Abstract
Objective:
Naltrexone and nalmefene are approved for the treatment of alcohol use disorders, in different countries. Naltrexone is also approved for the treatment for opioid use disorders, most recently in a depot formulation. These compounds target primarily μ(mu)- and κ(kappa)-opioid receptor systems, which are involved in the downstream neurobiological effects of alcohol and in the modulation of neuroendocrine Stress systems. The objective was to compare the neuroendocrine effects of naltrexone and nalmefene on adrenocorticotropic hormone (ACTH), cortisol, and prolactin, in normal volunteers.
Method:
Adult normal volunteers (n = 11 male and n = 9 female) were studied in a stress-minimized inpatient setting on three consecutive days, after intravenous saline, naltrexone HCl (10 mg), or nalmefene HCl (10 mg), in fixed order. ACTH, cortisol, and prolactin were analyzed pre-injection and up to 180 min post-injection.
Results:
Naltrexone and nalmefene caused elevations in ACTH and cortisol compared with saline. Nalmefene had a greater effect on ACTH and cortisol, compared with naltrexone. Both compounds also caused elevations in prolactin in males (females were not examined, due to the influence of menstrual cycle on prolactin).
Conclusions:
This study suggests that both nalmefene and naltrexone have effects potentially due to κ-partial agonism in humans, as well as antagonist effects at μ-receptors.
Keywords: ACTH, cortisol, nalmefene, naltrexone, neuroendocrine, prolactin
INTRODUCTION
Naltrexone and nalmefene are pharmacotherapies approved for the treatment of alcohol use disorders (AUD), in different countries (Castera et al., 2018; Mann, Bladstrom, Torup, Gual, & van den Brink, 2013; Soyka, Friede, & Schnitker, 2016). Naltrexone is also approved for the treatment of opioid use disorders, most recently as an intramuscular depot formulation (Lee et al., 2017; Woody & Metzger, 2011). Very recent studies have also examined whether novel formulations of naltrexone or nalmefene could be useful as potent and long-lasting medications against overdose caused by μ-agonists such as fentanyl (Krieter, Chiang, Gyaw, Skolnick, & Snyder, 2019; Krieter, Gyaw, Crystal, & Skolnick, 2019). Studies have detected changes in μ-receptor systems in the brain of humans with AUD (Hermann et al., 2017; Nestor et al., 2018). Exposure to either alcohol or other drugs of abuse can also result in upregulation in transcription of the gene coding for κ-receptors (OPRK1), and for the endogenous κ-agonist neuropeptides, the dynorphins (prodynorphin gene; Daunais, Roberts, & McGinty, 1993; Fagergren et al., 2003; Karkhanis, Holleran, & Jones, 2017; Mash & Staley, 1999; Spangler, Unterwald, & Kreek, 1993; Wee & Koob, 2010).
Naltrexone and nalmefene are potent μ-antagonists, in vitro and in vivo (Bart et al., 2005; Kaplan & Marx, 1993; Ko, Butelman, Traynor, & Woods, 1998; Wang, Sun, & Sadee, 2007; Wentland et al., 2009). Naltrexone and nalmefene also have considerable affinity at κ-receptors, where they can exert partial agonist effects in vitro (e.g., in GTPgammaS assays; Bart et al., 2005; Glass, Jhaveri, & Smith, 1994; Gual, He, Torup, van den Brink, & Mann, 2013; Remmers et al., 1999; Wentland et al., 2009). Studies in cloned human receptors indicate that the affinity of naltrexone is greater at μ- versus κ-receptors, although this finding is not as robust for nalmefene (Bart et al., 2005; Bidlack, 2014; Ghirmai, Azar, Polgar, Berzetei-Gurske, & Cashman, 2008; Wang et al., 2007). By comparison, both naltrexone and nalmefene have lower affinity at δ(delta)-opioid receptors (Bart et al., 2005; Wang et al., 2007; Wentland et al., 2009). Neuroendocrine effects are useful biomarkers for the investigation of pharmacotherapies which target opioid receptor systems. Hypothalamic–pituitary–adrenal (HPA) stress axis hormones (e.g., adrenocorticotropic hormone [ACTH] and cortisol in humans) are modulated by both μ and κ systems. Thus, μ-antagonists cause ACTH and cortisol release (Schluger et al., 1998). Selective κ-agonists also cause increases in HPA axis hormones (Maqueda et al., 2016; Pascoe et al., 2008). HPA axis hormones are associated with neurobehavioral aspects of both AUD and opioid use disorders (Sinha, Garcia, Paliwal, Kreek, & Rounsaville, 2006; Stephens & Wand, 2012). A prior study also shows that oral naltrexone administration results in an increase of ACTH and cortisol levels in humans with alcohol dependence (O’Malley, Krishnan-Sarin, Farren, Sinha, & Kreek, 2002). Furthermore, these cortisol levels were negatively correlated with alcohol craving (O’Malley et al., 2002). A very recent positron emission tomography neuroimaging study also showed that naltrexone-induced reduction in alcohol intake in heavy drinkers was negatively correlated with baseline κ-receptor availability in the striatum (de Laat et al., 2019). κ-agonists also cause dose-dependent increases in serum prolactin levels in vivo in humans and animals, and the potency and maximal effects of κ-receptor ligands on this neuroendocrine biomarker correlate with those observed in other assays (Butelman, Harris, & Kreek, 1999; Chang et al., 2011; Kreek, Schluger, Borg, Gunduz, & Ho, 1999). For example, partial κ-agonists cause smaller maximal prolactin release than high efficacy κ-agonists (Butelman et al., 1999). μ-agonists also cause dose-dependent increases in serum prolactin in humans (Hoehe, Duka, Doenicke, & Matussek, 1984; Kreek, 1978). However, comparative data on the effects of naltrexone and nalmefene in humans are extremely limited (Soyka et al., 2016). To our knowledge, there has been only one study that directly compared an effect of nalmefene and naltrexone in humans (Drobes, Anton, Thomas, & Voronin, 2004; Soyka et al., 2016). This is the first study to compare neuroendocrine effects of naltrexone and nalmefene in humans, using an intravenous (IV) route of administration. This route of administration should minimize difficulties in interpretation caused by differential oral pharmacokinetic and bioavailability profiles of these medications.
METHODS
Volunteers
This protocol, informed consent forms, and advertisements for the study were approved by the local Institutional Review Board, and in accordance with the Declaration of Helsinki. Male and female volunteers were recruited from the New York City area through Institutional Review Board-approved newspaper advertisements and flyers. The group studied herein was composed of n = 20 adult volunteers (n = 9 female), without any lifetime diagnosis of any substance use disorder, by DSM-IV criteria. Volunteers were examined in the hospital clinic on at least two occasions before participating in this inpatient study. Volunteers received medical and psychiatric evaluations by a clinician and included clinical interviews, a physical exam, and electrocardiogram. Laboratory testing included a complete blood count, blood chemistries, hepatitis serology, liver function, thyroid function testing, and an HIV antibody test. HIV-positive persons were excluded from this study, because HIV infection is known to alter endocrine function. Urine for toxicology from each clinic visit, as well as 24-hr urine samples while inpatient, were used to examine for illicit substances. Nicotine replacement therapy (e.g., nicotine patch) was offered to the volunteers while in study, because smoking was not allowed in the unit. A breathalyzer test was carried out on the night of admission, to confirm no recent ingestion of alcohol.
