Key Points
Question
Does treatment with semaglutide, 2.4 mg, once weekly affect the risk of developing symptoms of depression or suicidal ideation/behavior vs placebo in people with overweight or obesity without known major psychopathology?
Findings
In this post hoc analysis of the STEP 1, 2, and 3 trials and STEP 5 trial over 68 and 104 weeks, respectively, there were no clinically meaningful differences in mean Patient Health Questionnaire 9 scores throughout the trials between the semaglutide, 2.4 mg, and placebo groups. The proportion of participants reporting suicidal ideation/behavior (as assessed by the Columbia-Suicide Severity Rating Scale) was also similar between groups.
Meaning
The results of this post hoc analysis suggest that the risk of developing symptoms of depression or suicidal ideation/behavior was similar between semaglutide, 2.4 mg, and placebo.
Abstract
Importance
Obesity is associated with numerous psychosocial complications, making psychiatric safety a consideration for treating people with obesity. Few studies have investigated the psychiatric safety of newly available antiobesity medications.
Objective
To evaluate the psychiatric safety of subcutaneous semaglutide, 2.4 mg, once weekly in people without known major psychopathology.
Design, Setting, and Participants
This post hoc analysis of pooled data from the randomized, double-blind, placebo-controlled, multicenter phase 3a STEP 1, 2, and 3 trials (68 weeks; 2018-2020) and phase 3b STEP 5 trial (104 weeks; 2018-2021) included adults with overweight or obesity; STEP 2 participants also had type 2 diabetes. Trial designs have been published previously.
Interventions
Semaglutide, 2.4 mg, vs placebo.
Main Outcomes and Measures
Depressive symptoms and suicidal ideation/behavior were assessed using the Patient Health Questionnaire (PHQ-9) and Columbia–Suicide Severity Rating Scale, respectively. Psychiatric and nervous system disorder adverse events were investigated.
Results
This analysis included 3377 participants in the STEP 1, 2, and 3 trials (2360 women [69.6%]; mean [SD] age, 49 [13] years) and 304 participants in STEP 5 (236 women [77.6%]; mean [SD] age, 47 [11] years). In the STEP 1, 2, and 3 trials, mean (SD) baseline PHQ-9 scores for the semaglutide, 2.4 mg, and placebo groups were 2.0 (2.3) and 1.8 (2.3), respectively, indicating no/minimal symptoms of depression. PHQ-9 scores at week 68 were 2.0 (2.9) and 2.4 (3.3), respectively; the estimated treatment difference (95% CI) between groups was −0.56 (−0.81 to −0.32) (P < .001). Participants treated with semaglutide vs placebo were less likely to shift (from baseline to week 68) to a more severe category of PHQ-9 depression (odds ratio, 0.63; 95% CI, 0.50-0.79; P < .001). Based on the Columbia–Suicide Severity Rating Scale, 1% or fewer of participants reported suicidal ideation/behavior during treatment, with no differences between semaglutide, 2.4 mg, and placebo. Psychiatric disorder adverse events were generally balanced between groups. Similar results were observed in STEP 5.
Conclusions and Relevance
The results of this post hoc analysis suggest that treatment with semaglutide, 2.4 mg, did not increase the risk of developing symptoms of depression or suicidal ideation/behavior vs placebo and was associated with a small but statistically significant reduction in depressive symptoms (not considered clinically meaningful). People with obesity should be monitored for mental health concerns so they can receive appropriate support and care.
Trial Registration
ClinicalTrials.gov Identifiers: STEP 1 (NCT03548935), 2 (NCT03552757), 3 (NCT03611582), and 5 (NCT03693430)
This post hoc analysis of 4 randomized clinical trials evaluates the psychiatric safety of subcutaneous semaglutide, 2.4 mg, once weekly in people with obesity or overweight without known major psychopathology.
Introduction
Obesity is associated with multiple health complications, including an increased risk of depressive disorders and other psychiatric illness, particularly in people with a body mass index (calculated as weight in kilograms divided by height in meters squared) of 40 or greater.1,2,3,4 Depression may partially result from the pervasive weight stigma directed at people with obesity,5,6 although there is a bidirectional relationship between these conditions in which depression increases the risk of subsequent weight gain.2,7,8 The increased inflammatory state in people with obesity, which is a known risk factor for depression, may also contribute to this relationship.9,10 Obesity-related complications, including type 2 diabetes (T2D) and osteoarthritis, further impair psychosocial status and quality of life.11,12,13
Concerns about potential psychiatric adverse events (AEs) in people with clinically significant depression have accompanied the development of medications for chronic weight management,14 which, except for orlistat,15 access brain regions that regulate energy intake and may interact with mood-influencing pathways.16 Rimonabant was approved for weight management in Europe in 200617 but was later withdrawn due to associations with depression and suicidal ideation (SI).18 These risks are formally assessed by regulatory authorities,19,20 and with most medications approved for chronic weight management, health care professionals are advised to monitor patients for emerging or worsening psychiatric symptoms, including unusual changes in mood.16,21
Semaglutide is a glucagon-like peptide-1 receptor agonist (GLP-1RA) that is approved globally for weight management at a once-weekly dosage of 2.4 mg. After 68 weeks, semaglutide, 2.4 mg, produces an approximately 10% to 15% mean reduction in baseline body weight, which is associated with clinically meaningful improvements in numerous health outcomes,21,22,23,24,25,26 including cardiovascular events in people with overweight or obesity and a history of a cardiovascular event (but not T2D).27 Neither semaglutide, 2.4 mg, nor liraglutide, 3.0 mg, another GLP-1RA approved for weight management, was associated with increased psychiatric AEs vs placebo in phase 3 trials.21,25,28,29,30 However, the European Medicines Agency (EMA) and US Food and Drug Administration (FDA) are actively monitoring the psychiatric safety of GLP-1RAs following postmarketing surveillance reports of depression, SI, and suicidal behavior in people prescribed these medications for T2D or weight management.20,31,32 To date, neither agency has found evidence of a causal association.20,32 This post hoc analysis examines psychiatric data from 4 Semaglutide Treatment Effect in People With Obesity (STEP) trials to assess symptoms of depression, SI, and suicidal behavior, which is critical for evaluating postmarketing reports of these events in the general population.
Methods
This post hoc analysis evaluated pooled data from the STEP 1, 2, and 3 trials and data from STEP 5 (Supplement 1, Supplement 2, Supplement 3, and Supplement 4). All trials were performed at clinical research sites per the Declaration of Helsinki and Good Clinical Practice guidelines. Respective protocols were approved by local institutional review boards and ethics committees.
Overview of the STEP 1, 2, 3, and 5 Trials
Trial Designs and Interventions
Trial designs for STEP 1, 2, 3, and 5 have been reported.22,23,24,33 These were 68-week phase 3a (STEP 1, 2, and 3) or 104-week phase 3b (STEP 5) double-blind, placebo-controlled, multicenter trials. Participants were randomized to receive subcutaneous semaglutide, 2.4 mg, once weekly or placebo (2:1 [STEP 1 and 3]; 1:1:1 to semaglutide, 2.4 mg; semaglutide, 1.0 mg; or placebo [STEP 2]; or 1:1 [STEP 5]) as an adjunct to a lifestyle intervention. Participants receiving semaglutide, 1.0 mg, in STEP 2 were excluded from this analysis. In STEP 3, semaglutide, 2.4 mg, was provided as an adjunct to intensive behavioral therapy.23 In all trials, the primary end point was change in body weight from baseline to the end of treatment.
