Abstract
The Pharma-safe Index Dataset comprises comprehensive information on several aspects of pharmaceuticals, including general medication data, usage guidelines, dosage, adverse effects, pricing, drug interactions, duration of use, composition, and contraindications. The dataset is provided in both CSV and JSON file formats, and it is available in both English and Bahasa Indonesia. By conducting interviews, distributing flyers, and using pharmacy books, the dataset was collected from over-the-counter medications that were sold at three pharmacies located in Yogyakarta, Indonesia. A medical professional performed cleansing, standardization, and validation on it before it was exported to JSON and CSV formats. The data collected on drug efficacy, safety, and patient outcomes in Indonesia can be utilized by researchers in order to uncover trends and developing patterns of prescription drug resistance. It is possible that this will direct future research, lead to improvements in drug formulations, treatment strategies, and public health policies, and expand our understanding of how drugs work and how they affect patient health.
Keywords: Over-the-counter medicine dataset, Self-medication, Indonesian medicine dataset, Decision support system, Expert system
Specifications Table
| Subject | Health Informatics, Data Mining and Statistical Analysis |
| Specific subject area | Public health; Pharmocology; Self-medicated; Decision Support System; Expert System |
| Type of data | Table Raw |
| Data collection | The dataset was collected from a list of over-the-counter drugs sold in three pharmacies located in the Yogyakarta Special Region, Indonesia. To complete the data, data collection was carried out through interviews with pharmacists and doctors, physical flyers from medicine boxes, and pharmacy books. Next, the data goes through a cleaning process and a structure standardization process using Microsoft Excel. The dataset results are vali3dated by a doctor to guarantee the correctness of the data. Next the author and doctor translate the dataset from Indonesian to English. The final process of forming the dataset is to export it to JSON and CSV format using the Python application. |
| Data source location | Over-The-Counter medicine data used in this data set is collected from pharmacies: - Apotek Primedika-Jongke, Sleman, Daerah Istimewa Yogyakarta, Indonesia - Apotek Bina Farma, Sleman, Daerah Istimewa Yogyakarta, Indonesia - Apotek Sinar Farma, Wonosari, Daerah Istimewa yogyakarta, Indonesia Books: - Informasi Socialite Obat Indonesia |
| Data accessibility | Repository name: Mendeley Data: Pharma-safe Index Dataset [1] Data identification number: 10.17632/m78j7ctwhn.1 Direct URL to data: https://data.mendeley.com/datasets/m78j7ctwhn/2 |
| Related research article |
1. Value of the Data
-
•
The data can be used in a knowledge-based system for the public, providing easy access to accurate and comprehensive information about medications, including proper dosages and potential side effects. It educates the public about contraindications, helping them avoid harmful drug interactions and allergic reactions. This empowers individuals to make informed decisions about their healthcare by understanding the benefits and risks associated with their medications. By offering detailed guidance, the system promotes medication safety and adherence, ultimately enhancing public health and enabling better self-management of chronic conditions.
-
•
Currently in Indonesia there is no database that provides complete information about over-the-counter medicines, most of which only contain the active ingredients [2]. This rich dataset is crucial for training AI dan machine learning models that can optimize treatment plans and improve diagnostic tools especially for diseases that can be treated with over-the-counter medicines, especially diseases that often occur in tropical areas like Indonesia [3].
-
•
These data can be reused by researchers as a rich source of real-world information on drug efficacy, safety, and patient outcomes, especially in Indonesia. Researchers can analyze these data to identify trends in medication use and emerging resistance patterns, providing insights that guide future research directions. This can lead to improved drug formulations, tailored treatment plans, and better public health policies. Additionally, the data can support studies on the effectiveness of different medications in diverse populations, contributing to a deeper understanding of how various factors impact drug performance and patient health.