Diagnostic criteria
Volunteers were initially categorized with the structured clinical interview for DSM-IV criteria (SCID-I), by a clinician (e.g., nurse practitioner). Alcohol and tobacco use were also characterized with Kreek–McHugh–Schluger–Kellogg scales (measuring maximum lifetime exposure to drugs), a dimensional measure of maximal self-exposure to specific substances (Butelman et al., 2018; Kellogg et al., 2003; Tang et al., 2011).
PROCEDURES
Volunteers were admitted to a smoke-free private room, the night prior to the first study day. All study procedures took place in the volunteer’s room, and no passes were allowed. On each of the three following mornings, an indwelling 20 Gauge IV catheter (BD Angiocath, Becton-Dickinson, NJ) was inserted at least an hour prior to testing. The catheter was used for injection of saline, naltrexone, or nalmefene solutions and for blood sampling. The volunteers were studied under three conditions on consecutive days, in fixed order (Day 1: IV isotonic saline; Day 2: IV naltrexone HCl 10 mg; and Day 3: IV nalmefene HCl 10 mg). This fixed order design was justified by the expected greater duration of action of IV nalmefene versus IV naltrexone (Dixon et al., 1986; Wall, Brine, & Perez-Reyes, 1981) and was selected in order to minimize the risk of “carryover” from one experimental day to the next. Naltrexone HCl and nalmefene HCl were compounded by Greenpark Pharmacy (Houston, TX). All solutions were injected IV over 2 min, at 10:00 (±30 min). Blood samples for neuroendocrine biomarker (ACTH, cortisol, and prolactin) analysis were obtained at specific time points: −10 min and immediately prior to IV injection (time “0”), and at 10, 20, 30, 40, 60, 75, 90, 120, and 180 min post-injection. Blood for plasma samples was placed in EDTA-coated vacutainer tubes and centrifuged within 30 min. Blood for serum samples was placed in a plain vacutainer and centrifuged. Plasma and serum samples were then immediately stored frozen at −40_C until neuroendocrine biomarker analysis (see below). Intra-assay and inter-assay coefficients of variation were recently reported (Reed, Butelman, Fry, Kimani, & Kreek, 2017).
Plasma ACTH was quantified by our laboratory with a radioimmunoassay kit (DiaSorin; Saluggia, Italy), following manufacturer’s instructions, on Packard/COBRA Gamma Counters. Cortisol Serum cortisol was quantified by the Clinical Chemistry Service at the Department of Laboratory Medicine, Memorial Sloan Kettering Cancer Center, New York, NY, USA. Measurements were obtained with an automated immunoassay analyzer (AIA 360, Tosoh Bioscience, South San Francisco, CA). Serum prolactin was quantified in duplicate in our laboratory, using human prolactin immunoradiometric kits (MP Biomedicals, Santa Ana, CA), following manufacturer’s instructions, on Packard/COBRA Gamma Counters.
Visual analog scales for subjective mood effects
Visual analog scales (10 cm length, unmarked) were provided for scoring by the volunteers, 20 min prior to injection and at specific time points after injection (i.e., 30, 60, 90, 120, 150, 240, and 360 min). The scales were marked for “mood” self-report (marked “terrible” on the left to “terrific” on the right). This acute study was designed to focus principally on the aforementioned neuroendocrine measures rather than on potential subjective effects of these compounds on mood scores.
Statistical analysis
Neuroendocrine data are presented graphically as full time courses and also as area under the time-effect curve (0–180 min after injection), calculated with the trapezoidal rule (Graphpad Prism). The hormone level at time “0” was used as the baseline for these area under the curve (AUC) calculations. Time course data were analyzed with two-way repeated measures analyses of variance (ANOVAs; treatment day × time). AUC data were analyzed with one-way repeated measures ANOVA. Time course data for mood scores were analyzed with a two-way repeated measures ANOVA (treatment day × time). Post hoc comparisons were used as applicable. The level for significance (α) was set at the p 0.05 level.
Missing data
For situations in which a missing value occurred at post-injection time points, the whole data set was excluded from analysis (i.e., case-wise deletion).
RESULTS
Adverse events
There were no serious adverse events in any of these studies, and none required volunteer exiting from study. Reported events included experiencing a hot flash, flushing and/or sweating, or change of mood (both positive and negative), as well as headache, dizziness, and drowsiness.
Demographic data
Summary demographic details are in Table 1. This group of normal volunteers reported a normative range of alcohol and tobacco exposure, as expected.
TABLE 1.
Demographicsa
| Normal volunteers (n = 20) |
| Sex Male (n = 11) |
| Female (n = 9) |
| Ethnicity Black non-Hispanic (n = 9) |
| Black Hispanic (n = 5) |
| White non-Hispanic (n = 2) |
| White Hispanic (n = 1) |
| Asian (n = 1) |
| Mixed/other (n = 2) |
| Mean age (SEM) 41 (2.3) |
| Alcohol KMSK scoreb: median (IQR) 4 (2–8.5) |
| Tobacco KMSK scoreb: median (IQR) 0 (0–4.5) |
Abbreviations:
IQR: interquartile range; KMSK: Kreek–McHugh–Schluger–Kellogg scale (measuring maximum lifetime exposure to drugs); SEM, standard error of the mean.
Missing KMSK scores for n = 3.
KMSK scales are ordinal integer measures of maximal self-exposure to each drug, for the period in a persons’ life when use was the heaviest. A score of “0” indicates no lifetime use of the drug; the maximum scores for alcohol and tobacco is 13 (Butelman et al., 2018; Kellogg et al., 2003; Tang et al., 2011).
Neuroendocrine data
ACTH
Both naltrexone and nalmefene caused time-dependent increases in ACTH, compared with saline. ACTH data from male and female volunteers were compared and did not differ from each other (not shown); therefore, we present results for males and females combined. Figure 1a shows the time course of ACTH levels. A two-way repeated measures ANOVA (treatment day × time) was significant for treatment day, F(2, 30) = 14.12, p < .001, and time, F(12, 180) = 4.25, p < .001 main effects, and for their interaction, F(24, 360) = 5.19, p < .001. Tukey’s tests show that nalmefene had greater effects than both naltrexone and saline and that naltrexone had greater effects than saline. More specifically, nalmefene was different from saline at all time points from 10 to 120 min after injection. Naltrexone was different from saline for a shorter period, 20 to 90 min after injection. Nalmefene was different from naltrexone for the time points 10 to 60 min after injection. These ACTH data were then analyzed as AUC for the period 0 to 180 min (see Figure 1b). A one-way repeated measures ANOVA for ACTH AUC data was significant for the effect of treatment day, F(2, 30) = 12.33, p < .001. Tukey’s tests for AUC data revealed that nalmefene was greater than both naltrexone and saline.