Participant Screening
Adults with overweight or obesity were recruited and provided written informed consent; STEP 2 also included adults with T2D. Validated mental health questionnaires were used to assess depression (major depressive disorder [MDD]), SI, and suicidal behavior. The Patient Health Questionnaire (PHQ-9) is a self-reported, 9-item screening tool that assesses symptoms of depression during the previous 2 weeks.34 Total scores range from 0 to 27. Scores of 0 to 4, 5 to 9, 10 to 14, 15 to 19, and 20 or greater suggest no/minimal, mild, moderate, moderately severe, and severe symptoms of depression, respectively.34 The Columbia-Suicide Severity Rating Scale (C-SSRS)35 is a clinician-administered questionnaire used to identify SI and suicidal behavior.36 SI is defined by the C-SSRS as (1) a wish to be dead; (2) nonspecific active suicidal thoughts; (3) active SI with any method (no plan) without intent to act; (4) active SI with some intent to act without a specific plan; and (5) active SI with a specific plan and intent. Suicidal behavior was also assessed. Additional C-SSRS methods are provided in the eMethods in Supplement 5.
Exclusion criteria included MDD during the previous 2 years or other severe psychiatric disorders, including, but not limited to, schizophrenia or bipolar disorder, or a screening PHQ-9 score of 15 or greater (moderately severe or greater depression), a lifetime suicide attempt, or SI with intent and with/without a specific plan or suicidal behavior (self-reported or in medical records) within 30 days before screening. Full inclusion and exclusion criteria have been reported.22,23,24,33
Psychiatric Safety Assessments
Mental Health Questionnaires
The PHQ-9 and C-SSRS were administered at screening (1 week before randomization); at baseline (week 0); at weeks 12, 20, 36, 52, and 68 in STEP 1, 2, 3, and 5; and at weeks 84 and 104 in STEP 5 only. At each visit, the investigator reviewed the PHQ-9 and C-SSRS, and neuropsychiatric AEs were assessed through open-ended questioning. At any visit, participants who had a PHQ-9 score of 15 or greater or type 4 or 5 SI or suicidal behavior on the C-SSRS were referred to a mental health professional. Investigators could decide whether participants with a PHQ-9 score of 10 to 14 required referral.
Reporting of Neuropsychiatric AEs
AEs in the system organ classes of psychiatric disorders and nervous system disorders were identified using predefined Medical Dictionary for Regulatory Activities coding (version 22.1 [STEP 1, 2, and 3] or 23.1 [STEP 5]; International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use). AEs included those occurring during the in-trial period, plus 7 weeks of follow-up after treatment was discontinued.
Statistical Methods
STEP 1, 2, and 3 had the same trial duration, so data were pooled; STEP 5 had a longer trial duration, so data were analyzed separately. PHQ-9 scores, the proportion of participants reporting SI/suicidal behavior on the C-SSRS, and neuropsychiatric AEs were analyzed using the safety analysis set (all participants exposed to ≥1 dose of trial product) for the in-trial period. Changes in PHQ-9 scores while receiving treatment were reported at the end of treatment, as well as categorical shifts in PHQ-9 scores from baseline to end of treatment. For STEP 1, 2, and 3, the proportion of participants with AEs and event rates were adjusted using the Cochran-Mantel-Haenszel method to account for between-trial differences.
Changes in PHQ-9 scores and categorical shifts in PHQ-9 scores from baseline to the end of treatment were analyzed using analysis of covariance and logistic regression, respectively (with randomized treatment as a factor and baseline value as a covariate). A multiple imputation approach was used, and missing data were imputed from end of treatment measurements from participants in the same treatment group. No statistical analyses of SI/suicidal behavior were performed, as there were too few events. All analyses were post hoc and not adjusted for multiplicity. Statistical significance was defined as P < .05, with no correction for multiplicity. Statistical analyses were conducted using SAS, version 8.4 (SAS Institute).
Results
Baseline Characteristics
This analysis included 3377 participants in STEP 1, 2, and 3 (pooled data) and 304 in STEP 5 (eFigure 2 in Supplement 5). Participants were predominantly female (2360 [69.9%] and 236 [77.6%], respectively) and White (2415 [71.5%] and 283 [93.1%], respectively; race was self-reported) (Table 1). Common comorbidities at screening included hypertension (1479 [43.8%] and 118 [38.8%], respectively) and dyslipidemia (1486 [44.0%] and 107 [35.2%], respectively).
Table 1. Baseline Characteristics of Participants in STEP 1, 2, 3, and 5.
| Characteristic | No. (%) | |||
|---|---|---|---|---|
| STEP 1, 2, and 3 (pooled data) | STEP 5 | |||
| Semaglutide, 2.4 mg (n = 2116) | Placebo (n = 1261) | Semaglutide, 2.4 mg (n = 152) | Placebo (n = 152) | |
| Age, mean (SD), y | 48 (13) | 50 (13) | 47 (12) | 47 (10) |
| Sex | ||||
| Female | 1492 (70.5) | 868 (68.8) | 123 (80.9) | 113 (74.3) |
| Male | 624 (29.5) | 393 (31.2) | 29 (19.1) | 39 (25.7) |
| Race | ||||
| American Indian or Alaska Native | 22 (1.0) | 12 (1.0) | 2 (1.3) | 1 (0.7) |
| Asian | 298 (14.1) | 194 (15.4) | 2 (1.3) | 0 |
| Black or African American | 186 (8.8) | 112 (8.9) | 7 (4.6) | 5 (3.3) |
| Native Hawaiian or other Pacific Islander | 3 (0.1) | 3 (0.2) | 0 | 0 |
| White | 1517 (71.7) | 898 (71.2) | 141 (92.8) | 142 (93.4) |
| Other | 52 (2.5) | 25 (2.0) | 0 | 4 (2.6) |
| Not applicablea | 38 (1.8) | 17 (1.3) | NA | NA |
| Body weight, kg | 104.6 (22.4) | 103.4 (21.6) | 105.6 (20.8) | 106.5 (23.1) |
| BMI | 37.5 (6.7) | 37.3 (6.7) | 38.6 (6.7) | 38.5 (7.2) |
| Waist circumference, cm | 114.4 (14.7) | 114.5 (14.6) | 115.8 (14.3) | 115.7 (15.5) |
| HbA1c, %b | 5.7 (0.3) | 5.7 (0.3) | 5.7 (0.3) | 5.7 (0.4) |
| Fasting plasma glucose, mg/dLb | 95.1 (10.3) | 94.6 (10.3) | 95.9 (9.5) | 95.1 (10.3) |
| Glycemic status | ||||
| Normoglycemic | 924 (43.7) | 488 (38.7) | 75 (49.3) | 88 (57.9) |
| Prediabetes | 789 (37.3) | 371 (29.4) | 77 (50.7) | 64 (42.1) |
| Type 2 diabetes | 403 (19.0) | 402 (31.9) | 0 | 0 |
| Diabetes durationb | 8.3 (6.2) | 8.1 (6.2) | NA | NA |
| Systolic blood pressure, mm Hg | 127 (14) | 127 (14) | 126 (14) | 125 (15) |
| Diastolic blood pressure, mm Hg | 80 (9) | 80 (10) | 80 (9) | 80 (10) |
| Lipids, geometric mean (CV), mg/dL | ||||
| Total cholesterol | 185.1 (21.2) | 184.6 (21.5) | 188.0 (20.9) | 184.8 (18.3) |
| LDL | 105.7 (33.4) | 104.8 (34.2) | 113.3 (30.1) | 112.3 (25.7) |
| HDL | 48.9 (25.2) | 47.8 (25.0) | 47.8 (25.2) | 47.1 (22.5) |
| VLDL | 24.7 (48.6) | 26.0 (48.9) | 22.3 (46.5) | 21.4 (47.4) |
| Triglycerides | 127.5 (50.5) | 134.2 (51.3) | 114.3 (46.6) | 109.7 (47.4) |
| Kidney function, eGFR, geometric mean (CV), mL/min/1.73m2c | 95.9 (19.9) | 94.9 (20.4) | 95.7 (17.4) | 92.9 (18.2) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CV, coefficient of variance; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; HDL, high-density lipoprotein; LDL, low-density lipoprotein; NA, not applicable; VLDL, very low-density lipoprotein.
SI conversion factors: to convert lipids to mmol/L, multiply by 0.0259; for glucose to mmol/L, multiply by 0.555; for HbA1c to the proportion of total hemoglobin, multiply by 0.01; for triglycerides to mmol/L, multiply by 0.0113.
