Abstract
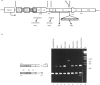
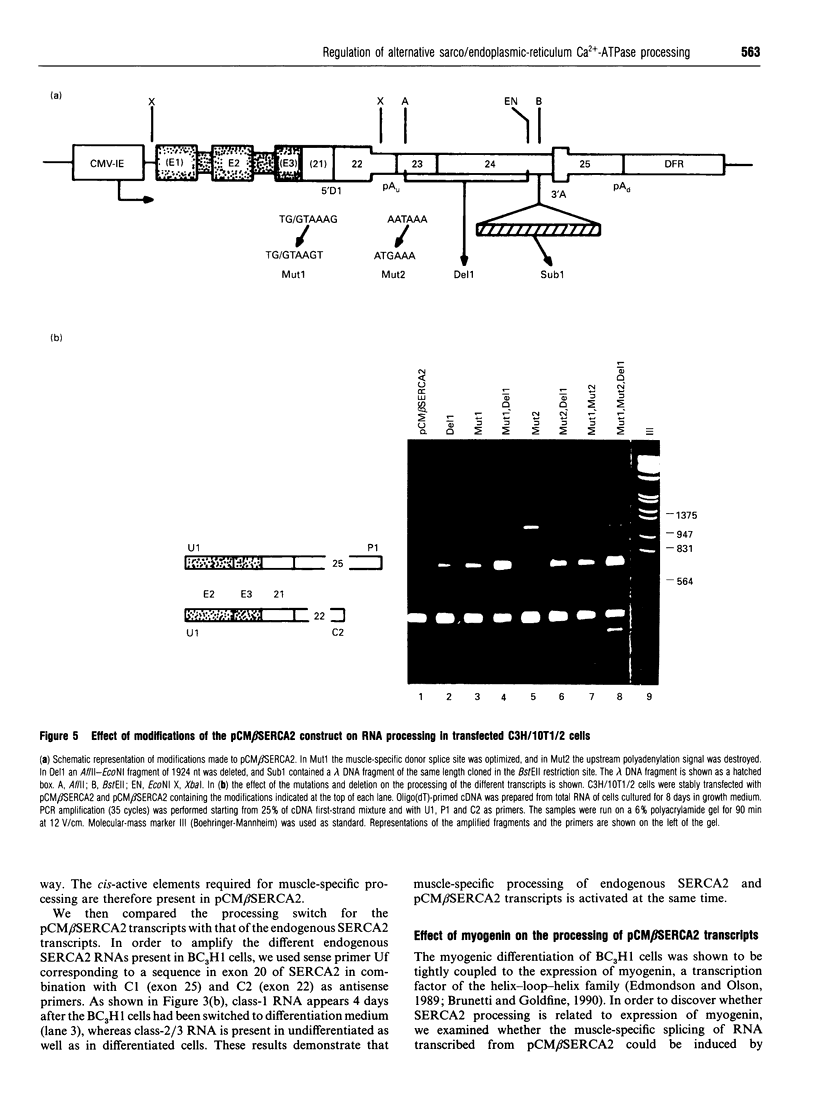
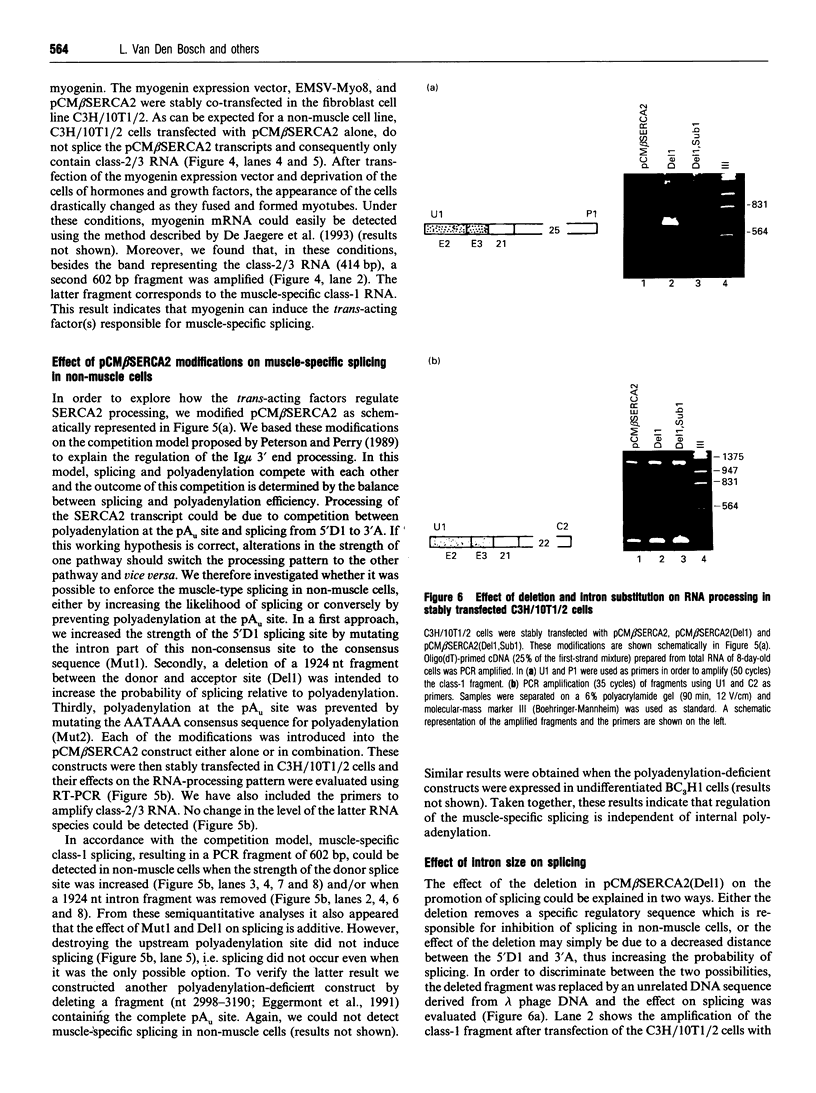
Tissue-specific alternative processing of sarco/endoplasmic reticulum Ca(2+)-ATPase 2 (SERCA2) transcripts generates functionally different Ca2+ pump isoforms in muscle compared with non-muscle tissues. In non-muscle cells, the SERCA2 pre-mRNA can be polyadenylated at a site located between the donor and acceptor splice site of an intron which is only removed in muscle tissues. To define the cis-active elements involved in differential processing, we constructed a minigene (pCM beta SERCA2) containing the 3' end of the SERCA2 gene. When stably transfected into a myogenic cell line, minigene transcripts were differentially processed depending on the differentiation state of the cells. This proves that the essential elements required for regulated processing are present in the construct. Furthermore, co-transfection of the pCM beta SERCA2 minigene and a myogenin expression vector in a fibroblast cell line induced muscle-specific splicing of transcripts from pCM beta SERCA2. This shows that trans-acting factor(s) responsible for muscle-specific processing can be induced by one of the important regulatory genes of muscle differentiation. Inactivation of the non-muscle poly(A) site did not induce splicing in non-muscle cells. This excludes a simple competition model between splicing and polyadenylation, but it is consistent with splicing being very inefficient in non-muscle cells. Moreover, splicing could be induced in non-muscle cells by optimizing the muscle-specific donor splice site and/or by shortening the intron length. We therefore propose that expression of the muscle-specific SERCA2a isoform is the result of activation of an otherwise inefficient splicing process.
