Abstract
BACKGROUND
No study has investigated the change regularity between age and subfoveal choroidal thickness (SFCT) in proliferative diabetic retinopathy (PDR).
AIM
To investigate the relationship between the SFCT and age in Chinese patients with PDR.
METHODS
This was a cross-sectional retrospective study. The participants were hospitalized individuals with type 2 diabetes who underwent vitrectomy for PDR. Con-tralateral eyes that met the criteria were included in the study. All necessary laboratory tests were performed at the time of admission. Central macular thickness (CMT) and SFCT were two quantitative assessments made using enhanced depth imaging optical coherence tomography. CMT was measured automatically and SFCT was measured manually with digital calipers provided by the Heidelberg Eye Explorer software.
RESULTS
The final analysis included a total of 234 individuals with PDR. The average age was 55.60 years old ± 10.03 years old, and 57.69% of the population was male. Univariate analysis revealed a significant negative connection between age and SFCT in patients with PDR [β = -2.44, 95% confidence interval (95%CI): -3.46 to -1.42; P < 0.0001]. In the fully adjusted model, the correlation between SFCT and age remained steady (β = -1.68, 95%CI: -2.97 to -0.39; P = 0.0117). Spline smoothing showed that the relationship between SFCT and age in patients with PDR was non-linear, with an inflection point at 54 years of age.
CONCLUSION
Our findings suggest that age is a key determinant of choroidal thickness. The non-linear link between SFCT and age in PDR patients should be taken into account.
Keywords: Age, Subfoveal choroidal thickness, Proliferative diabetic retinopathy, Optical coherence tomography, Central macular thickness
Core Tip: In the present study, the average subfoveal choroidal thickness (SFCT) of patients with proliferative diabetic retinopathy (PDR) was inversely correlated with age, and a reduction of 1.68 μm in SFCT per year was observed. Additionally, there was a progressively increasing trend in SFCT of 2.00 μm per year before the age of 54 years and a negative correlation of 4.89 μm per year after the age of 54 years; therefore, age around 54 years might be an important turning point. This is the first study to reveal a relationship between age and SFCT in patients with PDR.
INTRODUCTION
The choroid is a highly vascularized tissue in the eye that is affected by various systemic abnormalities affecting the blood vessels and is crucial to the physiology and pathogenesis of various ocular diseases[1]. As the only source of metabolic exchange in the avascular fovea, the choroid has the highest blood flow per unit weight of any human tissue[2]. The maintenance of retinal function requires structurally and functionally normal choroidal vessels. Impaired choroidal function may lead to photoreceptor dysfunction and death, eventually leading to vision loss[3].
Optical coherence tomography (OCT) provides high-resolution images of retinal microstructures in a non-invasive and rapid manner, which greatly improves the ability to diagnose and follow up fundus diseases, such as diabetic macular edema and diabetic retinopathy (DR)[4]. Moreover, OCT enables choroidal thickness quantification. Compared with traditional OCT, improved enhanced depth imaging OCT (EDI-OCT) can provide more reliable measurements of choroidal thickness by providing better resolution and depth of field[5]. The thickest component of the choroid in healthy humans is located in the macular avascular zone. Although there are considerable differences between individuals, the subfoveal choroidal thickness (SFCT) is approximately 350 μm[6,7]. The choroidal thickness in the periphery is approximately 264 μm[7].
Several studies have reported an association between the SFCT and age, sex, and ocular axial length. Studies at home and abroad have shown that the average loss rate of SFCT in healthy participants is 1.4-4.8 μm per year[8-15]. However, conflicting results have been reported in healthy participants. According to a study by Ding et al[16], in patients under the age of 60 years, SFCT showed no relationship with age. In participants beyond the age of 60 years, SFCT dramatically decreases by 5.40 m for every year of life and has a negative correlation with age. Recently, Huo et al[8] discovered that SFCT decreases linearly with age, with a more pronounced decline after reaching 50 years of age, which is associated with aging and a higher spherical equivalent in myopia but is not related to sex, foveal thickness, or intraocular pressure within the normal range. However, another study[17] showed that the mean SFCT demonstrated a significant positive correlation with refractive error but a significant negative correlation with age. This correlation was observed in patients under 60 years of age with respect to age, refractive error, and ocular axial length, but not in subjects aged > 60 years. These contradictory results indicate that although it is generally recognized that SFCT decreases with age in a healthy population, the change regularity of different ages and ethnic groups may differ.
Currently, there are no studies on the change regularity between age and SFCT in patients with proliferative DR (PDR), and the relationship between the both is often used only as a secondary supplement. Therefore, we used EDI-OCT to measure SFCT in Chinese patients with PDR and explored the change regularity between age and SFCT.
MATERIALS AND METHODS
Study population
A description of the study population can be found in the literature[18,19]. This was a retrospective and cross-sectional study. West China Hospital of Sichuan University provided ethical permission for the trial. The study was retrospective in nature and the obtained data were anonymized, thus the Institutional Review Board decided that informed consent was not necessary [No. 2020 (834)]. Patients who underwent vitrectomy for PDR with type 2 diabetes between May 2020 and February 2022 and were hospitalized to the West China Hospital of Sichuan University’s Ophthalmology Department served as the study’s participants. Patients with PDR and contralateral eyes that met the following criteria were included.
Inclusion criteria: (1) The contralateral eyes of patients with PDR were included, which were also graded as PDR with clear refractive media and no vitrectomy had been performed previously; and (2) Fasting blood glucose was < 8 mmol/L and blood glucose level was < 11 mmol/L two hours after three meals. In addition, blood glucose levels persisted for at least 7 days.
Exclusion criteria: (1) Ocular axial length more than 26.50 mm or refractive error exceeding ± 6.00 diopters; (2) Syphilis, leukemia, acquired immune deficiency syndrome, etc.; (3) Type 1 diabetes; (4) Retinal artery occlusion, retinal vein occlusion, neovascular glaucoma or iris neovascularization, paracentral acute middle maculopathy, uveitis, ocular trauma, endophthalmitis, age-related macular degeneration, and other ocular diseases; and (5) Poor-quality EDI-OCT images with no discernible chorioscleral interfaces.