In the STEP 1, 2, and 3 pooled data, race was recorded as not applicable for participants from France.
Participants from STEP 2 did not contribute to HbA1c and fasting plasma glucose levels. Only participants from STEP 2 contributed to the duration of diabetes.
Kidney function data for STEP 5 are from the screening visit (week −1).
In STEP 1, 2, and 3 and STEP 5, 533 (25%) and 49 (32%) participants receiving semaglutide, 2.4 mg, respectively, and 320 (25%) and 43 (28%) receiving placebo, respectively, reported concomitant psychiatric disorders (eTable 1 in Supplement 5). Information about antidepressant and other medication use, as well as concomitant nervous system disorders, is reported in eTable 1 in Supplement 5.
Psychiatric Safety Assessments
PHQ-9 Total Scores
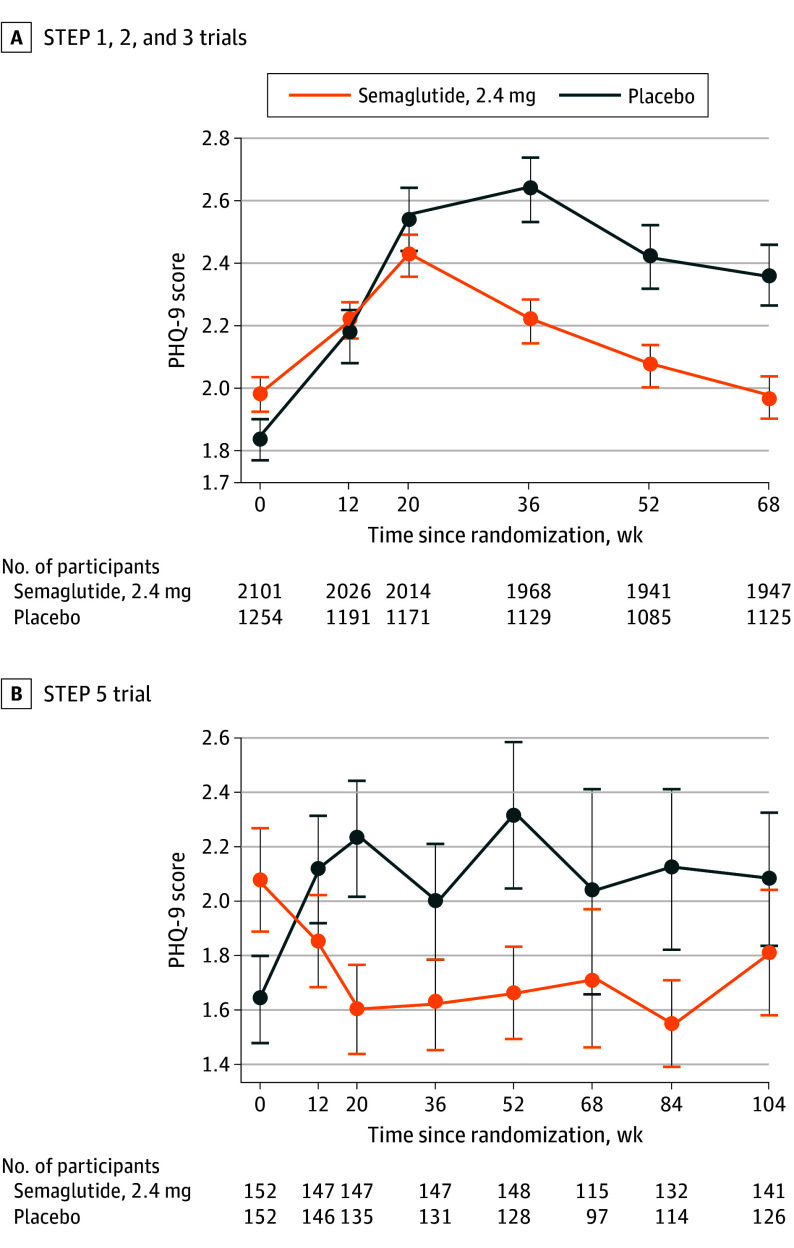
In-trial changes in mean PHQ-9 scores over time were minimal in both groups (eTable 2 in Supplement 5). In STEP 1, 2, and 3, mean (SD) PHQ-9 scores at baseline were 2.0 (2.3) and 1.8 (2.3) for the semaglutide, 2.4 mg, and placebo groups, respectively, which at week 68 were 2.0 (2.9) and 2.4 (3.3) (eFigure 1A in Supplement 5). The estimated treatment difference between groups for change in PHQ-9 scores from baseline to week 68 was −0.56 (95% CI, −0.81 to −0.32; P < .001), favoring semaglutide, 2.4 mg (eTable 3 in Supplement 5). Mean scores in both groups increased marginally through week 52 (Figure, A) but remained in the range of no/minimal symptoms of depression (eFigure 1A, eTable 2A in Supplement 5). Results were broadly similar for participants in STEP 5, although not statistically significant (estimated treatment difference, −0.20; 95% CI, −0.98 to 0.59; P < .63) (Figure, B; eFigure 1B, eTable 2B, and eTable 3 in Supplement 5).
Figure. Patient Health Questionnaire 9 (PHQ-9) Scores Over Time During the STEP 1, 2, 3, and 5 Trials.
Data are mean PHQ-9 scores over time from baseline to week 68 for STEP 1, 2, and 3 (A) and to week 104 for STEP 5 (B). Error bars represent the SD of the mean. Data are for the in-trial period for all participants in the safety analysis set.
In STEP 1, 2, and 3, participants assigned to semaglutide, 2.4 mg, who lost less than 5% of their body weight from baseline to week 68 had a mean (SD) increase of 0.5 (3.5) points on the PHQ-9 at end of treatment, similar to the mean (SD) increase of 0.8 (3.5) points in placebo-treated participants. eTable 4 in Supplement 5 shows additional data by percentage body weight loss.
Categorical Shift in PHQ-9 Scores
The categorical shifts in PHQ-9 scores from baseline to end of treatment for STEP 1, 2, and 3 and STEP 5 are summarized in Table 2. In STEP 1, 2, and 3, among the 1697 participants treated with semaglutide, 2.4 mg, who reported no/minimal symptoms of depression at baseline, 1520 (89.6%) reported the same at week 68, 147 (8.7%) reported mild symptoms, 22 (1.3%) moderate symptoms, and 8 (0.5%) moderately severe or severe symptoms (Table 2). A similar but somewhat less favorable pattern was observed in those receiving placebo. From baseline to week 68, participants receiving semaglutide, 2.4 mg, vs placebo were less likely to shift to a more severe category of depression on the PHQ-9 (odds ratio [OR], 0.63; 95% CI, 0.50-0.79; P < .001) (eTable 5 in Supplement 5). Similar but nonsignificant findings were observed in STEP 5 (OR, 0.74; 95% CI, 0.34-1.60; P = .44) (eTable 5 in Supplement 5).
