomatic knee osteoarthritis; RHOA, radiographic hand osteoarthritis; SHOA, symptomatic hand osteoarthritis.
We further investigated whether OA has a stronger bidirectional relationship (greater OR value) with synovitis exhibiting positive inflammatory activity, an indicator of severity. Participants were categorized into three groups based on different inflammatory activity status, control (i.e., without synovitis), synovitis without PDS, and synovitis with PDS. We then examined the bidirectional associations of the inflammatory activity of synovitis with the incidence of RKOA, SKOA, RHOA, and SHOA using separate GEE models. However, the analysis of the association between the inflammatory activity of hand synovitis at baseline and the incidence of hand OA could only be performed on RHOA, as the sample size for incident SHOA was limited in the PDS group. In addition, we did not evaluate the bidirectional associations between the presence of synovitis and the severity of OA, as few participants progressed from no OA to severe OA during the three-year follow-up. All statistical analyses were conducted using SAS V.9.4 (SAS Institute, Cary, NC, USA). A P value < 0.05 (two-sided) was considered statistically significant.
MR analysis
We obtained the WGS data from the XO Study and conducted several genome-wide association studies (GWASs) to obtain instrumental variables and GWAS summary statistics for subsequent MR analyses. Qualified individuals were included in the GWAS analysis, and the SAIGE method was implemented to efficiently control for potential case–control imbalance and sample relatedness while simultaneously adjusting for covariates.17 Separate one-sample MR analyses were then performed to investigate the potential bidirectional causal association between synovitis and OA. The core assumptions for the MR analysis were checked (details for GWAS, MR analysis, and validation of the core assumptions are provided in the Appendix, pp 6–10). The MR estimates were presented as the ORs with their 95% CIs and were interpreted as OA risk per unit increase in the log odds of synovitis, and vice versa.18
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Characteristics of the study population
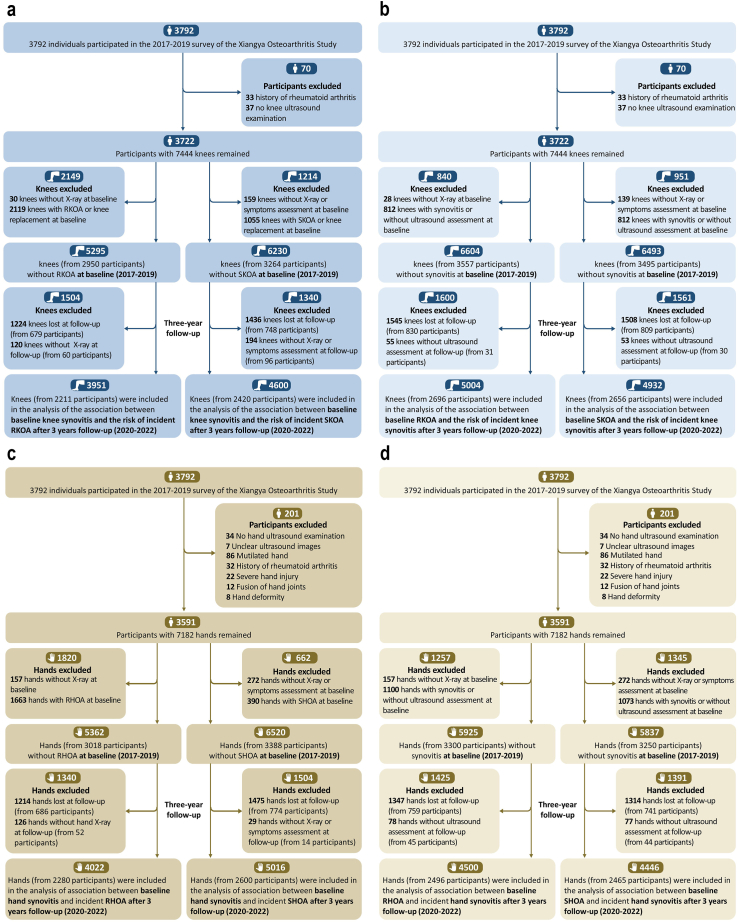
A flow chart depicting the participant selection process is shown in Fig. 2. A total of 2211 participants (contributing 3951 knees) and 2420 participants (contributing 4600 knees) from three sub-cohorts were included in analyses of the associations between baseline knee synovitis and the risk of incident RKOA or SKOA, respectively (Table 1). Among the participants eligible for incident RKOA analysis, the mean age was 62.7 years (SD = 8.4), the mean BMI was 24.0 kg/m2 (SD = 3.4), and 53.8% were female. Among the participants eligible for incident SKOA analysis, the mean age was 63.3 years (SD = 8.5), the mean BMI was 24.0 kg/m2 (SD = 3.4), and 55.2% were female (Table 1). A total of 1581 participants were excluded from the RKOA analysis and 1372 from the SKOA analysis. However, no statistically significant differences in characteristics were found between the included and excluded individuals, except for age (Appendix pp 14–15, P > 0.05).