Full text
PDF
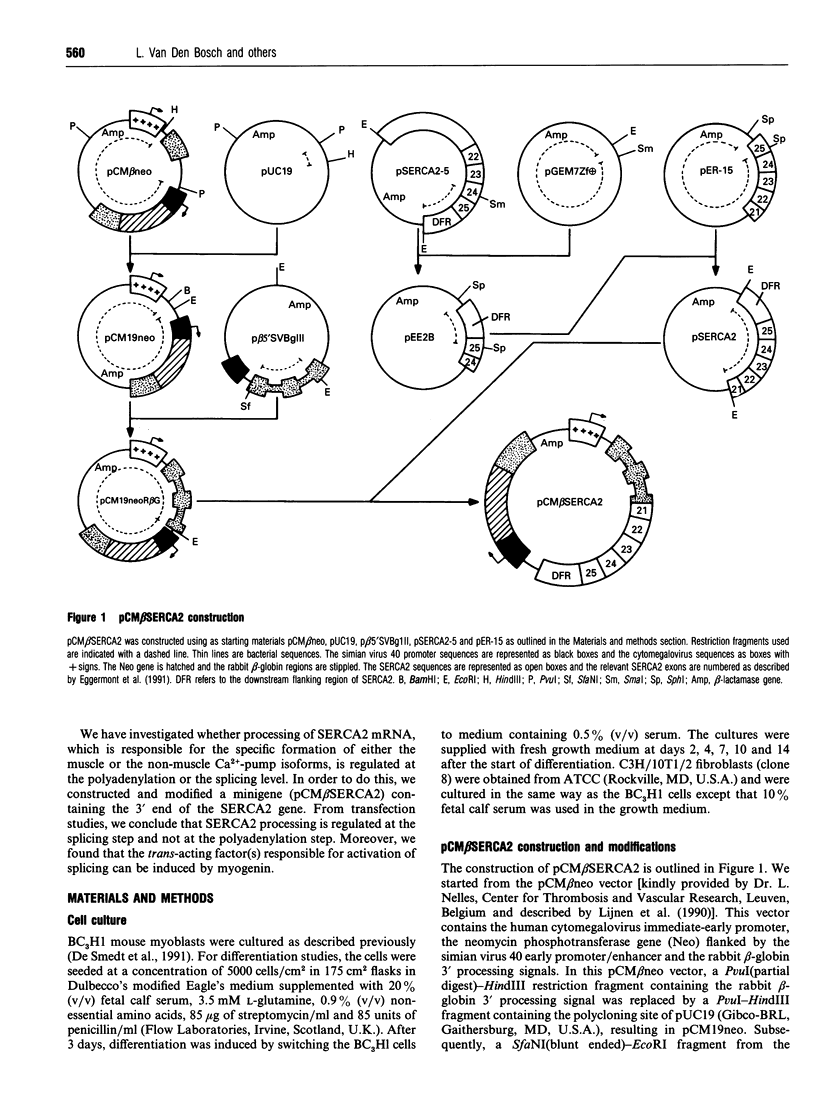

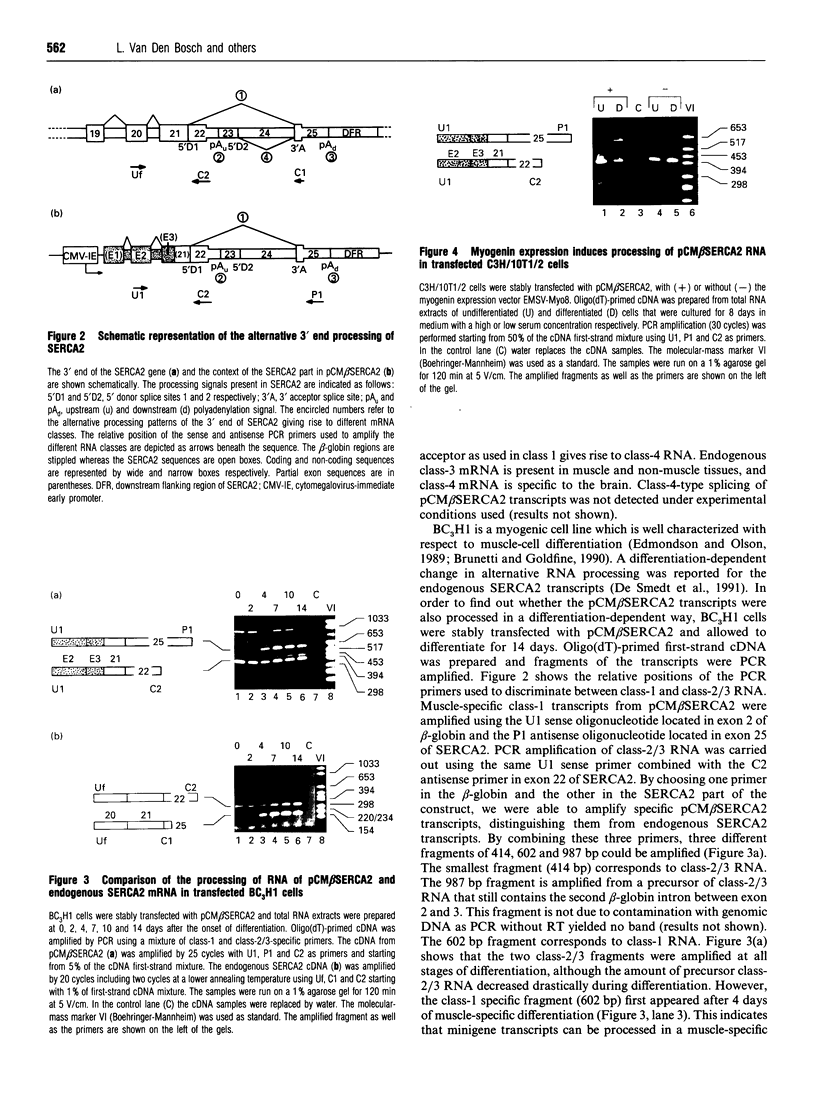


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Bothwell A. L., Knapp M., Siden E., Mather E., Koshland M., Baltimore D. Synthesis of secreted and membrane-bound immunoglobulin mu heavy chains is directed by mRNAs that differ at their 3' ends. Cell. 1980 Jun;20(2):293–301. doi: 10.1016/0092-8674(80)90615-7. [DOI] [PubMed] [Google Scholar]
- Arai M., Otsu K., MacLennan D. H., Periasamy M. Regulation of sarcoplasmic reticulum gene expression during cardiac and skeletal muscle development. Am J Physiol. 1992 Mar;262(3 Pt 1):C614–C620. doi: 10.1152/ajpcell.1992.262.3.C614. [DOI] [PubMed] [Google Scholar]
- Brandl C. J., Green N. M., Korczak B., MacLennan D. H. Two Ca2+ ATPase genes: homologies and mechanistic implications of deduced amino acid sequences. Cell. 1986 Feb 28;44(4):597–607. doi: 10.1016/0092-8674(86)90269-2. [DOI] [PubMed] [Google Scholar]
- Brandl C. J., deLeon S., Martin D. R., MacLennan D. H. Adult forms of the Ca2+ATPase of sarcoplasmic reticulum. Expression in developing skeletal muscle. J Biol Chem. 1987 Mar 15;262(8):3768–3774. [PubMed] [Google Scholar]
- Brunetti A., Goldfine I. D. Role of myogenin in myoblast differentiation and its regulation by fibroblast growth factor. J Biol Chem. 1990 Apr 15;265(11):5960–5963. [PubMed] [Google Scholar]
- Burk S. E., Lytton J., MacLennan D. H., Shull G. E. cDNA cloning, functional expression, and mRNA tissue distribution of a third organellar Ca2+ pump. J Biol Chem. 1989 Nov 5;264(31):18561–18568. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Danner D., Leder P. Role of an RNA cleavage/poly(A) addition site in the production of membrane-bound and secreted IgM mRNA. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8658–8662. doi: 10.1073/pnas.82.24.8658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jaegere S., Wuytack F., De Smedt H., Van den Bosch L., Casteels R. Alternative processing of the gene transcripts encoding a plasma-membrane and a sarco/endoplasmic reticulum Ca2+ pump during differentiation of BC3H1 muscle cells. Biochim Biophys Acta. 1993 May 28;1173(2):188–194. doi: 10.1016/0167-4781(93)90180-l. [DOI] [PubMed] [Google Scholar]
- Edmondson D. G., Olson E. N. A gene with homology to the myc similarity region of MyoD1 is expressed during myogenesis and is sufficient to activate the muscle differentiation program. Genes Dev. 1989 May;3(5):628–640. doi: 10.1101/gad.3.5.628. [DOI] [PubMed] [Google Scholar]