Table 2. Categorical Shift in Patient Health Questionnaire 9 (PHQ-9) Score From Baseline to End of Treatment in the STEP 1, 2, 3, and 5 Trialsa,b.
| STEP 1, 2, and 3: baseline PHQ-9 category by scorec | No. (%) | End of treatment PHQ-9 category at week 68 for semaglutide (n = 1947) or placebo (n = 1125), No. (%) | |||||
|---|---|---|---|---|---|---|---|
| None (0-4) | Mild (5-9) | Moderate (10-14) | Moderately severe (15-19) | Severe (20-27) | |||
| None (0-4) | |||||||
| Semaglutide | 1697 (87.2) | 1520 (89.6)d | 147 (8.7)e | 22 (1.3)e | 5 (0.3)e | 3 (0.2)e | |
| Placebo | 999 (88.8) | 848 (84.9)d | 123 (12.3)e | 15 (1.5)e | 11 (1.1)e | 2 (0.2)e | |
| Mild (5-9) | |||||||
| Semaglutide | 215 (11.0) | 139 (64.7)f | 60 (27.9)d | 14 (6.5)e | 1 (0.5)e | 1 (0.5)e | |
| Placebo | 109 (9.7) | 61 (56.0)f | 38 (34.9)d | 6 (5.5)e | 3 (2.8)e | 1 (0.9)e | |
| Moderate (10-14) | |||||||
| Semaglutide | 26 (1.3) | 11 (42.3)f | 9 (34.6)f | 5 (19.2)d | 1 (3.8)e | 0 | |
| Placebo | 12 (1.1) | 8 (66.7)f | 0 | 4 (33.3)d | 0 | 0 | |
| STEP 5: baseline PHQ-9 category by score g | No. (%) | End of treatment PHQ-9 category at week 104 for semaglutide (n = 141) or placebo (n = 126), No. (%) | |||||
| None (0-4) | Mild (5-9) | Moderate (10-14) | Moderately severe (15-19) | Severe (20-27) | |||
| None (0-4) | |||||||
| Semaglutide | 119 (84.4) | 110 (92.4)d | 8 (6.7)e | 1 (0.8)e | 0 | 0 | |
| Placebo | 113 (89.7) | 97 (85.8)d | 12 (10.6)e | 4 (3.5)e | 0 | 0 | |
| Mild (5-9) | |||||||
| Semaglutide | 21 (14.9) | 15 (71.4)f | 4 (19.0)d | 1 (4.8)e | 0 | 1 (4.8)e | |
| Placebo | 12 (9.5) | 9 (75.0)f | 2 (16.7)d | 1 (8.3)e | 0 | 0 | |
| Moderate (10-14) | |||||||
| Semaglutide | 1 (0.7) | 1 (100)f | 0 | 0 | 0 | 0 | |
| Placebo | 1 (0.8) | 1 (100)f | 0 | 0 | 0 | 0 | |
Observed data from the in-trial period. No. represents the number of participants with an observation at the visit. Percentages are in relation to participants with respective treatment and PHQ-9 category at baseline. Participants were excluded if they had a baseline PHQ-9 score of 15 or greater (suggesting the need for further psychiatric evaluation and treatment).
See eTable 10 in Supplement 5 for a version of this table with shading relating to baseline PHQ-9 score.
For the STEP 1, 2, and 3 trials, the total number of participants with an improvement was 159 (8.2%) in the semaglutide, 2.4 mg, group and 69 (6.1%) in the placebo group, the total with no change was 1585 (81.4%) for semaglutide, 2.4 mg, and 890 (79.1%) for placebo, and the total with worsening was 194 (10.0%) for semaglutide, 2.4 mg, and 161 (14.3%) for placebo.
Experienced no change from baseline values of the PHQ-9 score.
Experienced worsening from baseline values of the PHQ-9 score.
Experienced improvement from baseline values of the PHQ-9 score.
For the STEP 5 trial, the total number of participants with an improvement was 16 (11.3%) in the semaglutide, 2.4 mg, group and 10 (7.9%) in the placebo group, the total with no change was 114 (80.9%) for semaglutide, 2.4 mg, and 99 (78.6%) for placebo, and the total with worsening was 11 (7.8%) for semaglutide, 2.4 mg, and 17 (13.5%) for placebo.
In STEP 1, 2, and 3, examining maximum PHQ-9 scores occurring at any postbaseline visit, 60 participants (2.9%) assigned to semaglutide vs 54 (4.4%) receiving placebo had a PHQ-9 score of 15 or greater at 1 or more assessments (eTable 6 in Supplement 5). Semaglutide was associated with a reduced risk of this outcome (OR, 0.54; 95% CI, 0.37-0.80; P = .002) (eTable 5 in Supplement 5). Corresponding values in STEP 5 were 1 (0.7%) and 3 participants (2.0%), respectively (OR, 0.76; 95% CI, 0.10-5.80; P = .79). Across the trials, 12 (0.6%) and 17 participants (1.4%) receiving semaglutide, 2.4 mg, and placebo, respectively, had a PHQ-9 score of 15 or greater at the end of treatment.
SI and Suicidal Behavior as Assessed by C-SSRS
In STEP 1, 2, and 3, 30 participants (1.4%) receiving semaglutide, 2.4 mg, and 28 (2.2%) receiving placebo had a history of low- to moderate-risk SI as determined by the C-SSRS; corresponding numbers in STEP 5 were 4 (2.6%) and 2 participants (1.3%), respectively (Table 3). At baseline in STEP 1, 2, and 3, 1 participant receiving semaglutide, 2.4 mg, and 2 receiving placebo reported SI (low risk) (eResults in Supplement 5). No participants in STEP 5 reported SI at baseline.
Table 3. Proportion of Participants With Suicidal Ideation During the Studies as Assessed by the Columbia Suicide-Severity Rating Scale (C-SSRS)a.
| C-SSRS assessment (in trial) | No. (%) | |||
|---|---|---|---|---|
| STEP 1, 2, and 3 | STEP 5 | |||
| Semaglutide, 2.4 mg (n = 2116) | Placebo (n = 1261) | Semaglutide, 2.4 mg (n = 152) | Placebo (n = 152) | |
| Lifetime assessment, No. | 2116 | 1260 | 152 | 152 |
| Participants with suicidal ideation | 30 (1.4) | 28 (2.2) | 4 (2.6) | 2 (1.3) |
| Low risk | ||||
| 1. Wish to be dead | 23 (76.7) | 23 (82.1) | 3 (75.0) | 2 (100) |
| 2. Nonspecific active suicidal thought | 15 (50.0) | 13 (46.4) | 1 (25.0) | 0 |
| Moderate risk | ||||
| 3. Active suicidal ideation with any method (no plan) without intent to act | 5 (16.7) | 5 (17.9) | 0 | 0 |
| High risk | ||||
| 4. Active suicidal ideation with some intent to act without specific plan | 0 | 0 | 0 | 0 |
| 5. Active suicidal ideation with specific plan and intent | 0 | 0 | 0 | 0 |
| At baseline, No. | 2104 | 1248 | 152 | 150 |
| Participants with suicidal ideation | 1 (0.0) | 2 (0.2) | 0 | 0 |
| Low risk | ||||
| 1. Wish to be dead | 1 (100) | 0 | 0 | 0 |
| 2. Nonspecific active suicidal thought | 0 | 2 (100) | 0 | 0 |
| Moderate risk | ||||
| 3. Active suicidal ideation with any method (no plan) without intent to act | 0 | 0 | 0 | 0 |
| High risk | ||||
| 4. Active suicidal ideation with some intent to act without specific plan | 0 | 0 | 0 | 0 |
| 5. Active suicidal ideation with specific plan and intent | 0 | 0 | 0 | 0 |
| Postbaseline, No. | 2066 | 1228 | 152 | 146 |
| Participants with suicidal ideation | 8 (0.4) | 7 (0.6) | 1 (0.7) | 2 (1.4) |
| Low risk | ||||
| 1. Wish to be dead | 8 (100) | 7 (100) | 1 (100) | 2 (100) |
| 2. Nonspecific active suicidal thought | 3 (37.5) | 2 (28.6) | 0 | 2 (100) |
| Moderate risk | ||||
| 3. Active suicidal ideation with any method (no plan) without intent to act | 1 (12.5) | 1 (14.3) | 0 | 0 |
| High risk | ||||
| 4. Active suicidal ideation with some intent to act without specific plan | 1 (12.5) | 0 | 0 | 0 |
| 5. Active suicidal ideation with specific plan and intent | 0 | 0 | 0 | 0 |
Percentages for participants with suicidal ideation are based on participants with an observation at a visit and percentages for participants answering yes to C-SSRS questions 1 to 5 are based on participants with suicidal ideation. Observed data from in-trial period. No. represents the number of participants with an observation at the visit. Lifetime assessment was performed at the screening visit. The baseline assessment was at the randomization visit. Postbaseline is the participant’s worst assessment after the randomization visit.