Variables
Upon admission, all required laboratory testing was completed. Fasting laboratory values included glycosylated hemoglobin A1c (HbA1c), serum lipid profile, renal profile [e.g., serum cystatin C, estimated glomerular filtration rate (eGFR), serum creatinine, serum blood urea nitrogen, and uric acid], hepatic function, and serum lipid profile. The electronic medical record system provides pertinent baseline characteristics that are essential for managing hospitalized patients with PDR. The demographic data encompassed age, gender, ethnicity, and educational attainment. The three categories for educational attainment were junior high school or below, senior high school, or college or above. Relevant medical histories included hypertension, diabetes duration, diabetic nephropathy, stroke, anemia, and heart disease. A history of hypertension was defined as the use of antihypertensive medications or a physician’s diagnosis. Diabetes mellitus was defined as the use of insulin/oral hypoglycemic agents or a physician’s diagnosis. The history of anti-vascular endothelial growth factor (VEGF) therapy and the degree of pan-retinal photocoagulation (PRP) in the target eyes of patients with PDR were documented. The degree of PRP was classified into three categories based on the scope of the laser: None, partial, or whole. Systemic medication history included insulin treatment, oral glucose-lowering drugs, and oral antihypertensive drugs (calcium antagonists). Height, weight, diastolic blood pressure (DBP), and systolic blood pressure (SBP) were taken at the time of initial presentation. Weight in kilograms divided by height in meters squared (kg/m2) yielded the body mass index.
eGFR was used as a stratification factor of renal function, which was divided into four groups [eGFR < 30, 30 ≤ eGFR < 60, 60 ≤ eGFR < 90, eGFR ≥ 90 (mL/minutes × 1.73 m2)]. Hypercholesterolemia was defined as fasting serum with a total cholesterol concentration greater than 6.22 mmol/L (240 mg/dL)[20], according to the Chinese Adult Dyslipidemia Prevention Guide (2007 edition).
EDI-OCT imaging
Every individual had a thorough ocular examination, including intraocular pressure, visual acuity, ocular axial length, slit-lamp examination, and EDI-OCT. The description of EDI-OCT imaging can be found in our previous literature[18,19]. After mydriasis, compound tropicamide eye drops (Mydrin-P; Santen, Osaka, Japan) were used for EDI-OCT. Standardized EDI-OCT images were obtained in the afternoon by technicians using spectral-domain OCT (Spectralis OCT; Heidelberg Engineering, Heidelberg, Germany) to avoid diurnal variations. The quantitative measurements, such as central macular thickness (CMT) and SFCT, were acquired using EDI-OCT with the horizontal/vertical scans intersecting the fovea center (ART 100 frames, High Speed). CMT was measured automatically. Using digital calipers that the Heidelberg Eye Explorer software supplied, the SFCT was measured manually. CMT was defined as the vertical distance from the inner limiting membrane to the retinal pigment epithelium (RPE) at the fovea. The SFCT was defined as the vertical distance from the outer surface of the RPE to the chorioscleral interface in the macula. Measurements were carried out separately by two skilled doctors who were blind to the patient’s clinical information, achieving 85% uniformity within a tolerance of ± 15 μm. Consequently, all measurements were averaged for the final statistical analysis.
Statistical analysis
Descriptive statistics were used to summarize demographic characteristics and study outcomes. Continuous variables were presented as mean ± SD, while the categorical variables were shown as frequencies and percentages. The χ² test or Kruskal-Wallis test was employed to assess variation. To address the nonlinearity between age and SFCT, a multivariate linear regression model with spline smoothing (penalized spline method) was utilized[21,22]. The inflection point was calculated using a recursive algorithm if nonlinearity was detected, and a two-piecewise multivariate linear regression model was developed[23]. The selection of confounders was based on their association with outcomes or changes in effect estimates > 10%[24,25]. Statistical analyses were performed using R software, version 3.4.3 (http://www.R-project.org/, The R Foundation) and Empower Stats (http://www.empowerstats.com; X&Y Solutions Inc., Boston, MA, United States). Statistical significance was defined as a two-sided P value of < 0.05.
RESULTS
Baseline characteristics of participants
The final analysis included a total of 234 patients with PDR. The characteristics of the participants with PDR are summarized in Table 1. With 57.69% of the population being male, the mean age was 55.60 years old ± 10.03 years old. The SFCT was 264.70 μm ± 83.28 μm, and the average ocular axial length was 23.17 mm ± 0.99 mm.
Table 1.
Baseline characteristics of participants
|
Item
|
mean ± SD/n (%)
|
| Age (year) | 55.60 ± 10.03 |
| SBP (mmHg) | 136.98 ± 20.75 |
| DBP (mmHg) | 82.92 ± 10.90 |
| Height (cm) | 161.72 ± 8.16 |
| Weight (kg) | 62.91 ± 10.83 |
| BMI (kg/m2) | 23.99 ± 3.07 |
| Duration of diabetes (year) | 12.27 ± 6.80 |
| Visual acuity (logMAR) | 0.73 ± 0.53 |
| IOP (mmHg) | 16.28 ± 4.16 |
| Fasting blood glucose (mmol/L) | 7.40 ± 2.87 |
| Serum urea nitrogen (mmol/L) | 8.47 ± 4.39 |
| Serum creatinine, µmol/L | 145.98 ± 182.39 |
| eGFR (mL/minutes × 1.73 m2) | 66.95 ± 30.47 |
| Uric acid (mmol/L) | 377.59 ± 87.59 |
| Triglyceride (mmol/L) | 1.50 ± 0.75 |
| Total cholesterol (mmol/L) | 4.60 ± 1.19 |
| HDL-C (mmol/L) | 1.31 ± 0.41 |
| LDL-C (mmol/L) | 2.68 ± 1.02 |
| HbA1c (%) | 7.73 ± 1.74 |
| Ocular axial length (mm) | 23.17 ± 0.99 |
| CMT (μm) | 283.76 ± 122.66 |
| SFCT (μm) | 264.70 ± 83.28 |
| Gender | |
| Female | 99 (42.31) |
| Male | 135 (57.69) |
| Education level | |
| Junior high school or below | 147 (63.64) |
| Senior high school | 40 (17.32) |
| College or above | 44 (19.05) |
| Hypertension history | |
| No | 126 (53.85) |
| Yes | 108 (46.15) |
| Diabetic nephropathy history | |
| No | 188 (80.34) |
| Yes | 46 (19.66) |
| Stroke history | |
| No | 222 (94.87) |
| Yes | 12 (5.13) |
| Anemia history | |
| No | 162 (69.23) |
| Yes | 72 (30.77) |
| Heart disease history | |
| No | 222 (94.87) |
| Yes | 12 (5.13) |
| PRP | |
| None | 111 (47.44) |
| Partial | 43 (18.38) |
| Whole | 80 (34.19) |
| Anti-VEGF therapy | |
| No | 175 (74.79) |
| Yes | 59 (25.21) |
| Insulin treatment | |
| No | 113 (48.29) |
| Yes | 121 (51.71) |
| Oral glucose-lowering drugs | |
| No | 59 (25.21) |
| Yes | 175 (74.79) |
| Calcium antagonists | |
| No | 183 (78.54) |
| Yes | 50 (21.46) |
SBP: Systolic blood pressure; DBP: Diastolic blood pressure; BMI: Body mass index; HbA1c: Hemoglobin A1c; logMAR: Logarithmic minimum resolution angle; IOP: Intraocular pressure; HDL-C: High-density lipoprotein cholesterol; LDL-C: Low-density lipoprotein cholesterol; eGFR: Estimated glomerular filtration rate; CMT: Central macular thickness; SFCT: Subfoveal choroidal thickness; VEGF: Vascular endothelial growth factor; PRP: Pan-retinal photocoagulation.