FIGURE 1:
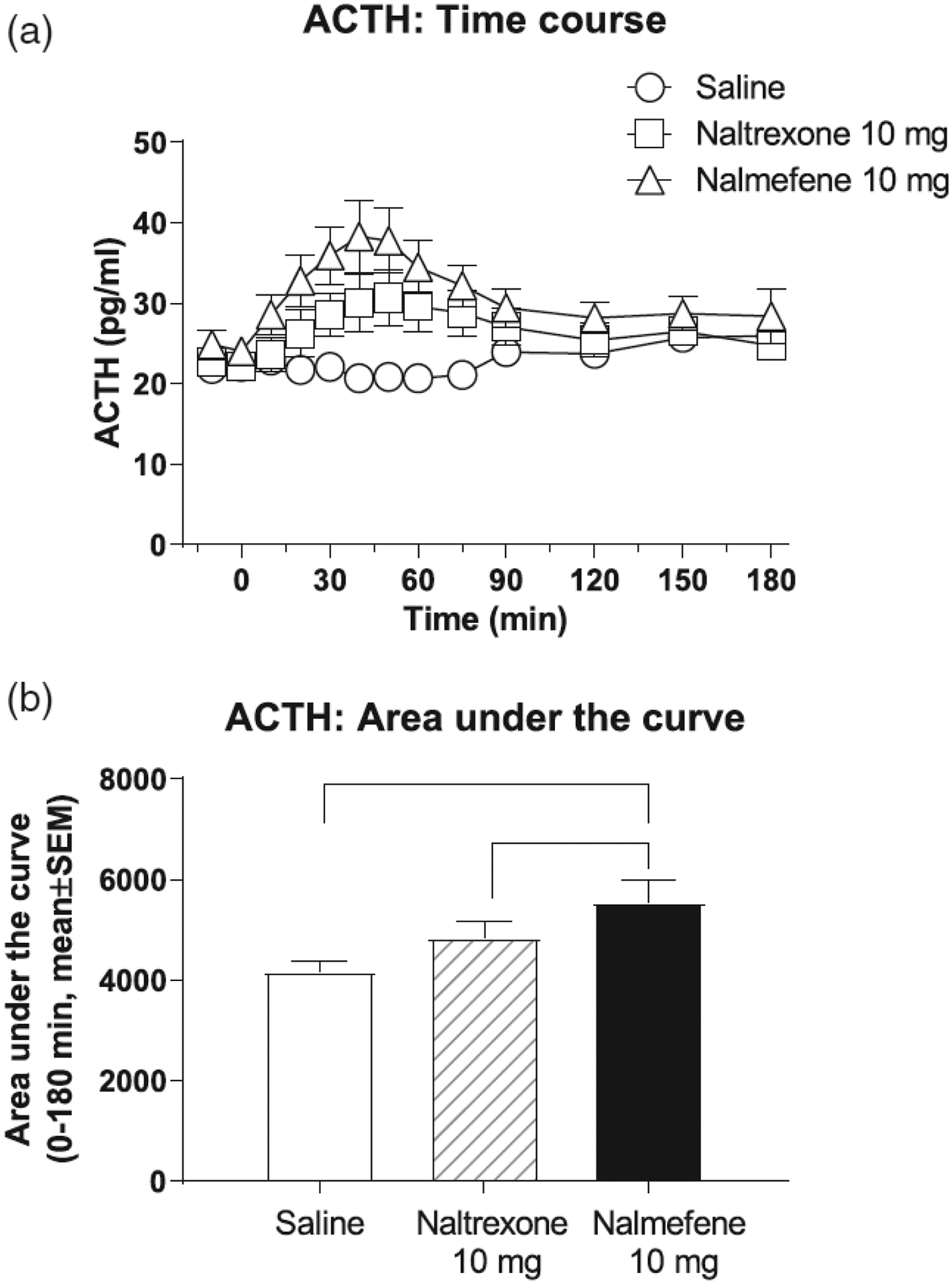
Effects of intravenous saline, naltrexone, and nalmefene on adrenocorticotropic hormone (ACTH), in male and female volunteers. Data (n = 16) are presented as mean ± standard error of the mean. Time course data (a): A two-way repeated measures ANOVA was significant for treatment day, F(2, 30) = 14.12, p < .001, and time, F(12, 180) = 4.25, p < .001, main effects, and for their interaction, F(24, 360) = 5.19, p < .001. Area under the curve (AUC) data for the period 0 to 180 min (b): A one-way repeated measures ANOVA was significant for treatment day, F(2, 30) = 12.33, p < .001. Black lines in panel (b) show significant Tukey post hoc tests.
Cortisol
Cortisol data from male and female volunteers were compared and did not differ from each other (not shown); therefore, we present results for males and females combined. Both naltrexone and nalmefene caused time-dependent increases in cortisol, compared with saline (Figure 2a). A two-way repeated measures ANOVA was significant for treatment, F(2, 34) = 21.73, p < .0001, and time, F(12, 204) = 4.30, p < .0001 main effects and for their interaction, F(24, 408) = 7.71, p < .0001. Tukey’s tests show that the effects of nalmefene were greater than saline for all time points from 20–180 min. Naltrexone had greater effects than saline for a shorter period, 30–90 min. Also, nalmefene had greater effects compared with naltrexone, for time points 20–150 min after injection. An AUC analysis of cortisol data was completed for the 0–180 min period (Figure 2b). A one-way repeated measures ANOVA for cortisol AUC data was significant for the effect of treatment day, F(2, 34) = 23.79, p < .0001. Tukey’s tests for AUC data revealed that each treatment day was different from each other.