From randomization through end of treatment in STEP 1, 2, and 3, 8 participants (0.4%) receiving semaglutide, 2.4 mg, and 7 (0.6%) receiving placebo reported incident SI; all but 1 case was characterized as low to moderate risk (Table 3). In STEP 5, 1 (0.7%) and 2 participants (1.4%) receiving semaglutide, 2.4 mg, and placebo, respectively, reported incident SI. Twelve of 18 SI cases (67%) had resolved by the end of the trial, spread evenly across groups; time of onset, nature, severity, and resolution are reported in eTable 7 in Supplement 5.
One participant who received semaglutide, 2.4 mg, and acknowledged incident SI (on the C-SSRS at week 36 and as a serious AE) also reported suicidal behavior (suicide attempt) at week 36 on the C-SSRS (STEP 1; eTable 7 in Supplement 5). Thereafter, the participant experienced low-risk SI at week 52. All reports of SI and suicidal behavior were resolved by the end of the trial. At baseline, the participant’s PHQ-9 score suggested mild symptoms of depression (total score 6), increasing to moderately severe symptoms (total score 16) at week 36 and returning to 6 at the end of the trial.
SI and Suicidal Behavior as Assessed by AE Reporting and the PHQ-9
During clinic visits in STEP 1, 2, and 3, 6 participants (0.3%) receiving semaglutide, 2.4 mg, and 2 (0.2%) receiving placebo reported AEs of SI (Table 4), 1 of which was classed as a serious AE in the semaglutide, 2.4 mg, group, as mentioned previously. No participants in STEP 5 reported these behaviors. One participant who received placebo reported a serious AE of suicide attempt during STEP 1 (Table 4). The participant had a history of depression and mood swings, and at the first visit had mild symptoms of depression (PHQ-9 score of 5). The participant did not show worsening on the PHQ-9 or report SI on the C-SSRS. At visits before and after the attempt, the PHQ-9 score remained at 2. The participant was hospitalized for 2 days.
Table 4. Psychiatric and Nervous System Disorder Adverse Events (AEs) by System Organ Class (SOC) and High-Level Group Term (HLGT)a.
| AEs by SOC and HLGT | STEP 1, 2, and 3 | STEP 5 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Semaglutide, 2.4 mg (n = 2116) | Placebo (n = 1261) | Semaglutide, 2.4 mg (n = 152) | Placebo (n = 152) | |||||||||
| No. (%)b | No. of events | Rb | No. (%)b | No. of events | Rb | No. (%)b | No. of events | Rb | No. (%)b | No. of events | Rb | |
| Psychiatric disorders | 214 (9.8) | 295 | 9.5 | 126 (10.5) | 168 | 10.1 | 26 (17.1) | 34 | 10.6 | 26 (17.1) | 31 | 10.3 |
| Sleep disorders and disturbances | 74 (3.4) | 86 | 2.8 | 43 (3.6) | 49 | 3.0 | 6 (3.9) | 7 | 2.2 | 5 (3.3) | 5 | 1.7 |
| Anxiety disorders and symptoms | 75 (3.3) | 84 | 2.7 | 50 (4.2) | 52 | 3.1 | 11 (7.2) | 13 | 4.0 | 14 (9.2) | 15 | 5.0 |
| Depressed mood disorders and disturbances | 61 (2.8) | 68 | 2.2 | 38 (3.2) | 40 | 2.4 | 7 (4.6) | 8 | 2.5 | 5 (3.3) | 5 | 1.7 |
| Mood disorders and disturbances | 19 (0.9) | 21 | 0.7 | 6 (0.5) | 7 | 0.4 | 1 (0.7) | 1 | 0.3 | 2 (1.3) | 2 | 0.7 |
| Sexual dysfunctions, disturbances, and gender identity disorders | 10 (0.4) | 10 | 0.3 | 2 (0.2) | 2 | 0.1 | 2 (1.3) | 2 | 0.6 | 0 | 0 | 0 |
| Suicidal and self-injurious behaviors | 6 (0.3) | 6 | 0.2 | 3 (0.3) | 5 | 0.3 | 0 | 0 | 0 | 0 | 0 | 0 |
| Suicidal ideationc | 6 (0.3) | 6 | 0.2 | 2 (0.2) | 4 | 0.2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Suicide attemptc | 0 | 0 | 0 | 1 (<0.1) | 1 | <0.1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Adjustment disorders | 5 (0.3) | 5 | 0.2 | 5 (0.4) | 5 | 0.3 | 1 (0.7) | 1 | 0.3 | 3 (2.0) | 3 | 1.0 |
| Changes in physical activity | 5 (0.2) | 5 | 0.2 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (0.7) | 1 | 0.3 |
| Cognitive and attention disorders and disturbances | 3 (0.1) | 4 | 0.1 | 3 (0.3) | 3 | 0.2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Delirium (including confusion) | 2 (<0.1) | 3 | <0.1 | 1 (<0.1) | 1 | <0.1 | 2 (1.3) | 2 | 0.6 | 0 | 0 | 0 |
| Manic and bipolar mood disorders and disturbances | 1 (<0.1) | 1 | <0.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Psychiatric and behavioral symptoms | 1 (<0.1) | 1 | <0.1 | 2 (0.2) | 2 | 0.1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Communication disorders and disturbances | 1 (<0.1) | 1 | <0.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Psychiatric disorders | 0 | 0 | 0 | 1 (<0.1) | 1 | <0.1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Somatic symptom and related disorders | 0 | 0 | 0 | 1 (<0.1) | 1 | <0.1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nervous system disorders | 644 (29.7) | 1220 | 39.2 | 262 (21.1) | 393 | 22.7 | 49 (32.2) | 114 | 35.4 | 40 (26.3) | 91 | 30.2 |
| Headaches | 371 (16.8) | 669 | 21.2 | 142 (11.7) | 185 | 10.9 | 23 (15.1) | 64 | 19.9 | 24 (15.8) | 43 | 14.3 |
| Neurological disorders | 307 (14.1) | 425 | 13.7 | 98 (7.8) | 129 | 7.3 | 30 (19.7) | 41 | 12.7 | 15 (9.9) | 28 | 9.3 |
| Spinal cord and nerve root disorders | 39 (1.8) | 42 | 1.4 | 21 (1.7) | 21 | 1.2 | 3 (2.0) | 3 | 0.9 | 6 (3.9) | 6 | 2.0 |
| Peripheral neuropathies | 25 (1.2) | 27 | 0.9 | 23 (1.8) | 25 | 1.4 | 1 (0.7) | 2 | 0.6 | 4 (2.6) | 6 | 2.0 |
| CNS vascular disorders | 12 (0.7) | 15 | 0.6 | 4 (0.3) | 5 | 0.2 | 0 | 0 | 0 | 3 (2.0) | 3 | 1.0 |
| Mental impairment disorders | 11 (0.5) | 11 | 0.4 | 10 (0.8) | 11 | 0.6 | 0 | 0 | 0 | 2 (1.3) | 2 | 0.7 |
| Movement disorders, including parkinsonism | 10 (0.4) | 11 | 0.3 | 3 (0.3) | 4 | 0.2 | 1 (0.7) | 1 | 0.3 | 1 (0.7) | 1 | 0.3 |
| Cranial nerve disorders (excluding neoplasms) | 7 (0.3) | 7 | 0.2 | 0 | 0 | 0 | 1 (0.7) | 1 | 0.3 | 2 (1.3) | 2 | 0.7 |
| Sleep disturbances | 5 (0.2) | 5 | 0.2 | 1 (<0.1) | 1 | <0.1 | 1 (0.7) | 1 | 0.3 | 0 | 0 | 0 |
| Encephalopathies | 2 (0.1) | 2 | <0.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Structural brain disorders | 1 (<0.1) | 1 | <0.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Increased intracranial pressure and hydrocephalus | 1 (<0.1) | 2 | <0.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Neurological disorders of the eye | 1 (<0.1) | 1 | <0.1 | 3 (0.2) | 3 | 0.2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Neuromuscular disorders | 1 (<0.1) | 1 | <0.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Demyelinating disorders | 1 (<0.1) | 1 | <0.1 | 2 (0.2) | 2 | 0.1 | 1 (0.7) | 1 | 0.3 | 0 | 0 | 0 |
| Seizures | 0 | 0 | 0 | 5 (0.4) | 7 | 0.4 | 0 | 0 | 0 | 0 | 0 | 0 |
Abbreviations: CNS, central nervous system; R, event rate per 100 patient-years of observation.