Univariate analysis in patients with PDR
In patients with PDR, age and SFCT showed a significant negative connection [β = -2.44, 95% confidence interval (95%CI): -3.46 to -1.42; P < 0.0001] according to the results of univariate analysis. Compared with women, men exhibited a positive association with SFCT (β = 31.08, 95%CI: 9.81-52.36; P = 0.0046). There were significant differences in DBP, height, weight, history of diabetic nephropathy, anti-VEGF therapy, calcium antagonists, insulin treatment, oral glucose-lowering drugs, serum creatinine, HDL-C, total cholesterol, and SFCT. Compared to non-PRP, the whole PRP was associated with SFCT. These results revealed that other factors were not significantly correlated with SFCT. The results of the univariate analysis are presented in Table 2.
Table 2.
Univariate linear regression analysis of subfoveal choroidal thickness measurements by enhanced depth imaging-optical coherence tomography
|
|
β, 95%CI
|
P value
|
| Male vs female | 31.08 (9.81 to 52.36) | 0.0046 |
| Age, per 1-year increase | -2.44 (-3.46 to -1.42) | < 0.0001 |
| Education level | ||
| Junior high school or below | Reference | |
| Senior high school | -19.95 (-48.87 to 8.97) | 0.1778 |
| College or above | 2.39 (-25.48 to 30.26) | 0.8666 |
| SBP, per 1 mmHg increase | 0.42 (-0.11 to 0.95) | 0.1222 |
| DBP, per 1 mmHg increase | 1.27 (0.26 to 2.27) | 0.0141 |
| Height, per 1 cm increase | 1.74 (0.41 to 3.08) | 0.0111 |
| Weight, per 1 cm increase | 1.17 (0.17 to 2.17) | 0.0234 |
| BMI, per 1 kg/m2 increase | 1.96 (-1.63 to 5.55) | 0.2865 |
| Duration of diabetes, per 1-year increase | -0.40 (-1.99 to 1.20) | 0.6269 |
| Hypertension vs absent | -2.97 (-24.42 to 18.48) | 0.7862 |
| Diabetic nephropathy history vs absent | 40.23 (13.83 to 66.64) | 0.0031 |
| Stroke history vs absent | 2.07 (-46.41 to 50.55) | 0.9333 |
| Anemia vs absent | 12.80 (-10.31 to 35.91) | 0.2788 |
| Heart disease vs absent | -19.42 (-67.84 to 28.99) | 0.4325 |
| PRP | ||
| None | Reference | |
| Partial | 4.02 (-24.99 to 33.03) | 0.7861 |
| Whole | 31.00 (7.32 to 54.69) | 0.0109 |
| Anti-VEGF therapy vs absent | 30.55 (6.24 to 54.86) | 0.0145 |
| Insulin vs absent | 23.81 (2.63 to 44.99) | 0.0286 |
| Oral glucose-lowering drugs vs absent | -28.96 (-53.31 to -4.62) | 0.0205 |
| Calcium antagonists vs absent | 40.49 (14.86 to 66.13) | 0.0022 |
| Visual acuity, per 1 LogMAR increase | -12.24 (-32.49 to 8.01) | 0.2374 |
| IOP, per 1 mmHg increase | 2.27 (-0.32 to 4.86) | 0.0876 |
| Fasting blood glucose, per 1 mmol/L increase | -2.34 (-6.06 to 1.39) | 0.2201 |
| Serum urea nitrogen, per 1 mmol/L increase | 1.63 (-0.81 to 4.06) | 0.1910 |
| Serum creatinine, per 1 μmol/L increase | 0.08 (0.02 to 0.14) | 0.0092 |
| eGFR, per 1 mL/minutes × 1.73m2 increase | -0.15 (-0.50 to 0.20) | 0.3938 |
| Uric acid, per 1 mmol/L | -0.01 (-0.14 to 0.11) | 0.8322 |
| Triglyceride, per 1 mmol/L increase | -3.01 (-24.36 to 18.34) | 0.7828 |
| Total cholesterol, per 1 mmol/L increase | -16.08 (-29.25 to -2.91) | 0.0181 |
| HDL-C, per 1 mmol/L increase | -42.66 (-81.44 to -3.89) | 0.0329 |
| LDL-C, per 1 mmol/L increase | -15.27 (-30.72 to 0.17) | 0.0548 |
| HbA1c, per 1 % increase | -5.36 (-16.77 to 6.04) | 0.3590 |
| CMT, per 1 μm increase | 0.02 (-0.06 to 0.11) | 0.5839 |
| Ocular axial length, per 1 mm increase | -18.57 (-34.96 to -2.18) | 0.0282 |
CI: Confidence interval; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; BMI: Body mass index; HbA1c: Hemoglobin A1c; logMAR: Logarithmic minimum resolution angle; IOP: Intraocular pressure; HDL-C: High-density lipoprotein cholesterol; LDL-C: Low-density lipoprotein cholesterol; eGFR: Estimated glomerular filtration rate; CMT: Central macular thickness; SFCT: Subfoveal choroidal thickness; VEGF: Vascular endothelial growth factor; PRP: Pan-retinal photocoagulation.
Relationship between age and SFCT in patients with PDR
As indicated in Table 3, we evaluated the relationship between age and the SFCT score using a multivariate linear regression model. We also present three adjusted models. In Model I (adjusted for sex and ocular axial length), the results revealed a significant negative relationship between age and SFCT (β = -2.23, 95%CI: -3.29 to -1.18; P < 0.0001). In Model II (adjusted for sex, ocular axial length, anti-VEGF therapy, PRP, calcium antagonists, insulin treatment, and oral glucose-lowering drugs), the relationship between age and SFCT was consistent (β = -1.96, 95%CI: -3.03 to -0.90; P = 0.0004). Furthermore, in Model III (adjusted for sex, ocular axial length, anti-VEGF therapy, PRP, calcium antagonists, insulin, oral glucose-lowering drugs, serum creatinine, duration of diabetes, HbA1c, DBP, and total cholesterol), the correlation between SFCT and age remained steady (β = -1.68, 95%CI: -2.97 to -0.39; P = 0.0117). Overall, age was negatively correlated with the average SFCT.
Table 3.