FIGURE 2:
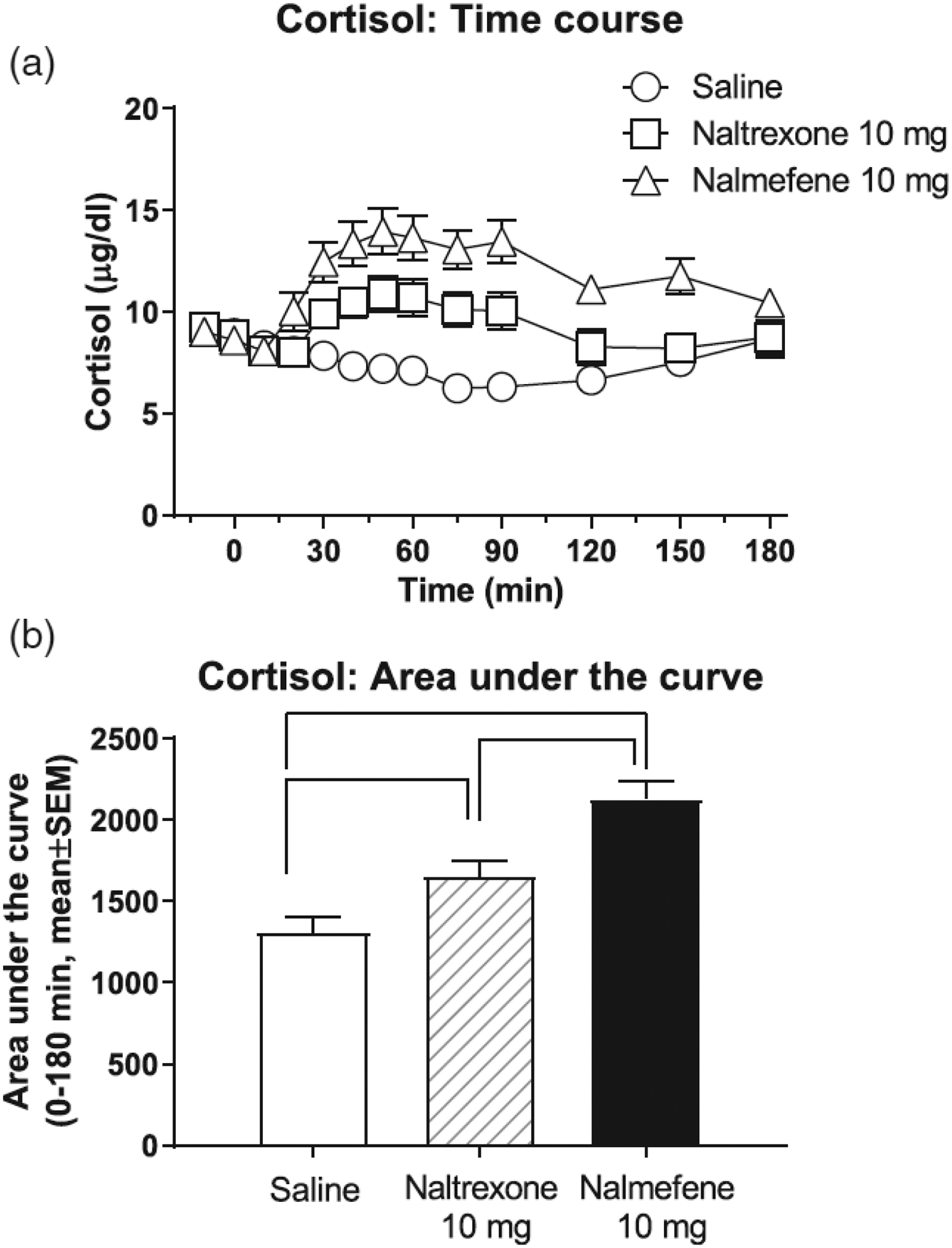
Effects of intravenous saline, naltrexone, and nalmefene on cortisol, in male and female volunteers (time course: panel a; area under the curve: panel b). Data (n = 18) are presented as mean ± standard error of the mean. Time course data (a): A two-way repeated measures ANOVA was significant for treatment, F (2, 34) = 21.73, p < .0001, and time, F(12, 204) = 4.30, p < .0001, main effects and for their interaction, F(24, 408) = 7.71, p < .0001. Area under the curve (AUC) data for the period 0 to 180 min (b): A one-way repeated measures ANOVA was significant for treatment day, F (2, 34) = 23.79, p < .0001. Black lines in panel (b) show significant Tukey post hoc tests.
Prolactin
Due to the well-known sexually dimorphic effects of opioids on prolactin levels and because of potential changes in prolactin across the menstrual cycle (Kreek et al., 1999; Roche & King, 2015), we analyzed only prolactin data for males (Figure 3a). Data from one male was excluded from analysis, because it was an outlier (i.e., pre-injection prolactin values were >2 standard deviations above the group mean). Another male was removed from analysis due to missing data. Overall, both naltrexone and nalmefene caused time-dependent increases in prolactin, compared with saline.
FIGURE 3:
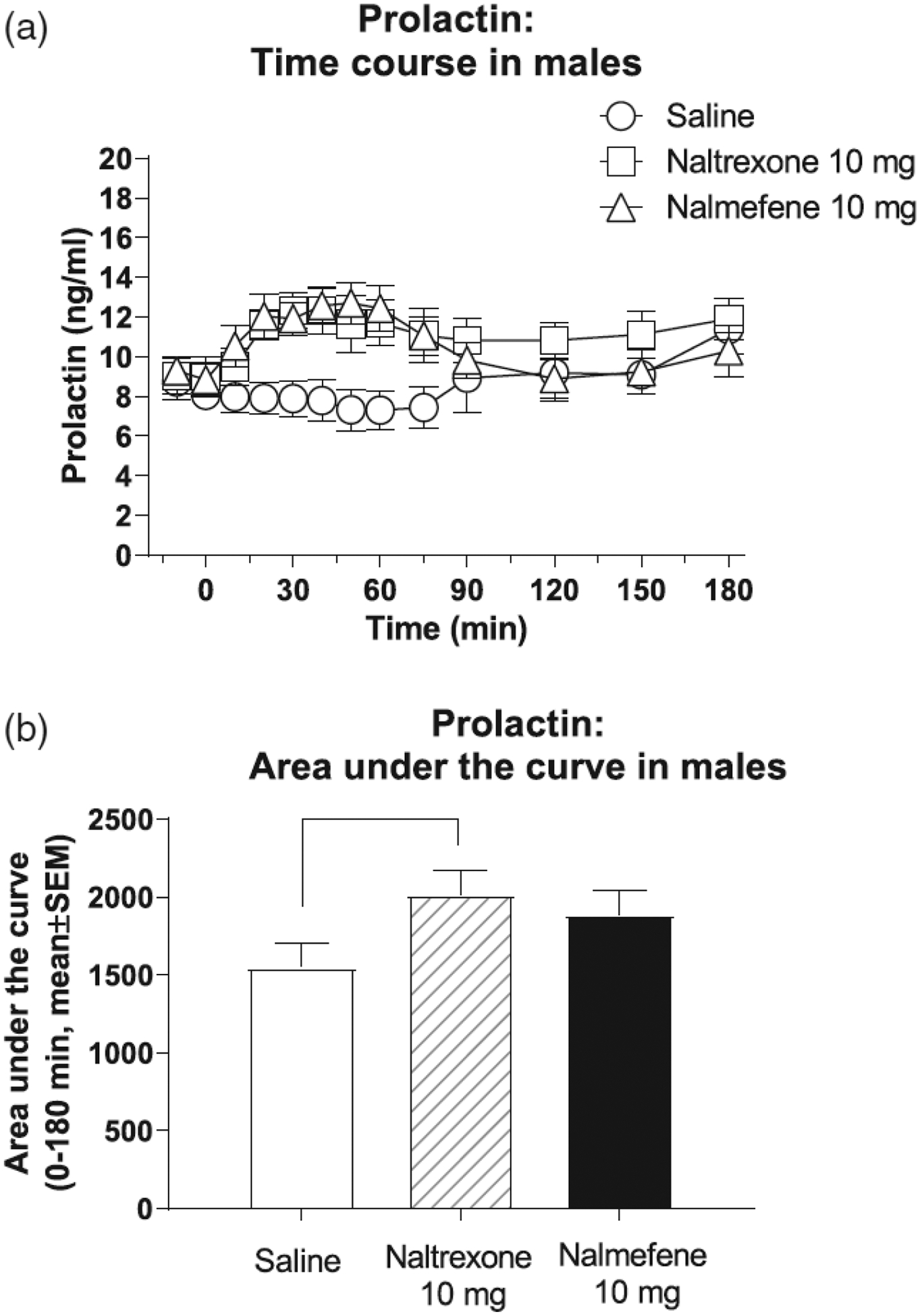
Effects of intravenous saline, naltrexone, and nalmefene on prolactin, in male volunteers only (time course: panel a; area under the curve: panel b). Data (n = 9) are presented as mean ± standard error of the mean. Time course data (a): A two-way repeated measures ANOVA was significant for main effects of treatment day, F(2, 16) = 9.37, p = .003, and time, F(12, 96) = 2.10, p < .03, and their interaction, F(24, 192) = 3.94, p < .0001. Area under the curve (AUC) data for the period 0 to 180 min (b): A one-way repeated measures ANOVA was significant, F(2, 16) = 5.44, p = .016. Black lines in the lower panel show significant Tukey post hoc tests.