2. Background
The rise in the utilization of over-the-counter medicine and the restricted availability of over-the-counter medicine for self-treatment can be attributed to various factors. These factors include the widespread access to information on the internet, the expensive nature of medical examinations at healthcare facilities, and the challenges associated with accessing healthcare services, such as distance and limited service hours [4,5]. There are several diseases that can be treated independently, such as flu, coughs, fever, pain, diarrhea, worm infections and gastritis. Over-the-counter medicines are accessible without the need for examination by a doctor or the use of a prescription, so there is a risk of errors in their use [6,7].
Accurate data sets are required in the fields of pharmacology, public health, and information science to validate the efficacy of over-the-counter drugs for self-medication [8]. From a pharmacological perspective, it is essential to comprehend the active components, modes of operation, and possible interactions of over-the-counter medications in order to guarantee their safe utilization and reduce the likelihood of adverse reactions. In public health theory emphasize the importance of informed self-care and the prevention of medication misuse and adverse reactions. Information science principles guide the organization, retrieval, and dissemination of data to maximize accessibility and usability.
3. Data Description
This “Pharma-safe Index Dataset” [1] is an over-the-counter drugs dataset consisting of 112 drugs data on medicinal products sold in the three pharmacist in Yogyakarta Special Region, Indonesia. The Pharma-safe Index dataset is available in Indonesian and English. The dataset is available in 2 formats, namely JSON and CSV formats. The two file formats are separated into 2 folders in the dataset (Table 1).
Table 1.
Folder structure.
 |
Table 2 shows the JSON Structure of the dataset. There is no difference in the JSON structure for Indonesian and English, what's different is the key-value pair data in it. There are 11 name-value pairs in JSON level 1, namely drug_name, disease_category, price, indication, composition, side_effects, contra_indications, contra_indications, drug_interactions, dosage, usage_rules, and usage_period. Data in the Pharma-safe Index already has a structured data form and reflects the relationships in each data record.
Table 2.
JSON structure.
 |
In the dataset there is some data that is related to one-to-many data, therefore there are several CSV files to accommodate these relationships. Each has different columns according to the information you want to store. An explanation of the columns in each CSV file can be seen in Table 3. The CSV file data-obat.csv is the main CSV file and stores OTC drug data information. The drug_id column is a column that shows the relationship to the CSV file data-obat.csv and is a column that accommodates one-to-many relationships.
Table 3.
Overview of column structure in CSV files.
| No | CSV File | CSV Column | Description |
|---|---|---|---|
| 1 | data-obat.csv | id | id or medicine code |
| nama_obat | Name of the medicine | ||
| kategori_penyakit | Medicine category | ||
| indikasi | Indiation | ||
| 2 | data-aturan.csv | id_obat | Id or medicine code; relation to data-obat.csv |
| aturan_pakai | How to use medicine | ||
| 3 | data-dosis.csv | id_obat | Id or medicine code; relation to data-obat.csv |
| profil | Profile type of medicine user | ||
| dosis | Dose | ||
| 4 | data-efeksamping.csv | id_obat | Id or medicine code; relation to data-obat.csv |
| efek_samping | Side effect | ||
| 5 | data-harga.csv | id_obat | Id or medicine code; relation to data-obat.csv |
| satuan | Medicine unit | ||
| harga | Medicine price | ||
| 6 | data-interaksiobat.csv | id_obat | Id or medicine code; relation to data-obat.csv |
| Interaksi_obat | Medicine interaction | ||
| 7 | data-jangkawaktupenggunaan.csv | id_obat | Id or medicine code; relation to data-obat.csv |
| jangka_waktu_penggunaan | Period of use | ||
| 8 | data-komposisi.csv | id_obat | Id or medicine code; relation to data-obat.csv |
| nama | Compound name | ||