Relationship between age and subfoveal choroidal thickness in different models
| Exposure |
Model I
|
Model II
|
Model III
|
|||
|
β (95%CI)
|
P value
|
β (95%CI)
|
P value
|
β (95%CI)
|
P value
|
|
| Age (year) | -2.23 (-3.29 to -1.18) | < 0.0001 | -1.96 (-3.03 to -0.90) | 0.0004 | -1.68 (-2.97 to -0.39) | 0.0117 |
Model I adjusted for sex and ocular axial length; Model II adjusted for sex, ocular axial length, anti-vascular endothelial growth factor (VEGF) therapy, pan-retinal photocoagulation (PRP), insulin treatment, oral glucose-lowering drugs, and calcium antagonists; Model III adjusted for sex, ocular axial length, anti-VEGF therapy, PRP, insulin treatment, oral glucose-lowering drugs, calcium antagonists, diastolic blood pressure, hemoglobin A1c, duration of diabetes, serum creatinine, and total cholesterol. CI: Confidence interval; DBP: Diastolic blood pressure; HbA1c: Hemoglobin A1c; SFCT: Subfoveal choroidal thickness; VEGF: Vascular endothelial growth factor; PRP: Pan-retinal photocoagulation.
Spline smoothing between age and SFCT in patients with PDR
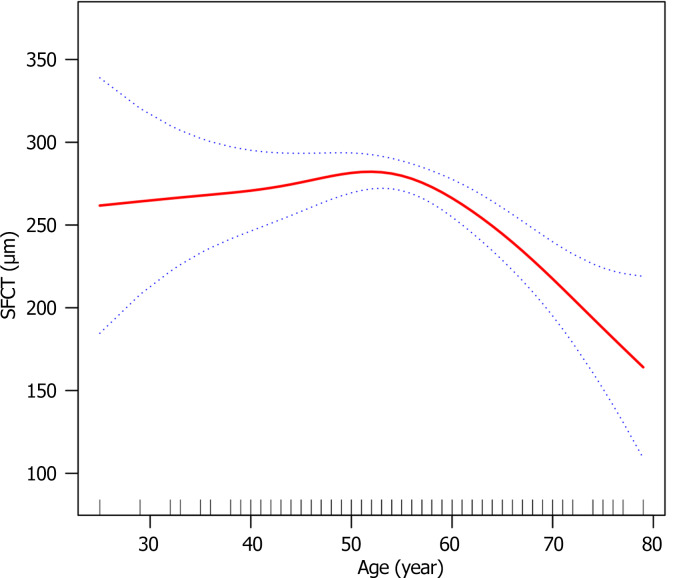
In patients with PDR, we found a non-linear connection between age and SFCT using a multivariate linear regression model with spline smoothing. The adjustment strategy was the same as that used in Model III, as shown in Figure 1. By employing the two-piecewise multivariate linear regression model, we determined that the inflection point was the age of 54 years, which showed a progressive increase trend in SFCT at a rate of 2.00 μm per year before the age of 54 years and a negative correlation with a decrease of 4.89 μm per year after the age of 54 years. These findings are summarized in Table 4.
Figure 1.
The multivariate linear regression model with spline smoothing showed that the relationship between age and subfoveal choroidal thickness in patients with proliferative diabetic retinopathy was non-linear. SFCT: Subfoveal choroidal thickness.
Table 4.
Non-linearity addressed by a two-piecewise linear regression model
|
For exposure: Age
|
SFCT (μm)
|
||
|
Outcome, Model
|
β
|
95%CI
|
P value
|
| Fitting model by standardized linear regression | -1.68 | -2.97 to -0.39 | 0.0117 |
| Fitting model by two-piecewise linear regression | |||
| Inflection point of age | 54.00 | ||
| ≤ 54 | 2.00 | -0.16 to 4.17 | 0.0712 |
| > 54 | -4.89 | -6.87 to -2.90 | < 0.0001 |
| Log likelihood ratio test | < 0.0010 | ||
The adjustment strategy was the same as the adjusted Model III.
Results of stratified analysis between age and SFCT in patients with PDR
The adjustment strategy for each stratification was identical to that of Model III except for the stratification factor. Table 5 displays all the findings from the stratified analysis. In patients with PDR, the downward trends in age and SFCT remained consistent across various stratifications, except for patients with a history of anemia.
Table 5.
Results of stratified analysis between age and subfoveal choroidal thickness in patients with proliferative diabetic retinopathy
|
Y = SFCT, μm
|
n
|
β, 95%CI
|
P value
|
| Gender | |||
| Female | 99 | -0.38 (-2.39 to 1.64) | 0.7157 |
| Male | 135 | -2.63 (-4.51 to -0.74) | 0.0074 |
| Hypertension history | |||
| No | 126 | -1.19 (-2.65 to 0.27) | 0.1137 |
| Yes | 108 | -3.27 (-5.42 to -1.12) | 0.0037 |
| Education level | |||
| Junior high school or below | 147 | -2.13 (-3.90 to -0.36) | 0.0198 |
| Senior high school | 40 | -0.72 (-4.44 to 3.00) | 0.7086 |
| College or above | 44 | -2.09 (-5.23 to 1.06) | 0.2054 |
| Diabetic nephropathy history | |||
| No | 188 | -1.76 (-3.06 to -0.46) | 0.0089 |
| Yes | 46 | -1.29 (-6.51 to 3.94) | 0.6341 |
| Stroke history | |||
| No | 222 | -1.48 (-2.80 to -0.16) | 0.0294 |
| Yes | 12 | Not applicable | |
| Heart disease history | |||
| No | 222 | -1.61 (-2.90 to -0.32) | 0.0153 |
| Yes | 12 | Not applicable | |
| Anemia history | |||
| No | 162 | -2.62 (-4.15 to -1.08) | 0.0011 |
| Yes | 72 | 1.16 (-1.62 to 3.93) | 0.4176 |
| PRP | |||
| None | 111 | 0.05 (-1.78 to 1.87) | 0.9602 |
| Partial | 43 | -3.64 (-6.62 to -0.66) | 0.0257 |
| Whole | 80 | -4.56 (-7.00 to -2.11) | 0.0006 |
| Anti-VEGF therapy | |||
| No | 175 | -1.03 (-2.56 to 0.50) | 0.1891 |
| Yes | 59 | -3.95 (-6.57 to -1.32) | 0.0055 |
| Insulin treatment | |||
| No | 113 | -1.16 (-2.82 to 0.49) | 0.1711 |
| Yes | 121 | -2.34 (-4.42 to -0.25) | 0.0304 |
| Oral glucose-lowering drugs | |||
| No | 59 | -2.85 (-5.15 to -0.56) | 0.0180 |
| Yes | 175 | -2.13 (-3.27 to -0.99) | 0.0003 |
| Calcium antagonists | |||
| No | 183 | -1.27 (-2.56 to 0.02) | 0.0550 |
| Yes | 50 | -3.00 (-7.47 to 1.48) | 0.1993 |
| Total cholesterol group (mmol/L) | |||
| < 6.22 | 119 | -1.78 (-3.74 to 0.18) | 0.0788 |
| ≥ 6.22 | 12 | Not applicable | |
| eGFR group (mL/minutes × 1.73 m2) | |||
| < 30 | 30 | -3.83 (-8.33 to 0.68) | 0.1198 |
| ≥ 30, < 60 | 61 | -3.45 (-7.12 to 0.22) | 0.0719 |
| ≥ 60, < 90 | 81 | -1.47 (-4.21 to 1.27) | 0.2976 |
| ≥ 90 | 62 | 0.11 (-1.87 to 2.08) | 0.9161 |
Not applicable means that the model failed because of the small sample size. The adjustment strategy for each stratification was identical to that of Model III except for the stratification factor. CI: Confidence interval; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; eGFR: Estimated glomerular filtration rate; SFCT: Subfoveal choroidal thickness; VEGF: Vascular endothelial growth factor; PRP: Pan-retinal photocoagulation.