A two-way repeated measures ANOVA was significant for main effects of treatment day, F(2, 16) = 9.37, p = .003, and time, F(12, 96) = 2.10, p < .03 main effects, and their interaction, F(24, 192) = 3.94, p < .0001. Tukey’s tests show that the effects of nalmefene were greater than saline for all time points in 10–90 min after injection. Naltrexone had greater effects than saline for a slightly shorter period, 20–90 min after injection. Nalmefene did not differ significantly from naltrexone at any time point. Prolactin data were then recalculated as AUC for the 0- to 180-min period and analyzed with a one-way repeated measures ANOVA (Figure 3b). This ANOVA was significant, F(2, 16) = 5.44, p = .016. Tukey’s tests indicate that naltrexone was significantly different from saline.
Effects of naltrexone and nalmefene effects on the “mood” visual analog scale
There were no robust effects of saline, naltrexone, or nalmefene injection in the visual analog scales for mood (Table 2). In a two-way repeated measures ANOVA (treatment day × time), there were no significant main effects of treatment day, F(2, 36) = 1.14, p = .33, time, F(7, 126) = 0.66, p = .55, or their interaction, F(14, 252) = 0.72, p = .93.
TABLE 2.
| Treatment day | Time from injection (min) | |||||||
|---|---|---|---|---|---|---|---|---|
| −20 | +30 | +60 | +90 | + 120 | + 150 | +240 | +360 | |
| Saline | ||||||||
| Mean | 7.1 | 7.1 | 7.0 | 7.0 | 6.9 | 7.0 | 7.2 | 7.1 |
| SEM | 0.5 | 0.4 | 0.4 | 0.5 | 0.4 | 0.4 | 0.4 | 0.5 |
| Naltrexone 10 mg | ||||||||
| Mean | 6.8 | 6.6 | 6.7 | 6.6 | 6.8 | 6.6 | 6.9 | 7.0 |
| SEM | 0.5 | 0.4 | 0.4 | 0.5 | 0.4 | 0.4 | 0.4 | 0.5 |
| Nalmefene 10 mg | ||||||||
| Mean | 6.9 | 6.6 | 6.9 | 6.9 | 6.7 | 6.7 | 7.0 | 7.1 |
| SEM | 0.4 | 0.4 | 0.4 | 0.4 | 0.5 | 0.5 | 0.5 | 0.4 |
Abbreviations: ANOVA, analysis of variance; SEM, standard error of the mean.
VAS scale was 10 cm long, labelled as “terrible” on the left and “terrific” on the right. Data are presented in cm.
A two-way repeated measures ANOVA (treatment day × time from injection) was non-significant (see Results).
DISCUSSION
To our knowledge, this is the first study in which naltrexone and nalmefene have been directly compared for their neuroendocrine effects in humans (Soyka et al., 2016).
Neuroendocrine effects
ACTH and cortisol
In the time course analyses, naltrexone and nalmefene caused increases in the HPA axis hormone ACTH and cortisol. The effects of nalmefene on ACTH and cortisol were of greater magnitude and duration than those of naltrexone. Also, based on the AUC analyses for both ACTH and cortisol, nalmefene caused greater HPA axis activation than naltrexone. We previously also observed that the effects of nalmefene on these hormones were greater than those of another shorter acting antagonist, naloxone (Schluger et al., 1998). It is known that both κ-agonism and μ-antagonism can cause activation of the HPA axis in humans and nonhuman primates (Maqueda et al., 2016; Pascoe et al., 2008; Schluger et al., 1998). The IV dose of both naltrexone and nalmefene administered here (10 mg) is expected to cause maximal occupancy of μ-receptor populations (Broksoe Kyhl et al., 2016; Ingman et al., 2005; Webster, Johnson, Stauffer, Setnik, & Ciric, 2011). The pharmacokinetic half-life of IV nalmefene in humans is thought to be longer than that of naltrexone (Dixon et al., 1986; Webster et al., 2011), although they have never been compared in the same study, to our knowledge. Such a difference in IV half-life could potentially influence neuroendocrine effects observed in a time course design, such as that used here. Overall, the greater HPA axis-activating effects of nalmefene versus naltrexone may be due to differences in their pharmacodynamics at κ- or μ-receptors, but we cannot fully exclude the potential influence of differences in IV pharmacokinetics.
Prolactin
Both μ-agonists and κ-agonists cause dose-dependent increases in prolactin levels in humans and other mammals, and therefore, this can be used as a neuroendocrine biomarker to interrogate ligand effects at these receptor systems (Bowen, Negus, Kelly, & Mello, 2002; Butelman et al., 1999; Chang et al., 2011; Hoehe, Duka, & Doenicke, 1988; Kreek et al., 1999). δ-agonists appear not to share this neuroendocrine effect (Butelman, Ball, & Kreek, 2002), and δ-receptor affinity of naltrexone and nalmefene is relatively low (Bart et al., 2005; Butelman et al., 2002; Wentland et al., 2009). Maximal prolactin-releasing effects of high efficacy κ-agonists are greater than those of partial κ-agonists (Butelman et al., 1999), and we have recently shown that a novel selective κ-antagonist (Rorick-Kehn et al., 2014) does not cause prolactin release in normal volunteers (Reed et al., 2017). In this study, prolactin levels were increased by both naltrexone and nalmefene. We have previously shown that nalmefene can increase prolactin levels in humans (Bart et al., 2005). These data show that IV naltrexone shares this neuroendocrine effect of nalmefene, consistent with a partial agonist signaling profile at κ-receptors, observed in vitro (Ghirmai, Azar, & Cashman, 2009; Wang et al., 2007; Wentland et al., 2009). However, other mechanisms for this effect, including actions at μ-receptors, cannot be fully excluded.