Fig. 2.
Flow chart of participants in the study according to onset of RKOA and SKOA (A), knee synovitis (B), RHOA and SHOA (C), and hand synovitis (D). RKOA, radiographic knee osteoarthritis; SKOA, symptomatic knee osteoarthritis; RHOA, radiographic hand osteoarthritis; SHOA, symptomatic hand osteoarthritis.
Table 1.
Baseline characteristics of participants in the analysis of the association between baseline synovitis and the risk of incident OA at both knee and hand sites over three years of follow-up.
| Participants without RKOAa | Participants without SKOAb | Participants without RHOAc | Participants without SHOAd | |
|---|---|---|---|---|
| Participants (knees or hands), n | 2211 (3951 knees) | 2420 (4600 knees) | 2280 (4022 hands) | 2600 (5016 hands) |
| Women, n (%) | 1189 (53.8%) | 1336 (55.2%) | 1325 (58.1%) | 1496 (57.5%) |
| Age, years (mean ± SD) | 62.7 ± 8.4 | 63.3 ± 8.5 | 62.8 ± 8.3 | 63.7 ± 8.6 |
| BMI, kg/m2 (mean ± SD) | 24.0 ± 3.4 | 24.0 ± 3.4 | 24.1 ± 3.5 | 24.1 ± 3.5 |
| Smoking status (%) | ||||
| Non-smoker | 62.0 | 63.5 | 65.3 | 65.4 |
| Ex-smoker | 4.6 | 4.5 | 4.2 | 4.4 |
| Current smoker | 33.4 | 32.0 | 30.5 | 30.2 |
| Alcohol drinking (%) | ||||
| Non-drinker | 52.1 | 52.3 | 53.3 | 53.7 |
| Ex-drinker | 10.3 | 10.6 | 9.8 | 10.8 |
| Current drinker | 37.6 | 37.1 | 36.9 | 35.5 |
| Joint injury history (%)e | 3.0 | 2.9 | 4.3 | 4.6 |
| Education (educated, %)f | 72.8 | 71.0 | 69.9 | 68.8 |
| Physical activity level (high, %) | 85.2 | 85.6 | 85.6 | 85.0 |
| Synovitis (%) | 7.0 | 8.4 | 8.9 | 13.7 |
n, number; SD, standard deviation; BMI, body mass index; OA, osteoarthritis; RKOA, radiographic knee OA; SKOA, symptomatic knee OA; RHOA, radiographic hand OA; SHOA, symptomatic hand OA.
Participants who underwent baseline knee joint ultrasound assessment without pre-existing RKOA at the joint level.
Participants who underwent baseline knee joint ultrasound assessment without pre-existing SKOA at the joint level.
Participants who underwent baseline hand joint ultrasound assessment without pre-existing RHOA at the joint level.
Participants who underwent baseline hand joint ultrasound assessment without pre-existing SHOA at the joint level.
Joint injury history was defined as a history of joint injury severely restricting function for at least one week.
Educated was defined as primary school or above.
Among 3591 individuals (7182 hands) enrolled at the baseline visit, 2280 participants (contributing 4022 hands) were included in analyses of the association between baseline hand synovitis and incident RHOA, and 2600 participants (contributing 5016 hands) were included in analyses of incident SHOA. Among the participants eligible for incident RHOA analysis, the mean age was 62.8 years (SD = 8.3), the mean BMI was 24.1 kg/m2 (SD = 3.5), and 58.1% were female. Among the participants eligible for incident SHOA, the mean age was 63.7 (SD = 8.6) years, the mean BMI was 24.1 (SD = 3.5) kg/m2, and 57.5% were female (Table 1). A total of 1512 participants were excluded from the RHOA analysis and 1192 participants from the SHOA analysis. However, no statistically significant differences in characteristics were found between the included and excluded individuals, except for age (Appendix pp 14–15, P > 0.05).
Over the three-year follow-up, incident RKOA occurred in 19.8% of individuals, SKOA in 9.6%, RHOA in 27.5%, and SHOA in 3.1%, respectively. The risk of RKOA, SKOA, and RHOA increased with age (all P for trend <0.01), while the odds of SHOA peaked in the 60–69 age group and then decreased after age 70. Compared with men, women had a higher risk of RKOA, SKOA, and SHOA (all P < 0.01), but not RHOA (P = 0.25) (Appendix p 13).
Associations of baseline synovitis with odds of incident OA at both knee and hand sites
As shown in Table 2, the odds of RKOA or SKOA in knees with baseline synovitis was significantly higher than those without synovitis, with aOR of 2.2 (95% CI: 1.7–3.1) and 2.0 (95% CI: 1.3–2.9), respectively. There was a strong dose–response association between the inflammatory activity of knee synovitis (i.e., no synovitis, synovitis without PDS, and synovitis with PDS) and incident RHOA. Compared with those without baseline synovitis, the risk of RKOA was much higher among knees with synovitis and PDS (aOR = 3.6) than knees with synovitis but without PDS (aOR = 2.1) (P for trend <0.01), and similar findings were observed for SKOA.