DISCUSSION
In the present study, age was inversely correlated with PDR patients’ average SFCT and a 1.68 μm annual decrease in SFCT. This study also showed a progressive increase trend in SFCT with 2.00 μm per year before the age of 54 years and a negative correlation with 4.89 μm per year after the age of 54 years, age around 54 years might be an important turning point. This is the first study to reveal a relationship between age and SFCT in patients with PDR.
In the present study, a yearly reduction of 1.68 μm in SFCT in PDR patients was found to be similar to the average rate of loss of 1.40-4.80 μm per year[8-15] in healthy participants, indicating that age plays a critical role in evaluating choroidal thickness. However, not all studies have reached the aforementioned conclusions. A study[26] that involved 140 healthy Saudi Arabian women aged 18-29 years found no significant association between SFCT and age. Additionally, Ding et al[16] found that SFCT was negatively correlated with age in healthy Chinese subjects over 60 years old, with an average annual loss of 5.40 μm per year, whereas no significant relationship was found in subjects aged < 60 years. Conversely, another study[17] showed that age was significantly correlated with the SFCT in participants aged < 60 years, whereas this correlation was not observed in participants aged > 60 years. Recently, Huo et al[8] found that the SFCT showed an age-related linear decrease with a steep decline after 50 years of age in healthy Chinese participants. Regarding the contradictory research results, we postulated that although it is generally recognized that SFCT decreases with age in a healthy population, the change regularity of different ages and ethnic groups may differ. In this present study, we found that around age 54 years might be an important turning point in patients with PDR and a progressive increase trend in SFCT with 2.00 μm per year before the age of 54 years; whereas after this age, a negative correlation with a loss of 4.89 μm per year was observed, which may also explain, to some extent, the conflicting results in different age groups in healthy subjects.
Hyperglycemia, in addition to age, can result in damage to the choroidal blood vessels[27]. The concept of diabetic choroidopathy (DC) was introduced by Saracco et al[28]. Subsequent investigations into the histopathological changes associated with DC revealed similarities with DR, such as the atrophy and loss of choroidal capillary endothelium, capillary stenosis and shedding, layered deposition, curved and beaded vessels, and choroidal neovascularization[29,30]. Further research by Bhutto et al[31] confirmed microvascular changes in the choroid including vascular tortuosity, changes in vascular diameter, and arterial stenosis in diabetic rat models. Histopathological findings align with en-face OCT imaging, which provides a clear view of vascular remodeling in DC. Ferrara et al[32] conducted a cross-sectional study involving 76 diabetic patients and found evidence of choroidal vascular remodeling, characterized by irregular, curved, beaded vessels, local dilatation, and stenosis in all patients at different stages of DR. Although OCT studies have attempted to determine the changing trend of choroidal thickness in diabetic patients, no consistent conclusion has been reached[33,34]. Several authors have found that decreased choroidal thickness is associated with diabetes[35-41], and studies[38,42,43] have found that choroidal thickness is significantly decreased in diabetic patients without clinical evidence of retinopathy indicating that reduced choroidal blood flow may be the initial event, which is consistent with the results of studies on choroidal vessels[44]. A recent study involving a large sample (1347 patients) of type 2 diabetic patients without any history of ocular treatment found that choroidal thickness initially increased in the early stages of DR and subsequently decreased as DR progressed[45]. However, in studies comparing the pathological changes in the retina and choroid of spontaneously diabetic torii (SDT) rats and normal Sprague-Dawley (SD) rats, the average thickness of the retina and choroid was significantly increased in SDT rats compared to normal SD rats, indicating choroidal edema in SDT rats[46]. Additionally, evidence suggests that the choroid may become thicker in patients with DR, rather than thinner[33,39,40,47-50]. Kim et al[47] found significant thickening of the choroid in the eyes of patients with DR, which increased with progression from severe non-PDR to untreated PDR severity. Another recent study by Zhang et al[48] identified a significant increase in choroidal thickness in the temporal (750 μm) and upper (1500 μm and 2250 μm) quadrants in diabetic subjects compared with healthy controls. Some studies have shown that choroidal thickness decreases in the early stages of DR and increases in the late stages[51,52].
The possible reasons for these different observations are as follows: First, the normal physiological fluctuation in choroidal thickness, which can be as high as 67 μm throughout the day regardless of the presence of diabetes, must be considered[53]. Other factors such as age and ocular axial length can also contribute to variations in choroidal thickness[54]. Additionally, different OCT systems and measurement parameters used in these studies may have affected our results[33,55]. Furthermore, the focus of the current studies on the measurement of SFCT may not fully capture the choroidal changes that occur throughout the central region and the mid-periphery in patients with diabetes. Moreover, studies on the effects of diabetes on choroidal vasculature often examine different stages of DR, leading to the inclusion of patients who have received previous DR treatments, such as anti-VEGF therapy or PRP. These treatments may affect choroidal thickness independently of diabetes-induced changes[32-34]. Manual measurement of choroidal thickness using EDI-OCT may result in a measurement error. Therefore, it is unclear whether diabetes directly affects choroidal thickness. To shed light on this matter, studies focusing on diabetic patients without any diabetes treatment or known ocular comorbidities may provide valuable insights[56].
Our study has several strengths. First, it represents a significant improvement in researching the nonlinearity of addressing compared with previous studies. We were able to identify the turning point between age and SFCT in PDR patients, which increased the statistical power and greatly enhanced the clinical significance. Second, although this was an observational study and, therefore, susceptible to potential confounding factors, we employed strict statistical adjustment to minimize any residual confounders. Finally, we ensure the reliability of the results by conducting a stratified analysis to test the robustness of our findings. Despite these intriguing results, this study has several limitations. First, the participants in this study consisted solely of Chinese PDR patients. Therefore, when interpreting our findings, it is essential to exercise caution owing to ethnic limitations and disease types. Second, the study population consisted of patients with PDR who underwent either anti-VEGF or PRP treatment, which could potentially affect choroidal thickness[57,58], as opposed to treatment-naïve patients. Although we considered these factors as confounding variables, our results may have been biased. Finally, similar to all observational studies, there is the possibility of uncontrolled confounders despite efforts to control for known potential confounding factors.