| jumlah | Quantity of compound | ||
| satuan | Compound unit | ||
| 9 | data-kontraindikasi.csv | id_obat | Id or medicine code; relation to data-obat.csv |
| kontra_indikasi | Contra indication |
4. Experimental Design, Materials and Methods
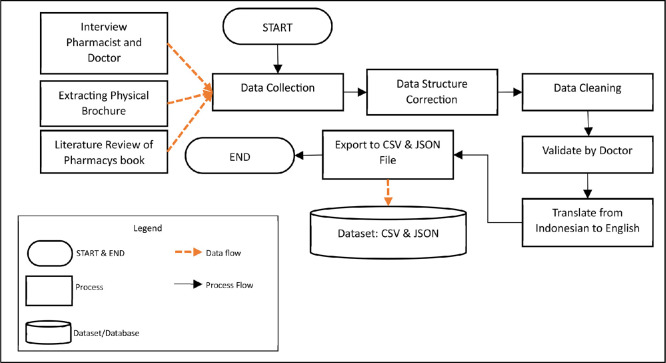
The research process was carried out in 5 main steps (Fig. 1). The research began with observing the locations of the three pharmacies that were the object of research, namely Primedika Jongke Pharmacy, Bina Farma Pharmacy, and Sinar Farma Pharmacy. Next, a list of drugs sold by the three pharmacies was collected. Next, the author determines the data source. Data sources were taken from interview pharmacist and doctor, the physical brocure from medicide box, and pharmacy books. The research steps are Data Collection (1), Data Structure Correction (2), Data Cleaning (3), Validated by Doctor (4), Translate from Indonesian to English (5) and Export to CSV and JSON File (6).
-
1.
Data Collection: Data collection is done in 3 ways, namely interview with pharmacist and doctor, collecting physical brochures from medicine box and collecting data from several pharmacy books [2]. The data that has been collected is stored in an Excel file format for further processing.
-
2.
Data Structure Correction: Text data collected from physical brochures and several pharmacy books have very diverse data structures. The second step of this research is to standardize the data structure [9]. Standardized data structures are used to determine the information to be stored into dataset.
-
3.
Data Cleaning: Every drug list and text data that has been collected is cleaned from characters that can interfere with the CSV and JSON format creation process. These characters are the quotation mark character (') and the double quotation mark (") [10]. In order not to lose the meaning of the sentence by removing characters, a back-slash character (\) is added to (\') and (\”).
-
4.
Validated by Doctor: After the data structure correction and data cleaning stages, the dataset is then validated by a doctor. The doctor examines the relationship of each existing data based on existing medical understanding. If there is an incorrect data, the data will be immediately corrected by a doctor based on medical knowledge.
-
5.
Translate from Indonesian to English: Lastly, we translate the dataset from Bahasa Indonesian to English. The translation process is carried out manually by researchers and doctors. Some translations are adapted to language commonly used in the medical area.
-
6.
Export to CSV & JSON: After the data is built, the final process is to create a dataset file in CSV and JSON format. A small program using the Python programming language was developed to build CSV and JSON files. The JSON structure can be seen at Table 1 and CSV file data can be seen at Table 2. The JSON and CSV data formats are data formats that have high access flexibility, where both formats can be used on various platforms [11].
Fig. 1.
Flowchart ilustrating the research process.
Limitations
Currently, the dataset is limited to Over-The-Counter/OTC drugs available at pharmacies in Yogyakarta Special Region, Indonesia (Apotek Primedika Jongke, Apotek Bina Farma, and Apotek Sinar Farma). The medicines chosen are medicines used for coughs, flu and stomachace in children and adults. There are still limitations regarding the data structure in some parts, such as drug dosage and side effects. Data Structure Standardize of drug dosages is difficult because there are many combinations of age divisions (1). For example, some say the children's ages are between 2 and 5 years, 2 and 6 years, over 4 years, and so on. Another challenge arises from the extensive usage of unstructured text in the side effects section, which often comprises complex words (2). For instance, in a certain brochure, the composition comprises the ramifications and the likelihood of its occurrence. While in other brochure, the structure is comprised of outcomes that are dependent on the dosage and usage, taking into account any pre-existing conditions. Therefore, it is difficult to classify and distinguish different side effects into separate categories.