Table 2.
Associations between baseline synovitis and its activity (status of PDS) and incident OA at both knee and hand sites over three years of follow-up.
| Baseline synovitis |
||||
|---|---|---|---|---|
| None baseline synovitis | Baseline synovitis | Synovitis without PDS | Synovitis with PDS | |
| Incident RKOA | ||||
| No, number (%) | 3117 (84.8) | 201 (72.6) | 180 (74.1) | 21 (61.8) |
| Yes, number (%) | 557 (15.2) | 76 (27.4) | 63 (25.9) | 13 (38.2) |
| Crude OR (95% CI) | 1.00 (reference) | 2.1 (1.6, 2.8) | 2.0 (1.4, 2.7) | 3.5 (1.7, 7.0) |
| Adjusted OR (95% CI)a | 1.00 (reference) | 2.2 (1.7, 3.1) | 2.1 (1.5, 2.9) | 3.6 (1.7, 7.3) |
| Incident SKOA | ||||
| No, number (%) | 3926 (93.2) | 338 (87.3) | 295 (88.1) | 43 (82.7) |
| Yes, number (%) | 287 (6.8) | 49 (12.7) | 40 (11.9) | 9 (17.3) |
| Crude OR (95% CI) | 1.00 (reference) | 2.0 (1.4, 2.8) | 1.8 (1.3, 2.7) | 2.9 (1.4, 6.0) |
| Adjusted OR (95% CI)a | 1.00 (reference) | 2.0 (1.3, 2.9) | 1.8 (1.2, 2.8) | 2.8 (1.3, 6.3) |
| Incident RHOA | ||||
| No, number (%) | 2978 (81.3) | 187 (52.2) | 184 (53.6) | 3 (20.0) |
| Yes, number (%) | 686 (18.7) | 171 (47.8) | 159 (46.4) | 12 (80.0) |
| Crude OR (95% CI) | 1.00 (reference) | 4.0 (3.2, 5.0) | 3.8 (3.0, 4.8) | 17.4 (5.2, 57.9) |
| Adjusted OR (95% CI)a | 1.00 (reference) | 3.4 (2.7, 4.4) | 3.3 (2.6, 4.3) | 8.1 (1.9, 35.5) |
| Incident SHOAb | ||||
| No, number (%) | 4251 (98.2) | 654 (94.9) | – | – |
| Yes, number (%) | 76 (1.8) | 35 (5.1) | – | – |
| Crude OR (95% CI) | 1.00 (reference) | 3.0 (1.9, 4.7) | – | – |
| Adjusted OR (95% CI)a | 1.00 (reference) | 2.4 (1.5, 3.8) | – | – |
CI, confidence interval; OA, osteoarthritis; RKOA, radiographic knee OA; SKOA, symptomatic knee OA; RHOA, radiographic hand OA; SHOA, symptomatic hand OA; OR, odds ratio; PDS, Power Doppler signal.
Adjusted for age (continuous variables), sex (male, female), BMI (continuous variables), smoking status (non-smokers, ex-smokers, current smokers), alcohol consumption (non-drinkers, ex-drinkers, current drinkers), educational level (educated or non-educated), physical activity (low or moderate, high) and knee or hand injury history (yes or no).
The analysis of the association between the inflammatory activity of hand synovitis at baseline and the incidence of hand OA could only be performed on RHOA since the sample size of incident SHOA was limited in the PDS group (n = 1).
Baseline hand synovitis was also associated with an increased odds of hand OA. The aORs of RHOA and SHOA for synovitis were 3.4 (95% CI: 2.7–4.4) and 2.4 (95% CI: 1.5–3.8), respectively. A strong dose–response association was also observed between the inflammatory activity of synovitis and incident RHOA (P for trend <0.01).
Associations of baseline OA with odds of incident synovitis at both knee and hand sites
In the study investigating the associations of baseline RKOA and SKOA with incident knee synovitis, 2696 individuals (contributing 5004 knees) and 2656 individuals (contributing 4932 knees) were included, with 1096 and 1136 participants excluded, respectively (Appendix p 16). No statistically significant differences in baseline characteristics were found between the included and excluded individuals except for age (Appendix pp 14–15, P > 0.05). Over the three years of follow-up, incident synovitis occurred in 32.8% of knees with RKOA, and in 34.7% of knees with SKOA, respectively (Table 3). Baseline RKOA and SKOA were significantly associated with increased odds of incident knee synovitis. Compared with no-RKOA (or no-SKOA), the aOR of incident synovitis was 3.4 (95% CI: 2.9–4.1) for RKOA and 2.7 (95% CI: 2.1–3.4) for SKOA, respectively. Baseline RKOA and SKOA were also significantly associated with incident inflammatory active synovitis (Table 3).
Table 3.