CONCLUSION
We found, in summary, that the average SFCT of individuals with PDR decreased by 1.68 μm year with age, and that age 54 may represent a significant turning point. Our findings imply that age has a significant role in choroidal thickness and that, when examining the relationship between SFCT and age in PDR patients, we should take the non-linear association into account.
ACKNOWLEDGEMENTS
The authors also want to thank all patients and their families who consented to participate in the study.
Footnotes
Institutional review board statement: This study was reviewed and approved by the Ethics Committee of the Ethical Committee of the West China Hospital of Sichuan University [Approval No. 2020(834)].
Informed consent statement: The data were anonymous and informed consent was waived by the approving Institutional Review Board because of the retrospective nature of the study.
Conflict-of-interest statement: The authors declare that they have no competing interests.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Ophthalmology
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade B, Grade C, Grade C
Novelty: Grade B, Grade B
Creativity or Innovation: Grade B, Grade C
Scientific Significance: Grade B, Grade B
P-Reviewer: Horowitz M; Unnikrishnan R; Yap E S-Editor: Chen YL L-Editor: A P-Editor: Cai YX
Contributor Information
Chun-Yan Lei, Department of Ophthalmology and Research Laboratory of Macular Disease, West China Hospital, Sichuan University, Chengdu 610041, Sichuan Province, China.
Jiang-Ying Xie, Sichuan University Operating Room, Department of Anesthesiology, West China Hospital, Sichuan University/West China School of Nursing, Sichuan University, Chengdu 610041, Sichuan Province, China.
Qi-Bo Ran, Department of Ophthalmology and Research Laboratory of Macular Disease, West China Hospital, Sichuan University, Chengdu 610041, Sichuan Province, China.
Mei-Xia Zhang, Department of Ophthalmology and Research Laboratory of Macular Disease, West China Hospital, Sichuan University, Chengdu 610041, Sichuan Province, China. zhangmeixia@scu.edu.cn.
Data sharing statement
Data will be made available on reasonable request.
References
- 1.Alm A, Bill A. Ocular and optic nerve blood flow at normal and increased intraocular pressures in monkeys (Macaca irus): a study with radioactively labelled microspheres including flow determinations in brain and some other tissues. Exp Eye Res. 1973;15:15–29. doi: 10.1016/0014-4835(73)90185-1. [DOI] [PubMed] [Google Scholar]
- 2.Mrejen S, Spaide RF. Optical coherence tomography: imaging of the choroid and beyond. Surv Ophthalmol. 2013;58:387–429. doi: 10.1016/j.survophthal.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Cao J, McLeod S, Merges CA, Lutty GA. Choriocapillaris degeneration and related pathologic changes in human diabetic eyes. Arch Ophthalmol. 1998;116:589–597. doi: 10.1001/archopht.116.5.589. [DOI] [PubMed] [Google Scholar]
- 4.Panozzo G, Cicinelli MV, Augustin AJ, Battaglia Parodi M, Cunha-Vaz J, Guarnaccia G, Kodjikian L, Jampol LM, Jünemann A, Lanzetta P, Löwenstein A, Midena E, Navarro R, Querques G, Ricci F, Schmidt-Erfurth U, Silva RMD, Sivaprasad S, Varano M, Virgili G, Bandello F. An optical coherence tomography-based grading of diabetic maculopathy proposed by an international expert panel: The European School for Advanced Studies in Ophthalmology classification. Eur J Ophthalmol. 2020;30:8–18. doi: 10.1177/1120672119880394. [DOI] [PubMed] [Google Scholar]
- 5.Spaide RF, Koizumi H, Pozzoni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008;146:496–500. doi: 10.1016/j.ajo.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 6.Shin JW, Shin YU, Lee BR. Choroidal thickness and volume mapping by a six radial scan protocol on spectral-domain optical coherence tomography. Ophthalmology. 2012;119:1017–1023. doi: 10.1016/j.ophtha.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 7.Hoseini-Yazdi H, Vincent SJ, Collins MJ, Read SA, Alonso-Caneiro D. Wide-field choroidal thickness in myopes and emmetropes. Sci Rep. 2019;9:3474. doi: 10.1038/s41598-019-39653-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huo Y, Guo Y, Wang H, Li L, Cao K, and Wang N. Change regularity of adult subfoveal choroidal thickness with age and its influencing factors. Zhongguo Shiyan Yanke Zazhi. 2021;39:29–33. [Google Scholar]
- 9.Moussa M, Sabry D, Soliman W. Macular choroidal thickness in normal Egyptians measured by swept source optical coherence tomography. BMC Ophthalmol. 2016;16:138. doi: 10.1186/s12886-016-0314-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei WB, Xu L, Jonas JB, Shao L, Du KF, Wang S, Chen CX, Xu J, Wang YX, Zhou JQ, You QS. Subfoveal choroidal thickness: the Beijing Eye Study. Ophthalmology. 2013;120:175–180. doi: 10.1016/j.ophtha.2012.07.048. [DOI] [PubMed] [Google Scholar]
- 11.Ikuno Y, Kawaguchi K, Nouchi T, Yasuno Y. Choroidal thickness in healthy Japanese subjects. Invest Ophthalmol Vis Sci. 2010;51:2173–2176. doi: 10.1167/iovs.09-4383. [DOI] [PubMed] [Google Scholar]
- 12.Margolis R, Spaide RF. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol. 2009;147:811–815. doi: 10.1016/j.ajo.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 13.Manjunath V, Taha M, Fujimoto JG, Duker JS. Choroidal thickness in normal eyes measured using Cirrus HD optical coherence tomography. Am J Ophthalmol. 2010;150:325–329.e1. doi: 10.1016/j.ajo.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shao L, Wang Y, Xu J, Chen C, You Q, Zhou J, Xu L, B Jonas J, and Wei W. Subfoveal choroidal thickness of Chinese aged over 50 years and patients with diabetes mellitus and glaucoma. Zhonghua Yanke Zazhi. 2014;6:414–420. [PubMed] [Google Scholar]