Subjective effects
In this stress-minimized inpatient setting, we did not observe robust subjective effects of acute IV naltrexone or nalmefene administration, in a “mood” scale. This is generally consistent with prior studies (Fudala, Heishman, Henningfield, & Johnson, 1991). It is possible that studies with behavioral or other stressors, or studies in other clinical populations may result in more robust subjective effects of acute naltrexone or nalmefene (Back et al., 2010; Sinha et al., 2006). 5.1.4 | Design considerations and limitations This study was designed with a fixed order of injections on consecutive days (Day 1: saline; Day 2: naltrexone; and Day 3: nalmefene), because nalmefene is thought to have a longer duration of action than naltrexone (Dixon et al., 1986; Wall et al., 1981), although this has never been directly compared in humans. However, it cannot be fully excluded that naltrexone administered on Day 2 may have caused some “carryover” on Day 3 (i.e., the nalmefene test day; Lee et al., 1988). It should be noted that ACTH, cortisol, and prolactin levels had returned to normal levels, in the pre-injection values on Day 3. Also, when administered on Day 3, IV nalmefene caused a rapid effect on the presently studied hormones, as had been observed in studies without prior naltrexone administration (Bart et al., 2005; Schluger et al., 1998). Therefore, it does not appear likely that such potential carryover effects substantially affected nalmefene data in this study. Future studies with different designs may further clarify this issue. The use of the IV route allowed for a comparison of the pharmacodynamics effects of naltrexone and nalmefene, decreasing the potential impact of different oral pharmacokinetics and bioavailability of these two compounds. Oral or IV pharmacokinetics and bioavailability of naltrexone and nalmefene have never been directly compared, to our knowledge (Broksoe Kyhl et al., 2016; Kogan, Verebey, & Mule, 1977). The selection of the 10-mg IV dose was guided by the goal of achieving comparability with prior studies (Bart et al., 2005; Schluger et al., 1998) and is substantially greater than IV doses of either naltrexone or nalmefene, which are needed to cause μ-receptor antagonism in humans (Moore, Bikhazi, Tuttle, & Weidler, 1990; Webster et al., 2011). Therefore, it can be expected that there would be a maximal blockade of μ-receptors by both naltrexone and nalmefene, with the IV dose used herein.
CONCLUSIONS
To our knowledge, this is the first direct comparison of the neuroendocrine effects of naltrexone and nalmefene in humans. Nalmefene produced greater HPA axis activation than naltrexone. One interpretation of this finding is a greater κ-partial agonist effect by nalmefene versus naltrexone, based on the in vitro profile of these compounds in major signaling assays (Bart et al., 2005; Ghirmai et al., 2009). Nalmefene and naltrexone also caused a time-dependent increase in prolactin levels, in male normal volunteers. This finding is also consistent with partial agonist effects of these compounds, because neither μ-antagonist not κ-antagonist actions are thought to result in prolactin release (Bowen et al., 2002; Reed et al., 2017). The μ-antagonist effects of naltrexone and nalmefene have received much attention in human research (Comer et al., 2002; Gal & DiFazio, 1986; Webster et al., 2011). The potential importance of κ-receptor mediated effects of these compounds in the treatment of AUD is the focus of more recent studies (de Laat et al., 2019). Overall, these data provide further insight into the neuroendocrine biomarker profile of these two pharmacotherapies. These data also provide a basis for design and interpretation of future studies with novel ligands with μ- and κ-receptor-mediated effects in humans, and of novel formulations.
ACKNOWLEDGEMENTS
The authors are very grateful to Joshua Altschuler, Konrad Ben, Alexandra Dunn, Jose’ Erazo, Catherine Guariglia, Michelle Morochnik, and Sage Rahm for technical assistance, conducting neuroendocrine assays, and data collection. Expert clinical assistance from Brenda Ray N.P. (in Memoriam) and Elizabeth Ducat N.P. is also gratefully acknowledged. This study was supported by the National Institutes of Health NIH-NCATS (5UL1TR001866–03), NIH-NIDA (RO1 DA018151), and the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation.
Footnotes
STATEMENT OF ETHICS
Subjects have given their written informed consent. The study protocol has been approved by the research institute’s committee on human research.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
REFERENCES
- Back SE, Hartwell K, DeSantis SM, Saladin M, McRae-Clark AL, Price KL, Brady KT (2010). Reactivity to laboratory stress provocation predicts relapse to cocaine. Drug and Alcohol Dependence, 106 (1), 21–27. 10.1016/j.drugalcdep.2009.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bart G, Schluger JH, Borg L, Ho A, Bidlack JM, & Kreek MJ (2005). Nalmefene induced elevation in serum prolactin in normal human volunteers: Partial kappa opioid agonist activity? Neuropsychopharmacology, 30(12), 2254–2262. 10.1038/sj.npp.1300811 [DOI] [PubMed] [Google Scholar]
- Bidlack JM (2014). Mixed kappa/mu partial opioid agonists as potential treatments for cocaine dependence. Advances in Pharmacology, 69, 387–418. 10.1016/b978-0-12-420118-7.00010-x [DOI] [PubMed] [Google Scholar]
- Bowen CA, Negus SS, Kelly M, & Mello NK (2002). The effects of heroin on prolactin levels in male rhesus monkeys: Use of cumulative dosing procedures. Psychoneuroendocrinology, 27(3), 319–336. [DOI] [PubMed] [Google Scholar]
- Broksoe Kyhl LE, Li S, Faerch KU, Soegaard B, Larsen F, & Areberg J (2016). Population pharmacokinetics of nalmefene in healthy subjects and its relation to mu-opioid receptor occupancy. British Journal of Clinical Pharmacology, 81, 290–300. 10.1111/bcp.12805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butelman ER, Ball JW, & Kreek MJ (2002). Comparison of the discriminative and neuroendocrine effects of centrally penetrating kappa-opioid agonists in rhesus monkeys. Psychopharmacology, 164(1), 115–120. 10.1007/s00213-002-1195-y [DOI] [PubMed] [Google Scholar]
- Butelman ER, Chen CY, Fry RS, Kimani R, Levran O, Ott J, Kreek MJ (2018). Re-evaluation of the KMSK scales, rapid dimensional measures of self-exposure to specific drugs: Gender-specific features. Drug & Alcohol Dependence, 190, 179–187. [DOI] [PubMed] [Google Scholar]
- Butelman ER, Harris TJ, & Kreek M (1999). Apparent efficacy of kappa-opioid receptor ligands on serum prolactin levels in rhesus monkeys. European Journal of Pharmacology, 383(3), 305–309. [DOI] [PubMed] [Google Scholar]
- Castera P, Stewart E, Großkopf J, Brotons C, Brix Schou M Zhang D, Meulien D (2018). Nalmefene, given as needed, in the routine treatment of patients with alcohol dependence: An interventional, open-label study in primary care. European Addiction Research, 24(6), 293–303. 10.1159/000494692 [DOI] [PubMed] [Google Scholar]