Associations of baseline OA with the risk of incident synovitis, as well as activity of synovitis (status of PDS), at both knee and hand sites over three years of follow-up.
| Baseline RKOA | Baseline SKOA | Baseline RHOA | Baseline SHOA | |
|---|---|---|---|---|
| Incident synovitis | ||||
| Incident number (%) | 409 (32.8) | 200 (34.7) | 192 (24.7) | 41 (25.3) |
| Crude OR (95% CI) | 3.3 (2.8, 3.9) | 2.9 (2.4, 3.6) | 2.7 (2.2, 3.2) | 2.3 (1.6, 3.4) |
| Adjusted OR (95% CI)a | 3.4 (2.9, 4.1) | 2.7 (2.1, 3.4) | 2.3 (1.8, 2.9) | 1.9 (1.2, 2.8) |
| Incident of synovitis without PDS | ||||
| Incident number (%) | 301 (24.1) | 144 (25.0) | 171 (22.0) | 36 (22.2) |
| Crude OR (95% CI) | 2.8 (2.3, 3.4) | 2.5 (2.0, 3.1) | 2.6 (2.1, 3.1) | 2.2 (1.5, 3.3) |
| Adjusted OR (95% CI)a | 2.9 (2.4, 3.5) | 2.4 (1.8, 3.0) | 2.2 (1.7, 2.8) | 1.8 (1.2, 2.7) |
| Incident of synovitis with PDS | ||||
| Incident number (%) | 108 (8.7) | 56 (9.7) | 21 (2.7) | 5 (3.1) |
| Crude OR (95% CI) | 6.9 (5.0, 9.6) | 5.0 (3.5, 7.1) | 4.0 (2.3, 6.9) | 3.5 (1.4, 9.1) |
| Adjusted OR (95% CI)a | 7.1 (4.8, 10.3) | 4.3 (2.8, 6.5) | 4.2 (2.3, 7.9) | 2.9 (1.1, 7.8) |
CI, confidence interval; OA, osteoarthritis; RKOA, radiographic knee OA; SKOA, symptomatic knee OA; RHOA, radiographic hand OA; SHOA, symptomatic hand OA; OR, odds ratio; PDS, Power Doppler signal.
Adjusted for age (continuous variables), sex (male, female), BMI (continuous variables), smoking status (non-smokers, ex-smokers, current smokers), alcohol consumption (non-drinkers, ex-drinkers, current drinkers), educational level (educated or non-educated), physical activity (low or moderate, high) and knee or hand injury history (yes or no).
In the investigation of the associations of baseline RHOA or SHOA with incident hand synovitis, 2496 individuals (contributing 4500 hands) and 2465 individuals (contributing 4446 hands) were included, 1296 and 1327 participants were excluded, respectively (Appendix p 16). There were no statistically significant differences in baseline characteristics between the included and excluded individuals except for age (Appendix pp 14–15, P > 0.05). Over the three years of follow-up, incident synovitis occurred in 24.7% of hands with RHOA, and in 25.3% of hands with SHOA, respectively (Table 3). Baseline RHOA was significantly associated with an increased three-year odds of hand synovitis. The aOR of incident synovitis was 2.3 (95% CI: 1.8–2.9) for RHOA and 1.9 (95% CI: 1.2–2.8) for SHOA, respectively. Baseline RHOA and SHOA were also significantly associated with incident inflammatory active synovitis (Table 3).
MR analysis
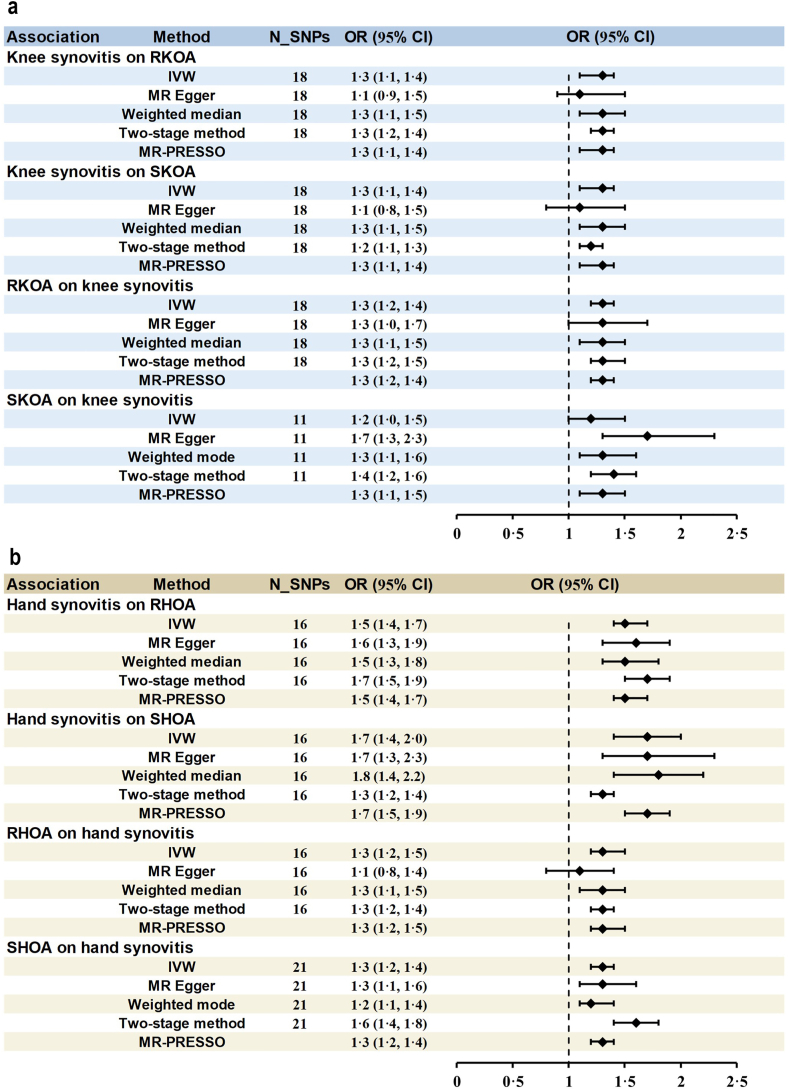
Genetic predisposition to synovitis significantly increased the odds of RKOA, SKOA, RHOA, and SHOA. Per one unit increase of log odds of synovitis, the ORs were 1.3 (95% CI: 1.1–1.4, P = 2.22 × 10−5), 1.3 (95% CI: 1.1–1.4, P = 8.11 × 10−4), 1.5 (95% CI: 1.4–1.7, P = 2.59 × 10−15) and 1.7 (95% CI: 1.4–2.0, P = 6.97 × 10−10) for RKOA, SKOA, RHOA and SHOA, respectively, respectively (Fig. 3, Appendix pp 17–18). Furthermore, the causal effects of OA on synovitis were also observed, with ORs being 1.3 (95% CI: 1.2–1.4, P = 6.46 × 10−6) per unit increase of log odds of RKOA, 1.2 (95% CI: 1.0–1.5, P = 1.8 × 10−2) per unit increase of log odds of SKOA, 1.3 (95% CI: 1.2–1.5, P = 9.05 × 10−6) per unit increase of log odds of RHOA and 1.3 (95% CI: 1.2–1.4, P = 1.24 × 10−9) per unit increase of log odds of SHOA, respectively (Fig. 3, Appendix pp 19–20). These bidirectional results were consistent across different MR methods (Fig. 3, Appendix pp 17–20). The characteristics of the instrumental variables used in MR analyses are elaborated in the Appendix (pp 21–34).