- 15.Pongsachareonnont P, Somkijrungroj T, Assavapongpaiboon B, Chitamara T, Chuntarapas M, Suwajanakorn D. Foveal and parafoveal choroidal thickness pattern measuring by swept source optical coherence tomography. Eye (Lond) 2019;33:1443–1451. doi: 10.1038/s41433-019-0404-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding X, Li J, Zeng J, Ma W, Liu R, Li T, Yu S, Tang S. Choroidal thickness in healthy Chinese subjects. Invest Ophthalmol Vis Sci. 2011;52:9555–9560. doi: 10.1167/iovs.11-8076. [DOI] [PubMed] [Google Scholar]
- 17.Kim M, Kim SS, Koh HJ, Lee SC. Choroidal thickness, age, and refractive error in healthy Korean subjects. Optom Vis Sci. 2014;91:491–496. doi: 10.1097/OPX.0000000000000229. [DOI] [PubMed] [Google Scholar]
- 18.Lei C, Gu J, Liu L, Zhang K, Zhang M. The correlation between peripheral complete blood count parameters and diabetic macular edema in proliferative diabetic retinopathy patients: a cross-sectional study. Front Endocrinol (Lausanne) 2023;14:1190239. doi: 10.3389/fendo.2023.1190239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lei C, Ran Q, Duan J, Zhang M. The Association Between Lipid Profile and Subfoveal Choroidal Thickness in Chinese Patients with Proliferative Diabetic Retinopathy Secondary to Type 2 Diabetes. Diabetes Metab Syndr Obes. 2023;16:2477–2489. doi: 10.2147/DMSO.S419794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joint Committee for Developing Chinese guidelines on Prevention and Treatment of Dyslipidemia in Adults. [Chinese guidelines on prevention and treatment of dyslipidemia in adults] Zhonghua Xin Xue Guan Bing Za Zhi. 2007;35:390–419. [PubMed] [Google Scholar]
- 21.Zhang J, Cao J, Zhang H, Jiang C, Lin T, Zhou Z, Song Y, Li Y, Liu C, Liu L, Wang B, Tang G, Li J, Zhang Y, Cui Y, Huo Y, Yang Y, Ling W, Yang J, Guo H, Wang X, Xu X, Qin X. Plasma copper and the risk of first stroke in hypertensive patients: a nested case-control study. Am J Clin Nutr. 2019;110:212–220. doi: 10.1093/ajcn/nqz099. [DOI] [PubMed] [Google Scholar]
- 22.Lyu Y, Shah PS, Ye XY, Warre R, Piedboeuf B, Deshpandey A, Dunn M, Lee SK Canadian Neonatal Network. Association between admission temperature and mortality and major morbidity in preterm infants born at fewer than 33 weeks’ gestation. JAMA Pediatr. 2015;169:e150277. doi: 10.1001/jamapediatrics.2015.0277. [DOI] [PubMed] [Google Scholar]
- 23.Fu W, Chen C, Chen XL, Wang K, Zuo P, Liu Y, Zhang M, Zhao X, Xie S, Zhang H, Geng Y, Liu C. A U-shaped association between baseline neutrophil count and COVID-19-related mortality: A retrospective cohort study. J Med Virol. 2021;93:4265–4272. doi: 10.1002/jmv.26794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jaddoe VW, de Jonge LL, Hofman A, Franco OH, Steegers EA, Gaillard R. First trimester fetal growth restriction and cardiovascular risk factors in school age children: population based cohort study. BMJ. 2014;348:g14. doi: 10.1136/bmj.g14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kernan WN, Viscoli CM, Brass LM, Broderick JP, Brott T, Feldmann E, Morgenstern LB, Wilterdink JL, Horwitz RI. Phenylpropanolamine and the risk of hemorrhagic stroke. N Engl J Med. 2000;343:1826–1832. doi: 10.1056/NEJM200012213432501. [DOI] [PubMed] [Google Scholar]
- 26.Zeried F, Ngozika E, Al-Anazi M, Mashige K, Osuagwu U. Choroidal Thickness Measured by Ocular Coherence Tomography (SD-OCT) and Body Mass Index in Healthy Saudi Women: A Cross-sectional Controlled Study. Curr Med Imaging. 2022;18:666–673. doi: 10.2174/1573405618666220131105957. [DOI] [PubMed] [Google Scholar]
- 27.Hidayat AA, Fine BS. Diabetic choroidopathy. Light and electron microscopic observations of seven cases. Ophthalmology. 1985;92:512–522. [PubMed] [Google Scholar]
- 28.Saracco JB, Gastaud P, Ridings B, Ubaud CA. [Preliminary study on diabetic choroidopathy] Bull Soc Ophtalmol Fr. 1982;82:451–454. [PubMed] [Google Scholar]
- 29.Lutty GA. Effects of diabetes on the eye. Invest Ophthalmol Vis Sci. 2013;54:ORSF81–ORSF87. doi: 10.1167/iovs.13-12979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McLeod DS, Lutty GA. High-resolution histologic analysis of the human choroidal vasculature. Invest Ophthalmol Vis Sci. 1994;35:3799–3811. [PubMed] [Google Scholar]
- 31.Bhutto IA, Lu ZY, Takami Y, Amemiya T. Retinal and choroidal vasculature in rats with spontaneous diabetes type 2 treated with the angiotensin-converting enzyme inhibitor cilazapril: corrosion cast and electron-microscopic study. Ophthalmic Res. 2002;34:220–231. doi: 10.1159/000063877. [DOI] [PubMed] [Google Scholar]
- 32.Ferrara D, Waheed NK, Duker JS. Investigating the choriocapillaris and choroidal vasculature with new optical coherence tomography technologies. Prog Retin Eye Res. 2016;52:130–155. doi: 10.1016/j.preteyeres.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 33.Melancia D, Vicente A, Cunha JP, Abegão Pinto L, Ferreira J. Diabetic choroidopathy: a review of the current literature. Graefes Arch Clin Exp Ophthalmol. 2016;254:1453–1461. doi: 10.1007/s00417-016-3360-8. [DOI] [PubMed] [Google Scholar]
- 34.Lutty GA. Diabetic choroidopathy. Vision Res. 2017;139:161–167. doi: 10.1016/j.visres.2017.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Regatieri CV, Branchini L, Carmody J, Fujimoto JG, Duker JS. Choroidal thickness in patients with diabetic retinopathy analyzed by spectral-domain optical coherence tomography. Retina. 2012;32:563–568. doi: 10.1097/IAE.0b013e31822f5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Unsal E, Eltutar K, Zirtiloğlu S, Dinçer N, Ozdoğan Erkul S, Güngel H. Choroidal thickness in patients with diabetic retinopathy. Clin Ophthalmol. 2014;8:637–642. doi: 10.2147/OPTH.S59395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Esmaeelpour M, Brunner S, Ansari-Shahrezaei S, Nemetz S, Povazay B, Kajic V, Drexler W, Binder S. Choroidal thinning in diabetes type 1 detected by 3-dimensional 1060 nm optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53:6803–6809. doi: 10.1167/iovs.12-10314. [DOI] [PubMed] [Google Scholar]