- Chang C, Byon W, Lu Y, Jacobsen LK, Badura LL, Sawant-Basak A, Maurer TS (2011). Quantitative PK-PD model-based translational pharmacology of a novel kappa opioid receptor antagonist between rats and humans. The AAPS Journal, 13, 565–575. 10.1208/s12248-011-9296-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Collins ED, Kleber HD, Nuwayser ES, Kerrigan JH, & Fischman MW (2002). Depot naltrexone: Long-lasting antagonism of the effects of heroin in humans. Psychopharmacology, 159(4), 351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daunais JB, Roberts DC, & McGinty JF (1993). Cocaine self-administration increases preprodynorphin, but not c-fos, mRNA in rat striatum. Neuroreport, 4(5), 543–546. [DOI] [PubMed] [Google Scholar]
- Dixon R, Howes J, Gentile J, Hsu HB, Hsiao J, Garg D, Tuttle R (1986). Nalmefene: Intravenous safety and kinetics of a new opioid antagonist. Clinical Pharmacology and Therapeutics, 39(1), 49–53. [DOI] [PubMed] [Google Scholar]
- Drobes DJ, Anton RF, Thomas SE, & Voronin K (2004). Effects of naltrexone and nalmefene on subjective response to alcohol among non-treatment-seeking alcoholics and social drinkers. Alcoholism, Clinical and Experimental Research, 28(9), 1362–1370. [DOI] [PubMed] [Google Scholar]
- Fagergren P, Smith HR, Daunais JB, Nader MA, Porrino LJ, & Hurd YL (2003). Temporal upregulation of prodynorphin mRNA in the primate striatum after cocaine self-administration. The European Journal of Neuroscience, 17(10), 2212–2218. [DOI] [PubMed] [Google Scholar]
- Fudala PJ, Heishman SJ, Henningfield JE, & Johnson RE (1991). Human pharmacology and abuse potential of nalmefene. Clinical Pharmacology and Therapeutics, 49(3), 300–306. [DOI] [PubMed] [Google Scholar]
- Gal TJ, & DiFazio CA (1986). Prolonged antagonism of opioid action with intravenous nalmefene in man. Anesthesiology, 64(2), 175–180. [DOI] [PubMed] [Google Scholar]
- Ghirmai S, Azar MR, & Cashman JR (2009). Synthesis and pharmacological evaluation of 6-naltrexamine analogs for alcohol cessation. Bioorganic & Medicinal Chemistry, 17(18), 6671–6681. 10.1016/j.bmc.2009.07.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghirmai S, Azar MR, Polgar WE, Berzetei-Gurske I, & Cashman JR (2008). Synthesis and biological evaluation of α- and β-6-amido derivatives of 17-cyclopropylmethyl-3, 14β-dihydroxy-4, 5α-epoxymorphinan: Potential alcohol-cessation agents. Journal of Medicinal Chemistry, 51(6), 1913–1924. 10.1021/jm701060e [DOI] [PubMed] [Google Scholar]
- Glass PS, Jhaveri RM, & Smith LR (1994). Comparison of potency and duration of action of nalmefene and naloxone. Anesthesia and Analgesia, 78(3), 536–541. [DOI] [PubMed] [Google Scholar]
- Gual A, He Y, Torup L, van den Brink W, & Mann K (2013). A randomised, double-blind, placebo-controlled, efficacy study of nalmefene, as-needed use, in patients with alcohol dependence. European Neuropsychopharmacology, 23, 1432–1442. 10.1016/j.euroneuro.2013.02.006 [DOI] [PubMed] [Google Scholar]
- Hermann D, Hirth N, Reimold M, Batra A, Smolka MN, Hoffmann S, Hansson AC (2017). Low mu-opioid receptor status in alcohol dependence identified by combined positron emission tomography and postmortem brain analysis. Neuropsychopharmacology, 42(3), 606–614. 10.1038/npp.2016.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehe M, Duka T, & Doenicke A (1988). Human studies on the mu opiate receptor agonist fentanyl: Neuroendocrine and behavioral responses. Psychoneuroendocrinology, 13(5), 397–408. [DOI] [PubMed] [Google Scholar]
- Hoehe M, Duka T, Doenicke A, & Matussek N (1984). Dose-dependent influence of fentanyl on prolactin, growth hormone, and mood. Neuropeptides, 5(1–3), 269–272 doi: 0143–4179(84) 90079–9 [pii]. [DOI] [PubMed] [Google Scholar]
- Ingman K, Hagelberg N, Aalto S, Nagren K, Juhakoski A, Karhuvaara S, … Scheinin H (2005). Prolonged central mu-opioid receptor occupancy after single and repeated nalmefene dosing. Neuropsychopharmacology, 30(12), 2245–2253. 10.1038/sj.npp.1300790 [DOI] [PubMed] [Google Scholar]
- Kaplan JL, & Marx JA (1993). Effectiveness and safety of intravenous nalmefene for emergency department patients with suspected narcotic overdose: A pilot study. Annals of Emergency Medicine, 22(2), 187–190. [DOI] [PubMed] [Google Scholar]
- Karkhanis A, Holleran KM, & Jones SR (2017). Dynorphin/kappa opioid receptor Signaling in preclinical models of alcohol, drug, and food addiction. International Review of Neurobiology, 136, 53–88. 10.1016/bs.irn.2017.08.001 [DOI] [PubMed] [Google Scholar]
- Kellogg SH, McHugh PF, Bell K, Schluger JH, Schluger RP, LaForge KS, … Kreek MJ (2003). The Kreek–McHugh–Schluger–Kellogg scale: A new, rapid method for quantifying substance abuse and its possible applications. Drug and Alcohol Dependence, 69(2), 137–150. [DOI] [PubMed] [Google Scholar]
- Ko MC, Butelman ER, Traynor JR, & Woods JH (1998). Differentiation of kappa opioid agonist-induced antinociception by naltrexone apparent pA2 analysis in rhesus monkeys. The Journal of Pharmacology and Experimental Therapeutics, 285(2), 518–526. [PMC free article] [PubMed] [Google Scholar]
- Kogan MJ, Verebey K, & Mule SJ (1977). Estimation of the systemic availability and other pharmacokinetic parameters of naltrexone in man after acute and chronic oral administration. Research Communications in Chemical Pathology and Pharmacology, 18(1), 29–34. [PubMed] [Google Scholar]
- Kreek MJ (1978). Medical complications in methadone patients. Annals of the new York Academy of Sciences, 311, 110–134. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Schluger J, Borg L, Gunduz M, & Ho A (1999). Dynorphin A1–13 causes elevation of serum levels of prolactin through an opioid receptor mechanism in humans: Gender differences and implications for modulation of dopaminergic tone in the treatment of addictions. Journal of Pharmacology and Experimental Therapeutics, 288(1), 260–269. [PubMed] [Google Scholar]
- Krieter P, Gyaw S, Crystal R, & Skolnick P (2019). Fighting fire with fire: Development of intranasal nalmefene to treat synthetic opioid overdose. The Journal of Pharmacology and Experimental Therapeutics, 371, 409–415. 10.1124/jpet.118.256115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieter PA, Chiang CN, Gyaw S, Skolnick P, & Snyder R (2019). Pharmacokinetic interaction of naloxone and naltrexone following intranasal administration to healthy subjects. Drug Metabolism and Disposition, 47, 690–698. 10.1124/dmd.118.085977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Laat B, Goldberg A, Shi J, Tetrault JM, Nabulsi N, Zheng MQ, Krishnan-Sarin S (2019). The kappa opioid receptor is associated with naltrexone-induced reduction of drinking and craving. Biol Psychiatry, 86, 864–871. 10.1016/j.biopsych.2019.05.021 [DOI] [PubMed] [Google Scholar]