Data are for the in-trial period for the safety analysis set. No. represents the number of participants with an observation at the visit. AEs were classed according to the Medical Dictionary for Regulatory Activities, version 22.1 (STEP 1, 2, and 3) or 23.1 (STEP 5).
The percentage of participants experiencing an AE and the event rate were adjusted using the Cochran-Mantel-Haenszel method to account for differences between trials.
The AE of suicidal and self-injurious behaviors is displayed by preferred term in addition to SOC. eTable 8 in Supplement 5 provides the full list of psychiatric disorder AEs by SOC, HLGT, and preferred term.
eTable 6 in Supplement 5 summarizes the number of participants who reported “thoughts that you would be better off dead, or hurting yourself in some way” on the PHQ-9 postbaseline. In STEP 1, 2, and 3, smaller proportions of participants receiving semaglutide, 2.4 mg, than placebo acknowledged these thoughts (3.4% vs 5.0%). Conversely, in STEP 5, a slightly higher proportion of participants treated with semaglutide, 2.4 mg, experienced these thoughts vs placebo (2.6% vs 2.0%).
Neuropsychiatric AEs
Reports of psychiatric disorder AEs were balanced between the semaglutide, 2.4 mg, and placebo groups in all trials (Table 4; eTable 8 in Supplement 5), including disorders of sleep, anxiety, and depressed mood. Slight imbalances were observed in nervous system disorder AEs between treatment groups, driven mainly by an increased incidence of headaches (STEP 1, 2, and 3) and dizziness (STEP 1, 2, 3, and 5) in the semaglutide, 2.4 mg, group (Table 4; eTable 9 in Supplement 5).
Discussion
In this post hoc analysis of the STEP 1, 2, 3, and 5 trials, semaglutide, 2.4 mg, relative to placebo, was not associated with increases in clinically significant symptoms of depression or in SI or suicidal behavior. Occurrence of these events, as assessed by PHQ-9 and C-SSRS, was low in both groups. Across the 4 trials, only 2.8% and 4.1% of semaglutide-treated and placebo-treated participants, respectively, had a postbaseline PHQ-9 score of 15 or greater, which is suggestive of moderately severe or greater symptoms of depression and requires evaluation by a mental health professional. Both percentages are consistent with the known risk of depression in people with obesity in the general population.2,3,4 Most participants in both groups reported no change or improvement in their mood during the in-trial period, results that are similar to those observed with behavioral weight loss interventions.14,37,38 Although in some analyses semaglutide, 2.4 mg, showed statistically greater improvements in depressive symptoms than placebo, we do not consider these differences to be clinically meaningful.
Incident SI, as assessed by the C-SSRS, was low (≤1%) in all trials, and no imbalance was observed between treatment groups, or as ascertained by AE reporting or question 9 of the PHQ-9. More participants in both groups acknowledged suicidal thoughts when completing the self-reported PHQ-9 than when responding to the clinician-administered C-SSRS. This discrepancy appears attributable to patient discomfort in directly disclosing sensitive information to health care professionals.39 There were 2 reports of suicide attempts in the trials, 1 per treatment group. The participant receiving semaglutide, 2.4 mg, reported depressive symptoms related to life stressors; the participant who received placebo had a history of depression and mood swings, but not SI/suicidal behavior, and the event was potentially linked to life stressors.
Our findings are consistent with a pooled analysis of 5 trials (N = 5325) investigating liraglutide, 3.0 mg, another GLP-1RA approved for chronic weight management.28,29,30 No differences were observed in symptoms of depression, SI, or suicidal behavior between recipients of liraglutide, 3.0 mg, and placebo, as assessed by the same validated measures used in the STEP trials.29 However, AE reporting revealed a minimally higher rate of SI/suicidal behavior with liraglutide, 3.0 mg, than placebo (0.3% vs 0.1%).29 Other reports have identified a slightly higher frequency of SI in people receiving semaglutide and liraglutide compared with other GLP-1RAs or metformin/insulin, although no causality was suggested, and investigations were deferred to the EMA and FDA.40,41
Randomized clinical trials are critical for understanding postmarketing reports of depression and suicidality in the general population prescribed GLP-1RAs for T2D or weight management. After reviewing several datasets, the EMA concluded that “the available evidence does not support a causal association between the GLP-1RAs and suicide and self-injurious thoughts and actions.”20 The FDA, in a preliminary evaluation of similar psychiatric events in individuals prescribed GLP-1RAs or similar incretin-based medications, concluded that it had “not found evidence that use of these medicines causes suicidal thoughts or actions.”32 However, regulators were unable to “definitively rule out that a small risk may exist” due to the small number of SI/suicidal behavior events in controlled and observational trials; surveillance and evaluation will continue.32 We encourage clinicians to monitor mental health in people with overweight or obesity, including those prescribed semaglutide, 2.4 mg, or similar medications. This is essential for ensuring that these individuals obtain the psychiatric care they require to manage anxiety, depression, and related psychiatric complications, which may occur in the presence or absence of weight management interventions.42
Our analysis of SI and suicidal behavior used descriptive statistics rather than formal hypothesis testing due to an inadequate number of events. Other investigators addressed this challenge by using large, aggregated electronic health records. In an observational, retrospective analysis of 52 783 people receiving semaglutide for weight management, semaglutide, compared with non–GLP-1RA antiobesity medications, was associated with a 73% reduction in the risk of SI during the first 6 months of treatment.43 Similar effects of semaglutide in relation to incident anxiety and depression were reported in a nationwide study in Taiwan.44
Other psychiatric safety concerns were not apparent in our analysis, based on the number and type of AEs reported. The incidents of anxiety disorders, depression, and sleep disorders and disturbances were broadly similar between groups. Some nervous system disorders, including dizziness and headaches (known AEs of this treatment), were generally more frequent with semaglutide, 2.4 mg, than placebo.21,22,23,24,25,26 A recent 2-year European database study showed that among 31 444 AE reports submitted for semaglutide, liraglutide, and tirzepatide, only 1.2% were psychiatric AEs.45
Strengths and Limitations
Strengths of our analysis included its relatively large sample size, use of randomized clinical trials, and inclusion of well-validated measures of depressive symptoms, SI, and suicidal behavior, which were administered frequently. The trials also had high retention in both treatment arms (eFigure 2 in Supplement 5), thus limiting the risk of bias due to discontinuations. Limitations included the exclusion of individuals with a lifetime suicide attempt, current high-risk SI, recently diagnosed MDD, and other clinically significant psychopathology. These individuals could be among the most vulnerable in the general population to experiencing psychiatric AEs while taking semaglutide, 2.4 mg, or other weight management medications. A recent pooled analysis found that semaglutide, 2.4 mg, was safe and effective in patients who at baseline took antidepressant medication.46 Controlled investigations and continued postmarketing surveillance are needed to assess the safety and effectiveness of semaglutide, 2.4 mg, and similar medications in people with obesity and major psychopathology.
Conclusions
This post hoc analysis of the STEP 1, 2, 3, and 5 trials found that semaglutide, 2.4 mg, did not increase the risk of developing symptoms of depression, SI, or suicidal behavior relative to placebo in people with overweight or obesity without known major psychopathology. A small number of patients taking semaglutide, 2.4 mg, may experience depression or SI, which may be related to psychosocial complications of obesity or other factors, including life stressors.