- Edmondson D. G., Olson E. N. Helix-loop-helix proteins as regulators of muscle-specific transcription. J Biol Chem. 1993 Jan 15;268(2):755–758. [PubMed] [Google Scholar]
- Eggermont J. A., Wuytack F., Casteels R. Characterization of the 3' end of the pig sarcoplasmic/endoplasmic-reticulum Ca2+ pump gene 2. Biochim Biophys Acta. 1991 Mar 26;1088(3):448–451. doi: 10.1016/0167-4781(91)90143-a. [DOI] [PubMed] [Google Scholar]
- Eggermont J. A., Wuytack F., Casteels R. Characterization of the mRNAs encoding the gene 2 sarcoplasmic/endoplasmic-reticulum Ca2+ pump in pig smooth muscle. Biochem J. 1990 Mar 15;266(3):901–907. [PMC free article] [PubMed] [Google Scholar]
- Eggermont J. A., Wuytack F., De Jaegere S., Nelles L., Casteels R. Evidence for two isoforms of the endoplasmic-reticulum Ca2+ pump in pig smooth muscle. Biochem J. 1989 Jun 15;260(3):757–761. doi: 10.1042/bj2600757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emeson R. B., Hedjran F., Yeakley J. M., Guise J. W., Rosenfeld M. G. Alternative production of calcitonin and CGRP mRNA is regulated at the calcitonin-specific splice acceptor. Nature. 1989 Sep 7;341(6237):76–80. doi: 10.1038/341076a0. [DOI] [PubMed] [Google Scholar]
- Eperon I. C., Ireland D. C., Smith R. A., Mayeda A., Krainer A. R. Pathways for selection of 5' splice sites by U1 snRNPs and SF2/ASF. EMBO J. 1993 Sep;12(9):3607–3617. doi: 10.1002/j.1460-2075.1993.tb06034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli G., Guise J., Tucker P. W., Nevins J. R. Poly(A) site choice rather than splice site choice governs the regulated production of IgM heavy-chain RNAs. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2439–2443. doi: 10.1073/pnas.85.8.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosveld G. C., de Boer E., Shewmaker C. K., Flavell R. A. DNA sequences necessary for transcription of the rabbit beta-globin gene in vivo. Nature. 1982 Jan 14;295(5845):120–126. doi: 10.1038/295120a0. [DOI] [PubMed] [Google Scholar]
- Gunteski-Hamblin A. M., Greeb J., Shull G. E. A novel Ca2+ pump expressed in brain, kidney, and stomach is encoded by an alternative transcript of the slow-twitch muscle sarcoplasmic reticulum Ca-ATPase gene. Identification of cDNAs encoding Ca2+ and other cation-transporting ATPases using an oligonucleotide probe derived from the ATP-binding site. J Biol Chem. 1988 Oct 15;263(29):15032–15040. [PubMed] [Google Scholar]