Ethics Statement
The authors hereby confirm that we have thoroughly read and adhere to the ethical guidelines for publishing in Data in Brief. Furthermore, we affirm that the ongoing project does not entail the use of human subjects, animal experimentation, or any data obtained from social media platforms.
CRediT Author Statement
Danny Sebastian: Conceptialization, Methodology, Software, Writing – Original Draft; Restyandito: Conceptialization, Methodology, Writing – Review & Editing; Justinus Putranto Agung Nugroho: Resources, Validation, Writing – Review & Editing.
Acknowledgments
Medicine Knowledge Contributors: Apt. Assysifa Septyani Putri, S.Farm., as Pharmacist, apt. Fretty Widadi, S.Farm., as Pharmacist, Apt. Aloysius Bimo Tiar Nugroho, S.Farm., as Pharmacist, dr. Oscar Gilang Purnajati, MHPE as Validator. This research is supported by Institute of Research and Community of Service Universitas Kristen Duta Wacana (LPPM-UKDW), Contract Number: 109/D01/LPPM/2024.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data Availability
Pharma-safe Index Dataset (Original data) (Mendeley Data).
References
- 1.Sebastian D., Restyandito R., Nugroho J.P.A. Pharma-safe index dataset. Mendeley Data. 2024 doi: 10.17632/m78j7ctwhn.2. [DOI] [Google Scholar]
- 2.Ikatan Apoteker Indonesia, Informasi Socialite Obat Indonesia, vol. 53. PT Pharma Tekno Solusi, 2021.
- 3.Rajpurkar P., Chen E., Banerjee O., Topol E.J. AI in health and medicine. Nat. Med. 2022;28(1):31–38. doi: 10.1038/s41591-021-01614-0. [DOI] [PubMed] [Google Scholar]
- 4.Brahmbhatt S.V., Dave V.D. A survey study on knowledge, attitude, and practice toward self-medication practice with over-the-counter drugs among under graduate dental students. Asian J. Pharm. Res. Health Care. 2023;15(4):359–363. doi: 10.4103/ajprhc.ajprhc_79_23. [DOI] [Google Scholar]
- 5.Mirdad O.A., et al. Over-the-counter medication use among parents in Saudi Arabia. Int. J. Environ. Res. Public Health. 2023;20(2):1193. doi: 10.3390/ijerph20021193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tesfamariam S., et al. Self-medication with over the counter drugs, prevalence of risky practice and its associated factors in pharmacy outlets of Asmara, Eritrea. BMC Public Health. 2019;19(1):159. doi: 10.1186/s12889-019-6470-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feli F., Pratiwi L., Rizkifani S. Analisis Tingkat Pengetahuan Mahasiswa Program Studi Farmasi Terhadap Swamedikasi Obat Bebas dan Bebas Terbatas. J. Syifa Sci. Clin. Res. 2022;4(2):275–286. doi: 10.37311/jsscr.v4i2.14027. [DOI] [Google Scholar]
- 8.Roughead E.E., Lim R. Preventing overdoses with over-the-counter medicines. Med. J. Aust. 2023;218(9):399–400. doi: 10.5694/mja2.51927. [DOI] [PubMed] [Google Scholar]
- 9.Gupta M.K., Chandra P. A comprehensive survey of data mining. Int. J. Inf. Technol. 2020;12(4):1243–1257. doi: 10.1007/s41870-020-00427-7. [DOI] [Google Scholar]
- 10.Wang J., Wang X., Yang Y., Zhang H., Fang B. A review of data cleaning methods for web information system. Comput., Mater. Continua. 2020;62(3):1053–1075. doi: 10.32604/cmc.2020.08675. [DOI] [Google Scholar]
- 11.Pezoa F., Reutter J.L., Suarez F., Ugarte M., Vrgoč D. Foundations of JSON schema. Proceedings of the 25th International Conference on World Wide Web; Republic and Canton of Geneva, Switzerland; Apr. 2016. pp. 263–273. International World Wide Web Conferences Steering Committee. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Pharma-safe Index Dataset (Original data) (Mendeley Data).