Fig. 3.
Bidirectional MR results of knee synovitis and knee OA (A), as well as hand synovitis and hand OA (B). MR, mendelian randomization; N_SNPs, number of single nucleotide polymorphisms; OR, odds ratio; CI, confidence interval; IVW, inverse-variance weighted; MR-PRESSO, MR pleiotropy residual sum outlier; RKOA, radiographic knee osteoarthritis; SKOA, symptomatic knee osteoarthritis; RHOA, Radiographic hand osteoarthritis; SHOA, symptomatic hand osteoarthritis.
Discussion
In this large general population-based cohort, synovitis was significantly associated with an increased risk of incident radiographic and symptomatic OA in both knee and hand joints, and vice versa. Such an association was more apparent for the active synovitis (e.g., synovitis with PDS). The bidirectional association between knee (or hand) OA and synovitis was further verified using bidirectional MR analyses.
Comparison with previous studies and potential mechanisms
Several longitudinal studies, including the Osteoarthritis Initiative (OAI), Multicenter Osteoarthritis Study (MOST), and Cohort Hip and Cohort Knee (CHECK) study, have reported that knee synovitis is associated with an increased risk of incident RKOA among high-risk populations of knee OA.19,20 Although no study has directly examined the relation of synovitis to the risk of symptomatic OA in both knee or hand joints, several studies have reported that synovitis and its change were associated with pain progression and fluctuation.21 These findings suggest that synovitis may serve as a potential target for treatment. However, current disease-modifying anti-inflammatory drugs have been unsuccessful in treating OA. This discrepancy suggests that the association between synovitis and OA may be more complex than previously recognized, and a one-way temporal relationship may not adequately explain the interplay of these two features.
Previous studies have reported that OA has a complex pathophysiology that involves all tissues in the joint, including hyperplasia, cellular infiltration and fibrosis of the synovium and thickening of the capsule, in what may be considered an adaptive response to joint insult.22 Some observational studies have reported more synovial inflammation in early compared with late KOA,23 whereas others have suggested that synovial inflammation accompanies the early structural changes of OA but becomes more common and severe in the later stages of OA.24 Indeed, two synovial transcriptomic analysis analyses detected some non-inflammatory or low-inflammatory profile in OA at the time of total joint replacement.25,26 Furthermore, even though synovitis is considered an inflammatory feature of OA, OA-related synovitis might be viewed as a secondary response.27 All these findings indicate that synovitis may accompany OA in a spiral progression and represent an integral component of OA. It is important to mention that synovitis and inflammation may play a stronger role compared with other traditional risk factors like joint overuse in generalized OA (multiple joint OA).28 This suggests that focusing on synovitis or systemic inflammation could offer insights into developing a universally effective treatment approach for various types of OA.