- Hasty P., Bradley A., Morris J. H., Edmondson D. G., Venuti J. M., Olson E. N., Klein W. H. Muscle deficiency and neonatal death in mice with a targeted mutation in the myogenin gene. Nature. 1993 Aug 5;364(6437):501–506. doi: 10.1038/364501a0. [DOI] [PubMed] [Google Scholar]
- Hoshijima K., Inoue K., Higuchi I., Sakamoto H., Shimura Y. Control of doublesex alternative splicing by transformer and transformer-2 in Drosophila. Science. 1991 May 10;252(5007):833–836. doi: 10.1126/science.1902987. [DOI] [PubMed] [Google Scholar]
- Leff S. E., Evans R. M., Rosenfeld M. G. Splice commitment dictates neuron-specific alternative RNA processing in calcitonin/CGRP gene expression. Cell. 1987 Feb 13;48(3):517–524. doi: 10.1016/0092-8674(87)90202-9. [DOI] [PubMed] [Google Scholar]
- Lijnen H. R., Nelles L., Van Hoef B., De Cock F., Collen D. Biochemical and functional characterization of human tissue-type plasminogen activator variants obtained by deletion and/or duplication of structural/functional domains. J Biol Chem. 1990 Apr 5;265(10):5677–5683. [PubMed] [Google Scholar]
- Lompre A. M., de la Bastie D., Boheler K. R., Schwartz K. Characterization and expression of the rat heart sarcoplasmic reticulum Ca2+-ATPase mRNA. FEBS Lett. 1989 May 22;249(1):35–41. doi: 10.1016/0014-5793(89)80010-9. [DOI] [PubMed] [Google Scholar]
- Lytton J., MacLennan D. H. Molecular cloning of cDNAs from human kidney coding for two alternatively spliced products of the cardiac Ca2+-ATPase gene. J Biol Chem. 1988 Oct 15;263(29):15024–15031. [PubMed] [Google Scholar]
- Lytton J., Westlin M., Burk S. E., Shull G. E., MacLennan D. H. Functional comparisons between isoforms of the sarcoplasmic or endoplasmic reticulum family of calcium pumps. J Biol Chem. 1992 Jul 15;267(20):14483–14489. [PubMed] [Google Scholar]
- Mayeda A., Krainer A. R. Regulation of alternative pre-mRNA splicing by hnRNP A1 and splicing factor SF2. Cell. 1992 Jan 24;68(2):365–375. doi: 10.1016/0092-8674(92)90477-t. [DOI] [PubMed] [Google Scholar]
- McKeown M. Alternative mRNA splicing. Annu Rev Cell Biol. 1992;8:133–155. doi: 10.1146/annurev.cb.08.110192.001025. [DOI] [PubMed] [Google Scholar]
- Nabeshima Y., Hanaoka K., Hayasaka M., Esumi E., Li S., Nonaka I., Nabeshima Y. Myogenin gene disruption results in perinatal lethality because of severe muscle defect. Nature. 1993 Aug 5;364(6437):532–535. doi: 10.1038/364532a0. [DOI] [PubMed] [Google Scholar]