Trial protocol and statistical analysis plan for STEP 1
Trial protocol and statistical analysis plan for STEP 2
Trial protocol and statistical analysis plan for STEP 3
Trial protocol and statistical analysis plan for STEP 5
eMethods. Additional methodology for the Columbia-Suicide Severity Rating Scale (C-SSRS)
eResults. Suicidal ideation and behavior measured by Columbia-Suicide Severity Rating Scale
eTable 1. Concomitant Psychiatric and Nervous System Disorders at Screening by System Organ Class and High-Level Group Term and Medication Use at Randomization in Participants in STEP 1–3 and STEP 5
eTable 2. PHQ-9 Score by Week. A) STEP 1–3 Trials and B) STEP 5 Trial
eTable 3. Change from baseline to end of treatment in total PHQ-9 score in STEP 1–3 and STEP 5 using the treatment policy
eTable 4. Change from baseline to end of trial in total PHQ-9 scores by category of body weight loss in STEP 1–3 and STEP 5
eTable 5. Treatment odds ratio of worsening PHQ-9 category and of reaching a total PHQ-9 score of ≥15 in STEP 1–3 and STEP 5
eTable 6. PHQ-9 Maximum Total Scores Post-Baseline Until End of Trial and Responses to Question 9 of the PHQ-9 at Any Time Post-Baseline Until End of Trial in STEP 1–3 and STEP 5
eTable 7. A) Suicidal Ideation and Behavior at Baseline and B) Post-Baseline
eTable 8. Psychiatric Adverse Events by System Organ Class, High-Level Group Term, and Preferred Term
eTable 9. Nervous System Adverse Events by System Organ Class, High-Level Group Term, and Preferred Term
eTable 10. Categorical Shift in Patient Health Questionnaire 9 (PHQ-9) Score from Baseline to End of Treatment in the STEP 1, 2, 3, and 5 Trials
eFigure 1. PHQ-9 Score by Week During the A) STEP 1-3 Trials (pooled) and B) STEP 5 Trial
eFigure 2. Participant flow in the A) pooled STEP 1–3 trials, and B) STEP 5 trial
Data sharing statement
References
- 1.Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15(5):288-298. doi: 10.1038/s41574-019-0176-8 [DOI] [PubMed] [Google Scholar]
- 2.Luppino FS, de Wit LM, Bouvy PF, et al. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry. 2010;67(3):220-229. doi: 10.1001/archgenpsychiatry.2010.2 [DOI] [PubMed] [Google Scholar]
- 3.Onyike CU, Crum RM, Lee HB, Lyketsos CG, Eaton WW. Is obesity associated with major depression? results from the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2003;158(12):1139-1147. doi: 10.1093/aje/kwg275 [DOI] [PubMed] [Google Scholar]
- 4.Simon GE, Von Korff M, Saunders K, et al. Association between obesity and psychiatric disorders in the US adult population. Arch Gen Psychiatry. 2006;63(7):824-830. doi: 10.1001/archpsyc.63.7.824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Emmer C, Bosnjak M, Mata J. The association between weight stigma and mental health: a meta-analysis. Obes Rev. 2020;21(1):e12935. doi: 10.1111/obr.12935 [DOI] [PubMed] [Google Scholar]
- 6.Papadopoulos S, Brennan L. Correlates of weight stigma in adults with overweight and obesity: a systematic literature review. Obesity (Silver Spring). 2015;23(9):1743-1760. doi: 10.1002/oby.21187 [DOI] [PubMed] [Google Scholar]
- 7.Milaneschi Y, Simmons WK, van Rossum EFC, Penninx BW. Depression and obesity: evidence of shared biological mechanisms. Mol Psychiatry. 2019;24(1):18-33. doi: 10.1038/s41380-018-0017-5 [DOI] [PubMed] [Google Scholar]
- 8.Zhang J. The bidirectional relationship between body weight and depression across gender: a simultaneous equation approach. Int J Environ Res Public Health. 2021;18(14):7673. doi: 10.3390/ijerph18147673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ouakinin SRS, Barreira DP, Gois CJ. Depression and obesity: integrating the role of stress, neuroendocrine dysfunction and inflammatory pathways. Front Endocrinol (Lausanne). 2018;9:431. doi: 10.3389/fendo.2018.00431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shelton RC, Miller AH. Inflammation in depression: is adiposity a cause? Dialogues Clin Neurosci. 2011;13(1):41-53. doi: 10.31887/DCNS.2011.13.1/rshelton [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, Lopez JM, Bolge SC, Zhu VJ, Stang PE. Depression among people with type 2 diabetes mellitus, US National Health and Nutrition Examination Survey (NHANES), 2005-2012. BMC Psychiatry. 2016;16:88. doi: 10.1186/s12888-016-0800-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zurita-Cruz JN, Manuel-Apolinar L, Arellano-Flores ML, Gutierrez-Gonzalez A, Najera-Ahumada AG, Cisneros-González N. Health and quality of life outcomes impairment of quality of life in type 2 diabetes mellitus: a cross-sectional study. Health Qual Life Outcomes. 2018;16(1):94. doi: 10.1186/s12955-018-0906-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Picavet HS, Hoeymans N. Health related quality of life in multiple musculoskeletal diseases: SF-36 and EQ-5D in the DMC3 study. Ann Rheum Dis. 2004;63(6):723-729. doi: 10.1136/ard.2003.010769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faulconbridge LF, Driscoll CFB, Hopkins CM, et al. Combined treatment for obesity and depression: a pilot study. Obesity (Silver Spring). 2018;26(7):1144-1152. doi: 10.1002/oby.22209 [DOI] [PubMed] [Google Scholar]
- 15.US Food and Drug Administration . Xenical (orlistat) prescribing information. Accessed March 18, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020766s029lbl.pdf
- 16.US Food and Drug Administration . Contrave (naltrexone-bupropion) prescribing information. Accessed March 18, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/200063s015lbl.pdf
- 17.Christensen R, Kristensen PK, Bartels EM, Bliddal H, Astrup A. Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet. 2007;370(9600):1706-1713. doi: 10.1016/S0140-6736(07)61721-8 [DOI] [PubMed] [Google Scholar]
- 18.Krentz AJ, Fujioka K, Hompesch M. Evolution of pharmacological obesity treatments: focus on adverse side-effect profiles. Diabetes Obes Metab. 2016;18(6):558-570. doi: 10.1111/dom.12657 [DOI] [PubMed] [Google Scholar]
- 19.US Food and Drug Administration Center for Drug Evaluation and Research . Suicidal ideation and behavior: prospective assessment of occurrence in clinical trials (draft guidance). Accessed October 27, 2023. http://www.fda.gov/downloads/Drugs/Guidances/UCM225130.pdf
- 20.European Medicines Agency . Meeting highlights from the Pharmacovigilance Risk Assessment Committee. Accessed June 6, 2024. https://www.ema.europa.eu/en/news/meeting-highlights-pharmacovigilance-risk-assessment-committee-prac-8-11-april-2024
- 21.US Food and Drug Administration . Wegovy (semaglutide) injection prescribing information, revised in December 2022. Accessed October 27, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215256s005lbl.pdf
- 22.Wilding JPH, Batterham RL, Calanna S, et al. ; STEP 1 Study Group . Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989-1002. doi: 10.1056/NEJMoa2032183 [DOI] [PubMed] [Google Scholar]
- 23.Wadden TA, Bailey TS, Billings LK, et al. ; STEP 3 Investigators . Effect of subcutaneous semaglutide vs placebo as an adjunct to intensive behavioral therapy on body weight in adults with overweight or obesity: the STEP 3 randomized clinical trial. JAMA. 2021;325(14):1403-1413. doi: 10.1001/jama.2021.1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garvey WT, Batterham RL, Bhatta M, et al. ; STEP 5 Study Group . Two-year effects of semaglutide in adults with overweight or obesity: the STEP 5 trial. Nat Med. 2022;28(10):2083-2091. doi: 10.1038/s41591-022-02026-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.European Medicines Agency . Wegovy summary of product characteristics. Accessed June 12, 2024. https://www.ema.europa.eu/en/documents/product-information/wegovy-epar-product-information_en.pdf