Typically, OA is considered to be primarily a “degenerative” arthritis of synovial joints with low-inflammatory characteristics because (1) inflammatory symptoms and signs are absent or minimal2; (2) leukocyte count of synovial fluid below the ‘inflammatory’ threshold (2000 cells per mm3)4; (3) the acute phase response is absent apart from minor increases in C-reactive protein; (4) low PDS prevalence of synovial inflammation in the context of OA.27 Several previous studies have reported that synovitis at the knee and hand associates more with radiographic OA than with pain.29,30 These evidence suggests that this low-grade inflammation is likely to be part of the joint's adaptive tissue response to various insults or the consequence of the inherent repair process of the synovial joint. Notably, it remains a question of whether specifically targeting low-grade inflammation could indeed prevent or slow down the development and progression of OA in humans.27
Clinical and research implications
Our findings of bidirectional relationships support the standpoint of low-grade synovitis being both a risk factor and a consequence of the OA (e.g., an integral component of the OA) rather than the prevailing view that synovitis is solely a risk factor for OA. The role of synovitis as a mere ‘risk factor’ fails to explain the bidirectional relationship of these two features thoroughly. Therefore, its suitability as a potential treatment target requires further consideration. To a certain extent, this also provides some explanation for the failure of current disease-modifying anti-inflammatory treatments. Future research should delve deeper into understanding the nature of synovitis in OA, potentially enhancing our understanding of the trajectory of OA and adjusting the current focus and strategy of disease-modifying anti-inflammatory treatments.
Strengths and limitations
Our study has several strengths. Firstly, we explored the temporal relationship between synovitis and OA in both weight-bearing (knee) and non-weight-bearing (hand) joint sites, enhancing the reliability and generalisability of the findings. Secondly, we provide the first-ever population-based study of hand OA incidence in Asians. By utilizing these epidemiological data, we can better understand the natural history of hand OA and identify differences in incidence among different populations. Although the risk of incident SHOA increases with age and peaks around 65 years, it gradually decreases thereafter. This pattern aligns with the occurrence characteristics of symptomatic OA and is partly influenced by the increased pain thresholds observed in elderly females. Thirdly, our study was well suited to investigating the complex association between synovitis and OA because it included repeated measures of both features over time. Fourthly, we adjusted for many covariates in the current analysis, including age, sex, BMI, smoking status, alcohol consumption, educational level, and joint injury history; thus minimizing potential bias from major confounders. Finally, we conducted an MR study using the available genomic data from the XO Study to further investigate the bidirectional causal relationship between synovitis and OA.
However, our study has several limitations. Firstly, the lack of estimates for the association between hand synovitis with PDS and SHOA and the wide confidence intervals for the association between hand synovitis with PDS and OA may be due to the low prevalence of PDS in the general population. Secondly, radiographs are not sensitive to mild cartilage and bone changes; thus, our study cannot investigate the relationship between synovitis and earlier structural changes associated with OA. Thirdly, considering that the participants are from a general population cohort in rural mountainous areas of China, the findings could be generalizable to populations with similar characteristics. However, the generalizability of these findings to populations with different characteristics may be limited. Fourthly, while self-reported symptom assessment methods are commonly used in cohort studies, the evaluation results may be subject to recall bias. Furthermore, we used one-sample MR in our cohort for a causal association; further studies on two-sample MR would be helpful to confirm the findings. Lastly, we observed that the excluded individuals were older than the included ones, which may be attributed to difficulties in contact, inability to participate in follow-up, and higher mortality among the elderly. Therefore, there remains potential selection bias, which reduces the reliability of the results.
Conclusion
This general population-based cohort study found that the association between synovitis and OA is bidirectional. These findings indicate that synovitis could be a risk factor or a consequence of the OA rather than a sole risk factor for joint damage. Future research is needed on understanding the nature of OA especially inflammation, rather than simply adopting anti-inflammatory treatment from inflammatory arthritis to OA therapy.
Contributors
GL, CZ, and JW have full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. CZ, GL, and JW are co-corresponding authors, with GL being the lead contact. GL, CZ, JW, YZ, WZ, and MD conceived and designed the study. TJ performed the ultrasound examination and assessment. TY performed the radiographic examination and assessment. QW, KL, and QC collated the imaging data of ultrasound. TY, JL, YW, HL, QW, and KL collected the clinical data. JW, ZY, YZ, WZ, TJ, HH, JL, QW, and KL analyzed the data. JW, YZ, WZ, JX, and HH verified the Mendelian randomization analysis. TJ, QW, KL, and HH drafted the manuscript. All authors made critical and valuable comments on the manuscript. All authors were involved in the reviewing of the manuscript and approved the final version.