- Lee JD, Nunes EV Jr., Novo P, Bachrach K, Bailey GL, Bhatt S, Rotrosen J (2017). Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): A multicentre, open-label. Randomised Controlled Trial. Lancet, 391, 309–318. 10.1016/s0140-6736(17)32812-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MC, Wagner HN Jr., Tanada S, Frost JJ, Bice AN, & Dannals RF (1988). Duration of occupancy of opiate receptors by naltrexone. Journal of Nuclear Medicine, 29(7), 1207–1211. [PubMed] [Google Scholar]
- Mann K, Bladstrom A, Torup L, Gual A, & van den Brink W (2013). Extending the treatment options in alcohol dependence: A randomized controlled study of as-needed nalmefene. Biological Psychiatry, 73(8), 706–713. 10.1016/j.biopsych.2012.10.020 [DOI] [PubMed] [Google Scholar]
- Maqueda AE, Valle M, Addy PH, Antonijoan RM, Puntes M, Coimbra J, Riba J (2016). Naltrexone but not ketanserin antagonizes the subjective, cardiovascular and neuroendocrine effects of salvinorin-A in humans. The International Journal of Neuropsychopharmacology, 19, 1–13. 10.1093/ijnp/pyw016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mash DC, & Staley JK (1999). D3 dopamine and kappa opioid receptor alterations in human brain of cocaine-overdose victims. Annals of the new York Academy of Sciences, 877, 507–522. [DOI] [PubMed] [Google Scholar]
- Moore LR, Bikhazi GB, Tuttle RR, & Weidler DJ (1990). Antagonism of fentanyl-induced respiratory depression with nalmefene. Methods and Findings in Experimental and Clinical Pharmacology, 12(1),29–35. [PubMed] [Google Scholar]
- Nestor LJ, Paterson LM, Murphy A, McGonigle J, Orban C, Reed L, Nutt DJ (2018). Naltrexone differentially modulates the neural correlates of motor impulse control in abstinent alcohol-dependent and poly-substance dependent individuals. Eur J Neurosci, 50, 2311–2321. 10.1111/ejn.14262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley SS, Krishnan-Sarin S, Farren C, Sinha R, & Kreek MJ (2002). Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo–pituitary–adrenocortical axis. Psychopharmacology, 160(1), 19–29. 10.1007/s002130100919 [DOI] [PubMed] [Google Scholar]
- Pascoe JE, Williams KL, Mukhopadhyay P, Rice KC, Woods JH, & Ko MC (2008). Effects of mu, kappa, and delta opioid receptor agonists on the function of hypothalamic–pituitary–adrenal axis in monkeys. Psychoneuroendocrinology, 33(4), 478–486. 10.1016/j.psyneuen.2008.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed B, Butelman ER, Fry R, Kimani R, & Kreek MJ (2017). Repeated Administration of opra kappa (LY2456302), a novel, short-acting, selective KOP-r antagonist, in persons with and without cocaine dependence. Neuropsychopharmacology, 43, 739–750. 10.1038/npp.2017.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remmers AE, Clark MJ, Mansour A, Akil H, Woods JH, & Medzihradsky F (1999). Opioid efficacy in a C6 glioma cell line stably expressing the human kappa opioid receptor. Journal of Pharmacology and Experimental Therapeutics, 288(2), 827–833. [PubMed] [Google Scholar]
- Roche DJ, & King AC (2015). Sex differences in acute hormonal and subjective response to naltrexone: The impact of menstrual cycle phase. Psychoneuroendocrinology, 52, 59–71. 10.1016/j.psyneuen.2014.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorick-Kehn LM, Witkin JM, Statnick MA, Eberle EL, McKinzie JH, Kahl SD, McKinzie DL (2014). LY2456302 is a novel, potent, orally-bioavailable small molecule kappa-selective antagonist with activity in animal models predictive of efficacy in mood and addictive disorders. Neuropharmacology, 77, 131–144. 10.1016/j.neuropharm.2013.09.021 [DOI] [PubMed] [Google Scholar]
- Schluger JH, Ho A, Borg L, Porter M, Maniar S, Gunduz M, Kreek MJ (1998). Nalmefene causes greater hypothalamic-pituitaryadrenal axis activation than naloxone in normal volunteers: Implications for the treatment of alcoholism. Alcoholism-Clinical and Experimental Research, 22(7), 1430–1436. 10.1111/J.1530-0277.1998.Tb03931.X [DOI] [PubMed] [Google Scholar]
- Sinha R, Garcia M, Paliwal P, Kreek MJ, & Rounsaville BJ (2006). Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Archives of General Psychiatry, 63(3), 324–331. 10.1001/archpsyc.63.3.324 [DOI] [PubMed] [Google Scholar]
- Soyka M, Friede M, & Schnitker J (2016). Comparing Nalmefene and naltrexone in alcohol dependence: Are there any differences? Results from an Indirect Meta-Analysis. Pharmacopsychiatry. Doi, 49, 66–75. 10.1055/s-0035-1565184 [DOI] [PubMed] [Google Scholar]
- Spangler R, Unterwald EM, & Kreek MJ (1993). ‘Binge’ cocaine administration induces a sustained increase of prodynorphin mRNA in rat caudate-putamen. Brain Research. Molecular Brain Research, 19(4), 323–327. [DOI] [PubMed] [Google Scholar]
- Stephens MA, & Wand G (2012). Stress and the HPA axis: Role of glucocorticoids in alcohol dependence. Alcohol Research: Current Reviews, 34(4), 468–483. [PMC free article] [PubMed] [Google Scholar]
- Tang YL, Khoury L, Bradley B, Gillespie CF, Ressler KJ, & Cubells JF (2011). Substance use disorders assessed using the Kreek–McHugh–Schluger–Kellogg (KMSK) scale in an urban low income and predominantly African American sample of primary care patients. The American Journal on Addictions, 20(3), 292–299. 10.1111/j.1521-0391.2011.00121.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall ME, Brine DR, & Perez-Reyes M (1981). Metabolism and disposition of naltrexone in man after oral and intravenous administration. Drug Metabolism and Disposition, 9(4), 369–375. [PubMed] [Google Scholar]
- Wang D, Sun X, & Sadee W (2007). Different effects of opioid antagonists on mu-, delta-, and kappa-opioid receptors with and without agonist pretreatment. The Journal of Pharmacology and Experimental Therapeutics, 321(2), 544–552. 10.1124/jpet.106.118810 [DOI] [PubMed] [Google Scholar]
- Webster LR, Johnson FK, Stauffer J, Setnik B, & Ciric S (2011). Impact of intravenous naltrexone on intravenous morphine-induced high, drug liking, and euphoric effects in experienced, nondependent male opioid users. Drugs R D, 11(3), 259–275. 10.2165/11593390-000000000-00000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, & Koob GF (2010). The role of the dynorphin-kappa opioid system in the reinforcing effects of drugs of abuse. Psychopharmacology, 210, 121–135. 10.1007/s00213-010-1825-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentland MP, Lou R, Lu Q, Bu Y, Denhardt C, Jin J, Bidlack JM (2009). Syntheses of novel high affinity ligands for opioid receptors. Bioorganic & Medicinal Chemistry Letters, 19(8), 2289–2294. 10.1016/j.bmcl.2009.02.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woody GE, & Metzger DS (2011). Injectable extended-release naltrexone for opioid dependence. Lancet, 378(9792), 664–665. 10.1016/s0140-6736(11)61330-5 [DOI] [PubMed] [Google Scholar]