- Olson E. N. MyoD family: a paradigm for development? Genes Dev. 1990 Sep;4(9):1454–1461. doi: 10.1101/gad.4.9.1454. [DOI] [PubMed] [Google Scholar]
- Peterson M. L., Gimmi E. R., Perry R. P. The developmentally regulated shift from membrane to secreted mu mRNA production is accompanied by an increase in cleavage-polyadenylation efficiency but no measurable change in splicing efficiency. Mol Cell Biol. 1991 Apr;11(4):2324–2327. doi: 10.1128/mcb.11.4.2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson M. L., Perry R. P. Regulated production of mu m and mu s mRNA requires linkage of the poly(A) addition sites and is dependent on the length of the mu s-mu m intron. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8883–8887. doi: 10.1073/pnas.83.23.8883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson M. L., Perry R. P. The regulated production of mu m and mu s mRNA is dependent on the relative efficiencies of mu s poly(A) site usage and the c mu 4-to-M1 splice. Mol Cell Biol. 1989 Feb;9(2):726–738. doi: 10.1128/mcb.9.2.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plessers L., Eggermont J. A., Wuytack F., Casteels R. A study of the organellar Ca2(+)-transport ATPase isozymes in pig cerebellar Purkinje neurons. J Neurosci. 1991 Mar;11(3):650–656. doi: 10.1523/JNEUROSCI.11-03-00650.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryner L. C., Baker B. S. Regulation of doublesex pre-mRNA processing occurs by 3'-splice site activation. Genes Dev. 1991 Nov;5(11):2071–2085. doi: 10.1101/gad.5.11.2071. [DOI] [PubMed] [Google Scholar]
- Saitoh O., Olson E. N., Periasamy M. Muscle-specific RNA processing continues in the absence of myogenin expression. J Biol Chem. 1990 Nov 15;265(32):19381–19384. [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Hong G. F., Hill D. F., Petersen G. B. Nucleotide sequence of bacteriophage lambda DNA. J Mol Biol. 1982 Dec 25;162(4):729–773. doi: 10.1016/0022-2836(82)90546-0. [DOI] [PubMed] [Google Scholar]
- Shapiro M. B., Senapathy P. RNA splice junctions of different classes of eukaryotes: sequence statistics and functional implications in gene expression. Nucleic Acids Res. 1987 Sep 11;15(17):7155–7174. doi: 10.1093/nar/15.17.7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. W., Patton J. G., Nadal-Ginard B. Alternative splicing in the control of gene expression. Annu Rev Genet. 1989;23:527–577. doi: 10.1146/annurev.ge.23.120189.002523. [DOI] [PubMed] [Google Scholar]