Data sharing statement
The study data could be made available on reasonable request from the corresponding authors GL and CZ.
Declaration of interests
All authors have completed the ICMJE uniform disclosure form at http://www.icmje.org/disclosure-of-interest/and declare: support from the National Key Research and Development Plan, the National Natural Science Foundation of China, the Key Research and Development Program of Hunan Province, the Natural Science Foundation of Hunan Province, the Central South University Innovation-Driven Research Programme, and the Fundamental Research Funds for the Central Universities of Central South University for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Acknowledgements
This work was supported by the National Key Research and Development Plan (2022YFC2505500 and 2022YFC3601900), the National Natural Science Foundation of China (81930071, 82072502, 82372474 and U21A20352), the Key Research and Development Program of Hunan Province (2021SK2017), the Natural Science Foundation of Hunan Province (2022JJ20100, 2023JJ30893), and the Central South University Innovation-Driven Research Programme (2023CXQD031), and the Fundamental Research Funds for the Central Universities of Central South University (2024ZZTS0032). No funding bodies had any role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We acknowledge Aliya Sarmanova, Abasiama D. Obotiba, Michelle Hall, and Philip Courtney for their assistance with the standard protocol of ultrasound examination and assessment according to OMERACT atlas. We thank Bei Xu, Bin Zhou, Dongxing Xie, Haochen Wang, Jing Wu, Junyan Liu, Junyu Zhu, Ke He, Ning Wang, Xiang Ding, Xiaoxiao Li, Xin Huang, Xinjia Deng, Yilun Wang, Zhenglei Zhu, Zhichen Liu, Ziying Wu, and Wei Li for their contribution to the data collection of the XO Study.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanwpc.2024.101169.
Contributor Information
Jie Wei, Email: weij1988@csu.edu.cn.
Guanghua Lei, Email: lei_guanghua@csu.edu.cn.
Chao Zeng, Email: zengchao@csu.edu.cn.
Appendix A. Supplementary data
References
- 1.Global, regional, and national burden of osteoarthritis, 1990-2020 and projections to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023;5(9):e508–e522. doi: 10.1016/S2665-9913(23)00163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hunter D.J., Bierma-Zeinstra S. Osteoarthritis. Lancet (London, England) 2019;393(10182):1745–1759. doi: 10.1016/S0140-6736(19)30417-9. [DOI] [PubMed] [Google Scholar]
- 3.Weng Q., Chen Q., Jiang T., et al. Global burden of early-onset osteoarthritis, 1990-2019: results from the global burden of disease study 2019. Ann Rheum Dis. 2024;83:915. doi: 10.1136/ard-2023-225324. [DOI] [PubMed] [Google Scholar]
- 4.Sellam J., Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol. 2010;6(11):625–635. doi: 10.1038/nrrheum.2010.159. [DOI] [PubMed] [Google Scholar]
- 5.Conaghan P.G., Cook A.D., Hamilton J.A., Tak P.P. Therapeutic options for targeting inflammatory osteoarthritis pain. Nat Rev Rheumatol. 2019;15(6):355–363. doi: 10.1038/s41584-019-0221-y. [DOI] [PubMed] [Google Scholar]
- 6.Persson M.S.M., Sarmanova A., Doherty M., Zhang W. Conventional and biologic disease-modifying anti-rheumatic drugs for osteoarthritis: a meta-analysis of randomized controlled trials. Rheumatology. 2018;57(10):1830–1837. doi: 10.1093/rheumatology/key131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei J., Zhang Y., Dalbeth N., et al. Association between gut microbiota and elevated serum urate in two independent cohorts. Arthritis Rheumatol. 2022;74(4):682–691. doi: 10.1002/art.42009. [DOI] [PubMed] [Google Scholar]
- 8.Jiang T., Yang Z., Zhang Y., et al. Dysbiosis of gut microbiota, a potential mediator of bile acid compositions, and prevalence of hand synovitis: a community-based study. Rheumatology. 2023;62(9):3179–3187. doi: 10.1093/rheumatology/kead042. [DOI] [PubMed] [Google Scholar]
- 9.Hammer H.B., Bolton-King P., Bakkeheim V., et al. Examination of intra and interrater reliability with a new ultrasonographic reference atlas for scoring of synovitis in patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70(11):1995–1998. doi: 10.1136/ard.2011.152926. [DOI] [PubMed] [Google Scholar]
- 10.D'Agostino M.A., Conaghan P., Le Bars M., et al. EULAR report on the use of ultrasonography in painful knee osteoarthritis. Part 1: prevalence of inflammation in osteoarthritis. Ann Rheum Dis. 2005;64(12):1703–1709. doi: 10.1136/ard.2005.037994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang T., Yang T., Zhang W., et al. Prevalence of ultrasound-detected knee synovial abnormalities in a middle-aged and older general population-the Xiangya Osteoarthritis Study. Arthritis Res Ther. 2021;23(1):156. doi: 10.1186/s13075-021-02539-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kellgren J.H., Lawrence J.S. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang N., Xie M., Lei G., et al. A cross-sectional study of association between plasma selenium levels and the prevalence of osteoarthritis: data from the Xiangya osteoarthritis study. J Nutr Health Aging. 2022;26(2):197–202. doi: 10.1007/s12603-022-1739-2. [DOI] [PubMed] [Google Scholar]