- 26.Davies M, Færch L, Jeppesen OK, et al. ; STEP 2 Study Group . Semaglutide 2·4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet. 2021;397(10278):971-984. doi: 10.1016/S0140-6736(21)00213-0 [DOI] [PubMed] [Google Scholar]
- 27.Lincoff AM, Brown-Frandsen K, Colhoun HM, et al. ; SELECT Trial Investigators . Semaglutide and cardiovascular outcomes in obesity without diabetes. N Engl J Med. 2023;389(24):2221-2232. doi: 10.1056/NEJMoa2307563 [DOI] [PubMed] [Google Scholar]
- 28.European Medicines Agency . Saxenda 6 mg/mL summary of product characteristics. Accessed March 18, 2024. https://www.ema.europa.eu/en/documents/product-information/saxenda-epar-product-information_en.pdf
- 29.O’Neil PM, Aroda VR, Astrup A, et al. ; Satiety and Clinical Adiposity–Liraglutide Evidence in Individuals With and Without Diabetes (SCALE) Study Groups . Neuropsychiatric safety with liraglutide 3.0 mg for weight management: results from randomized controlled phase 2 and 3a trials. Diabetes Obes Metab. 2017;19(11):1529-1536. doi: 10.1111/dom.12963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.US Food and Drug Administration . Saxenda (liraglutide injection) prescribing information. Accessed March 18, 2024. https://www.novo-pi.com/saxenda.pdf
- 31.Respaurt R, Terhune C. Wegovy, other weight loss drugs scrutinized over reports of suicidal thoughts. Accessed March 18, 2024. https://www.reuters.com/business/healthcare-pharmaceuticals/wegovy-other-weight-loss-drugs-scrutinized-over-reports-suicidal-thoughts-2023-09-28/
- 32.US Food and Drug Administration . Update on FDA’s ongoing evaluation of reports of suicidal thoughts or actions in patients taking a certain type of medicines approved for type 2 diabetes and obesity. Accessed March 18, 2024. https://www.fda.gov/drugs/drug-safety-and-availability/update-fdas-ongoing-evaluation-reports-suicidal-thoughts-or-actions-patients-taking-certain-type
- 33.Kushner RF, Calanna S, Davies M, et al. Semaglutide 2.4 mg for the treatment of obesity: key elements of the STEP trials 1 to 5. Obesity (Silver Spring). 2020;28(6):1050-1061. doi: 10.1002/oby.22794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613. doi: 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Columbia Lighthouse Project . The Columbia protocol (C-SSRS). Accessed March 18, 2024. https://cssrs.columbia.edu/
- 36.Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168(12):1266-1277. doi: 10.1176/appi.ajp.2011.10111704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rubin RR, Wadden TA, Bahnson JL, et al. ; Look AHEAD Research Group . Impact of intensive lifestyle intervention on depression and health-related quality of life in type 2 diabetes: the Look AHEAD Trial. Diabetes Care. 2014;37(6):1544-1553. doi: 10.2337/dc13-1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pagoto S, Schneider KL, Whited MC, et al. Randomized controlled trial of behavioral treatment for comorbid obesity and depression in women: the Be Active Trial. Int J Obes (Lond). 2013;37(11):1427-1434. doi: 10.1038/ijo.2013.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao K, Wu R, Wang Z, et al. Disagreement between self-reported and clinician-ascertained suicidal ideation and its correlation with depression and anxiety severity in patients with major depressive disorder or bipolar disorder. J Psychiatr Res. 2015;60:117-124. doi: 10.1016/j.jpsychires.2014.09.011 [DOI] [PubMed] [Google Scholar]
- 40.Ruggiero R, Mascolo A, Spezzaferri A, et al. Glucagon-like peptide-1 receptor agonists and suicidal ideation: analysis of real-word data collected in the European pharmacovigilance database. Pharmaceuticals (Basel). 2024;17(2):147. doi: 10.3390/ph17020147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McIntyre RS, Mansur RB, Rosenblat JD, Kwan ATH. The association between glucagon-like peptide-1 receptor agonists (GLP-1 RAs) and suicidality: reports to the Food and Drug Administration Adverse Event Reporting System (FAERS). Expert Opin Drug Saf. 2024;23(1):47-55. doi: 10.1080/14740338.2023.2295397 [DOI] [PubMed] [Google Scholar]
- 42.Wadden TA, Chao AM, Moore M, et al. The role of lifestyle modification with second-generation anti-obesity medications: comparisons, questions, and clinical opportunities. Curr Obes Rep. 2023;12(4):453-473. doi: 10.1007/s13679-023-00534-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang W, Volkow ND, Berger NA, Davis PB, Kaelber DC, Xu R. Association of semaglutide with risk of suicidal ideation in a real-world cohort. Nat Med. 2024;30(1):168-176. doi: 10.1038/s41591-023-02672-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsai WH, Sung FC, Chiu LT, Shih YH, Tsai MC, Wu SI. Decreased risk of anxiety in diabetic patients receiving glucagon-like peptide-1 receptor agonist: a nationwide, population-based cohort study. Front Pharmacol. 2022;13:765446. doi: 10.3389/fphar.2022.765446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tobaiqy M, Elkout H. Psychiatric adverse events associated with semaglutide, liraglutide and tirzepatide: a pharmacovigilance analysis of individual case safety reports submitted to the EudraVigilance database. Int J Clin Pharm. 2024;46(2):488-495. doi: 10.1007/s11096-023-01694-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kushner RF, Fink-Jensen A, Frenkel O, et al. Efficacy and safety of semaglutide 2.4 mg according to antidepressant use at baseline: a post hoc subgroup analysis. Obesity (Silver Spring). 2024;32(2):273-280. doi: 10.1002/oby.23946 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol and statistical analysis plan for STEP 1
Trial protocol and statistical analysis plan for STEP 2
Trial protocol and statistical analysis plan for STEP 3
Trial protocol and statistical analysis plan for STEP 5
eMethods. Additional methodology for the Columbia-Suicide Severity Rating Scale (C-SSRS)
eResults. Suicidal ideation and behavior measured by Columbia-Suicide Severity Rating Scale
eTable 1. Concomitant Psychiatric and Nervous System Disorders at Screening by System Organ Class and High-Level Group Term and Medication Use at Randomization in Participants in STEP 1–3 and STEP 5
eTable 2. PHQ-9 Score by Week. A) STEP 1–3 Trials and B) STEP 5 Trial
eTable 3. Change from baseline to end of treatment in total PHQ-9 score in STEP 1–3 and STEP 5 using the treatment policy
eTable 4. Change from baseline to end of trial in total PHQ-9 scores by category of body weight loss in STEP 1–3 and STEP 5
eTable 5. Treatment odds ratio of worsening PHQ-9 category and of reaching a total PHQ-9 score of ≥15 in STEP 1–3 and STEP 5
eTable 6. PHQ-9 Maximum Total Scores Post-Baseline Until End of Trial and Responses to Question 9 of the PHQ-9 at Any Time Post-Baseline Until End of Trial in STEP 1–3 and STEP 5
eTable 7. A) Suicidal Ideation and Behavior at Baseline and B) Post-Baseline
eTable 8. Psychiatric Adverse Events by System Organ Class, High-Level Group Term, and Preferred Term
eTable 9. Nervous System Adverse Events by System Organ Class, High-Level Group Term, and Preferred Term
eTable 10. Categorical Shift in Patient Health Questionnaire 9 (PHQ-9) Score from Baseline to End of Treatment in the STEP 1, 2, 3, and 5 Trials
eFigure 1. PHQ-9 Score by Week During the A) STEP 1-3 Trials (pooled) and B) STEP 5 Trial
eFigure 2. Participant flow in the A) pooled STEP 1–3 trials, and B) STEP 5 trial
Data sharing statement