- Tian M., Maniatis T. Positive control of pre-mRNA splicing in vitro. Science. 1992 Apr 10;256(5054):237–240. doi: 10.1126/science.1566072. [DOI] [PubMed] [Google Scholar]
- Tsurushita N., Korn L. J. Effects of intron length on differential processing of mouse mu heavy-chain mRNA. Mol Cell Biol. 1987 Jul;7(7):2602–2605. doi: 10.1128/mcb.7.7.2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verboomen H., Wuytack F., De Smedt H., Himpens B., Casteels R. Functional difference between SERCA2a and SERCA2b Ca2+ pumps and their modulation by phospholamban. Biochem J. 1992 Sep 1;286(Pt 2):591–595. doi: 10.1042/bj2860591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K. D., Lytton J. Molecular cloning and quantification of sarcoplasmic reticulum Ca(2+)-ATPase isoforms in rat muscles. Am J Physiol. 1993 Feb;264(2 Pt 1):C333–C341. doi: 10.1152/ajpcell.1993.264.2.C333. [DOI] [PubMed] [Google Scholar]
- Wuytack F., Raeymaekers L., De Smedt H., Eggermont J. A., Missiaen L., Van Den Bosch L., De Jaegere S., Verboomen H., Plessers L., Casteels R. Ca(2+)-transport ATPases and their regulation in muscle and brain. Ann N Y Acad Sci. 1992 Nov 30;671:82–91. doi: 10.1111/j.1749-6632.1992.tb43786.x. [DOI] [PubMed] [Google Scholar]
- Zahler A. M., Lane W. S., Stolk J. A., Roth M. B. SR proteins: a conserved family of pre-mRNA splicing factors. Genes Dev. 1992 May;6(5):837–847. doi: 10.1101/gad.6.5.837. [DOI] [PubMed] [Google Scholar]
- Zahler A. M., Neugebauer K. M., Lane W. S., Roth M. B. Distinct functions of SR proteins in alternative pre-mRNA splicing. Science. 1993 Apr 9;260(5105):219–222. doi: 10.1126/science.8385799. [DOI] [PubMed] [Google Scholar]
- de Smedt H., Eggermont J. A., Wuytack F., Parys J. B., Van den Bosch L., Missiaen L., Verbis J., Casteels R. Isoform switching of the sarco(endo)plasmic reticulum Ca2+ pump during differentiation of BC3H1 myoblasts. J Biol Chem. 1991 Apr 15;266(11):7092–7095. [PubMed] [Google Scholar]
- de la Bastie D., Wisnewsky C., Schwartz K., Lompré A. M. (Ca2+ + Mg2+)-dependent ATPase mRNA from smooth muscle sarcoplasmic reticulum differs from that in cardiac and fast skeletal muscles. FEBS Lett. 1988 Feb 29;229(1):45–48. doi: 10.1016/0014-5793(88)80794-4. [DOI] [PubMed] [Google Scholar]
- van Ooyen A., van den Berg J., Mantei N., Weissmann C. Comparison of total sequence of a cloned rabbit beta-globin gene and its flanking regions with a homologous mouse sequence. Science. 1979 Oct 19;206(4416):337–344. doi: 10.1126/science.482942. [DOI] [PubMed] [Google Scholar]