- 14.Wei J., Zhang C., Zhang Y., et al. Association between gut microbiota and symptomatic hand osteoarthritis: data from the Xiangya osteoarthritis study. Arthritis Rheumatol. 2021;73(9):1656–1662. doi: 10.1002/art.41729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niu J., Clancy M., Aliabadi P., Vasan R., Felson D.T. Metabolic syndrome, its components, and knee osteoarthritis: the framingham osteoarthritis study. Arthritis Rheumatol. 2017;69(6):1194–1203. doi: 10.1002/art.40087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haugen I.K., Ramachandran V.S., Misra D., et al. Hand osteoarthritis in relation to mortality and incidence of cardiovascular disease: data from the Framingham heart study. Ann Rheum Dis. 2015;74(1):74–81. doi: 10.1136/annrheumdis-2013-203789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou W., Nielsen J.B., Fritsche L.G., et al. Efficiently controlling for case-control imbalance and sample relatedness in large-scale genetic association studies. Nat Genet. 2018;50(9):1335–1341. doi: 10.1038/s41588-018-0184-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burgess S., Labrecque J.A. Mendelian randomization with a binary exposure variable: interpretation and presentation of causal estimates. Eur J Epidemiol. 2018;33(10):947–952. doi: 10.1007/s10654-018-0424-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Atukorala I., Kwoh C.K., Guermazi A., et al. Synovitis in knee osteoarthritis: a precursor of disease? Ann Rheum Dis. 2016;75(2):390–395. doi: 10.1136/annrheumdis-2014-205894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Felson D.T., Niu J., Neogi T., et al. Synovitis and the risk of knee osteoarthritis: the MOST Study. Osteoarthritis Cartilage. 2016;24(3):458–464. doi: 10.1016/j.joca.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hill C.L., Hunter D.J., Niu J., et al. Synovitis detected on magnetic resonance imaging and its relation to pain and cartilage loss in knee osteoarthritis. Ann Rheum Dis. 2007;66(12):1599–1603. doi: 10.1136/ard.2006.067470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doherty M., Hunter D.J., Bijlsma H., Arden N., Dalbeth N. 3 edn. Oxford University Press; 2016. Oxford textbook of osteoarthritis and crystal arthropathy. [Google Scholar]
- 23.Benito M.J., Veale D.J., FitzGerald O., van den Berg W.B., Bresnihan B. Synovial tissue inflammation in early and late osteoarthritis. Ann Rheum Dis. 2005;64(9):1263–1267. doi: 10.1136/ard.2004.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scanzello C.R., Goldring S.R. The role of synovitis in osteoarthritis pathogenesis. Bone. 2012;51(2):249–257. doi: 10.1016/j.bone.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steinberg J., Southam L., Fontalis A., et al. Linking chondrocyte and synovial transcriptional profile to clinical phenotype in osteoarthritis. Ann Rheum Dis. 2021;80(8):1070–1074. doi: 10.1136/annrheumdis-2020-219760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orange D.E., Agius P., DiCarlo E.F., et al. Identification of three rheumatoid arthritis disease subtypes by machine learning integration of synovial histologic features and RNA sequencing data. Arthritis Rheumatol. 2018;70(5):690–701. doi: 10.1002/art.40428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanchez-Lopez E., Coras R., Torres A., Lane N.E., Guma M. Synovial inflammation in osteoarthritis progression. Nat Rev Rheumatol. 2022;18(5):258–275. doi: 10.1038/s41584-022-00749-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moradi K., Kwee R.M., Mohajer B., et al. Erosive hand osteoarthritis and sarcopenia: data from Osteoarthritis Initiative cohort. Ann Rheum Dis. 2024;83(6):799–806. doi: 10.1136/ard-2023-224997. [DOI] [PubMed] [Google Scholar]
- 29.Hall M., Doherty S., Courtney P., Latief K., Zhang W., Doherty M. Synovial pathology detected on ultrasound correlates with the severity of radiographic knee osteoarthritis more than with symptoms. Osteoarthritis Cartilage. 2014;22(10):1627–1633. doi: 10.1016/j.joca.2014.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Obotiba A.D., Swain S., Kaur J., et al. Synovitis and bone marrow lesions associate with symptoms and radiographic progression in hand osteoarthritis: a systematic review and meta-analysis of observational studies. Osteoarthritis Cartilage. 2021;29(7):946–955. doi: 10.1016/j.joca.2021.03.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.