- 38.Esmaeelpour M, Považay B, Hermann B, Hofer B, Kajic V, Hale SL, North RV, Drexler W, Sheen NJ. Mapping choroidal and retinal thickness variation in type 2 diabetes using three-dimensional 1060-nm optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52:5311–5316. doi: 10.1167/iovs.10-6875. [DOI] [PubMed] [Google Scholar]
- 39.Lee HK, Lim JW, Shin MC. Comparison of choroidal thickness in patients with diabetes by spectral-domain optical coherence tomography. Korean J Ophthalmol. 2013;27:433–439. doi: 10.3341/kjo.2013.27.6.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vujosevic S, Martini F, Cavarzeran F, Pilotto E, Midena E. Macular and peripapillary choroidal thickness in diabetic patients. Retina. 2012;32:1781–1790. doi: 10.1097/IAE.0b013e31825db73d. [DOI] [PubMed] [Google Scholar]
- 41.Huang X, Zhang P, Zou X, Xu Y, Zhu J, He J, Zhang B, Lu L, Zou H. Thinner Average Choroidal Thickness Is a Risk Factor for the Onset of Diabetic Retinopathy. Ophthalmic Res. 2020;63:259–270. doi: 10.1159/000504756. [DOI] [PubMed] [Google Scholar]
- 42.Querques G, Lattanzio R, Querques L, Del Turco C, Forte R, Pierro L, Souied EH, Bandello F. Enhanced depth imaging optical coherence tomography in type 2 diabetes. Invest Ophthalmol Vis Sci. 2012;53:6017–6024. doi: 10.1167/iovs.12-9692. [DOI] [PubMed] [Google Scholar]
- 43.Endo H, Kase S, Saito M, Yokoi M, Takahashi M, Ishida S, Kase M. Choroidal Thickness in Diabetic Patients Without Diabetic Retinopathy: A Meta-analysis. Am J Ophthalmol. 2020;218:68–77. doi: 10.1016/j.ajo.2020.05.036. [DOI] [PubMed] [Google Scholar]
- 44.Nagaoka T, Kitaya N, Sugawara R, Yokota H, Mori F, Hikichi T, Fujio N, Yoshida A. Alteration of choroidal circulation in the foveal region in patients with type 2 diabetes. Br J Ophthalmol. 2004;88:1060–1063. doi: 10.1136/bjo.2003.035345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang W, Liu S, Qiu Z, He M, Wang L, Li Y, Huang W. Choroidal Thickness in Diabetes and Diabetic Retinopathy: A Swept Source OCT Study. Invest Ophthalmol Vis Sci. 2020;61:29. doi: 10.1167/iovs.61.4.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Toyoda F, Tanaka Y, Shimmura M, Kinoshita N, Takano H, Kakehashi A. Diabetic Retinal and Choroidal Edema in SDT Rats. J Diabetes Res. 2016;2016:2345141. doi: 10.1155/2016/2345141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim JT, Lee DH, Joe SG, Kim JG, Yoon YH. Changes in choroidal thickness in relation to the severity of retinopathy and macular edema in type 2 diabetic patients. Invest Ophthalmol Vis Sci. 2013;54:3378–3384. doi: 10.1167/iovs.12-11503. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Y, Qin Y, Wang S, Liu Y, Li X, Sun X. Higher choroidal thickness and lower choriocapillaris blood flow signal density based on optical coherence tomography angiography in diabetics. Sci Rep. 2021;11:5799. doi: 10.1038/s41598-021-85065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rewbury R, Want A, Varughese R, Chong V. Subfoveal choroidal thickness in patients with diabetic retinopathy and diabetic macular oedema. Eye (Lond) 2016;30:1568–1572. doi: 10.1038/eye.2016.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tavares Ferreira J, Vicente A, Proença R, Santos BO, Cunha JP, Alves M, Papoila AL, Abegão Pinto L. Choroidal thickness in diabetic patients without diabetic retinopathy. Retina. 2018;38:795–804. doi: 10.1097/IAE.0000000000001582. [DOI] [PubMed] [Google Scholar]
- 51.Kase S, Endo H, Yokoi M, Kotani M, Katsuta S, Takahashi M, Kase M. Choroidal thickness in diabetic retinopathy in relation to long-term systemic treatments for diabetes mellitus. Eur J Ophthalmol. 2016;26:158–162. doi: 10.5301/ejo.5000676. [DOI] [PubMed] [Google Scholar]
- 52.Ghassemi F, Berijani S, Babeli A, Faghihi H, Gholizadeh A, Sabour S. The quantitative measurements of choroidal thickness and volume in diabetic retinopathy using optical coherence tomography and optical coherence tomography angiography; correlation with vision and foveal avascular zone. BMC Ophthalmol. 2022;22:3. doi: 10.1186/s12886-021-02178-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Han YS, Lim HB, Lee SH, Kim JY. Diurnal Variation in Choroidal and Retinal Thickness of the Early Treatment of Diabetic Retinopathy Study Macular Subfields Determined Using Swept-Source Optical Coherence Tomography. Ophthalmologica. 2015;233:192–197. doi: 10.1159/000375538. [DOI] [PubMed] [Google Scholar]
- 54.Tan CS, Cheong KX. Macular choroidal thicknesses in healthy adults--relationship with ocular and demographic factors. Invest Ophthalmol Vis Sci. 2014;55:6452–6458. doi: 10.1167/iovs.13-13771. [DOI] [PubMed] [Google Scholar]
- 55.Brown JS, Flitcroft DI, Ying GS, Francis EL, Schmid GF, Quinn GE, Stone RA. In vivo human choroidal thickness measurements: evidence for diurnal fluctuations. Invest Ophthalmol Vis Sci. 2009;50:5–12. doi: 10.1167/iovs.08-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Campos A, Campos EJ, Martins J, Ambrósio AF, Silva R. Viewing the choroid: where we stand, challenges and contradictions in diabetic retinopathy and diabetic macular oedema. Acta Ophthalmol. 2017;95:446–459. doi: 10.1111/aos.13210. [DOI] [PubMed] [Google Scholar]
- 57.Ohara Z, Tabuchi H, Nakakura S, Yoshizumi Y, Sumino H, Maeda Y, Kiuchi Y. Changes in choroidal thickness in patients with diabetic retinopathy. Int Ophthalmol. 2018;38:279–286. doi: 10.1007/s10792-017-0459-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nourinia R, Ahmadieh H, Nekoei E, Malekifar P, Tofighi Z. Changes in central choroidal thickness after treatment of diabetic macular edema with intravitreal bevacizumab correlation with central macular thickness and best-corrected visual acuity. Retina. 2018;38:970–975. doi: 10.1097/IAE.0000000000001645. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on reasonable request.