Abstract
Background/Aim
This study examined the influence of preoperative MRI on the choice of implant volume in patients undergoing subcutaneous mastectomy with immediate breast reconstruction. It was postulated that preoperative MRI scans can adequately estimate glandular tissue, which in turn correlates with implant size.
Patients and Methods
Preoperative and postoperative MRI scans were used in oncological and prophylactical subcutaneous mastectomy scenarios in 67 cases at the Department of Gynaecology, Breast Cancer Center, University of Cologne, Germany. The preoperative MRI was used to estimate the resected tissue and the postoperative MRI was used to scan for residual glandular tissue. In addition, a correlation found by Malter et al. in 2021 was evaluated with the available data.
Results
Preoperative MRIs result in an adequate estimation of resected tissue. This in turn correlates with implant volume. The correlation by Malter et al. also holds when estimating implant volume. The likelihood of residual gland was low if the preoperatively estimate volume was removed.
Conclusion
Our results indicate that the use of preoperative and postoperative MRI scans for subcutaneous mastectomies is advantageous. We suggest a routine estimation of glandular tissue, especially for small breasts.
Keywords: Breast reconstruction, breast implant, implant volume, subcutaneous mastectomy, breast MRI, breast cancer, breast surgery
The standard surgical procedure in modern breast cancer treatment is breast conserving surgery (1-6). In case this is not possible or in prophylactic intention, mastectomy procedures such as subcutaneous mastectomy or nipple sparing mastectomy are sometimes required (7,8). Mastectomy with immediate reconstruction is a safe and cosmetically advantageous method used in both oncological and prophylactic scenarios (9). Currently, implant size is estimated preoperatively with the help of physical examination, which is subjective and often highly dependent on the surgeons` experience. Imaging methods like ultrasound or x-ray-based methods may be used additionally (5). Most commonly however, surgeons use sizers to scale the pocket dimensions and decide on an implant type and volume intraoperatively. This requires a large quantity of implants available on site. Thus, an adequate preoperative estimation of implant volume would be useful for surgical planning and therefore reducing operating times and logistical effort. Cases with significant residual glandular parenchyma in the follow-up MRI, which occur in about 40% of patients after subcutaneous mastectomy, could also be reduced to improve oncological safety (10).
Despite many advantages, determining the volume of the breast tissue in a preoperative MRI is not a standard procedure (10-13). MRIs are routinely used to evaluate tumor size and dispersion in special cases; they can however also be used to preoperatively evaluate and quantify the glandular tissue. Thus, preoperative MRIs might be able to predict the glandular parenchyma to be removed and thus implant size. In addition, a threshold value is set for the surgeon for the minimum amount of tissue to be to be removed.
The aim of this retrospective study was to evaluate the usefulness of preoperative MR mammography for estimating the removed breast tissue and the required implant volume.
We investigated three main hypotheses. First, we investigated the usefulness of pre- and postoperative MRI scans to determine residual glandular parenchyma after subcutaneous mastectomy. We hypothesized that the likelihood of residual glandular parenchyma would be low if the minimum volume of glandular tissue estimated on MRI was removed. We also hypothesized that MRI-estimated glandular parenchyma correlates with implant volume and that the correlation found by Malter et al. on intraoperative implant choice is applicable to our data (14).
Patients and Methods
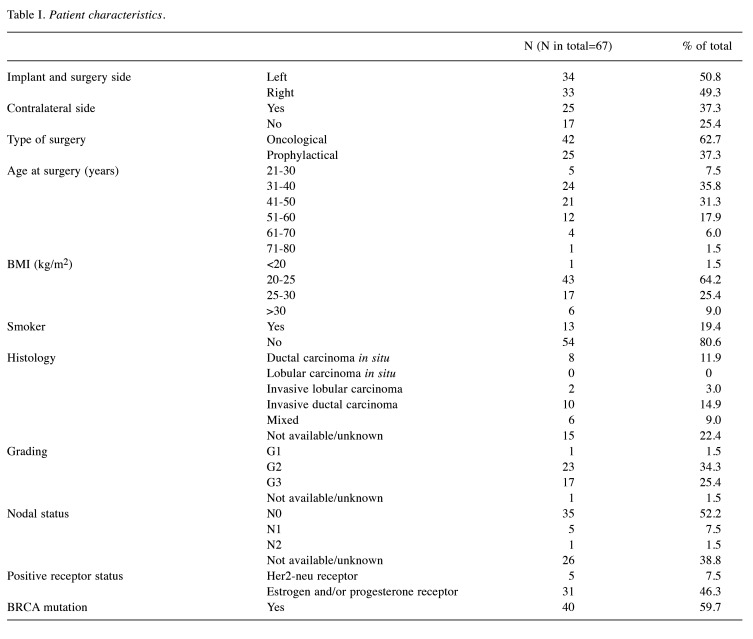
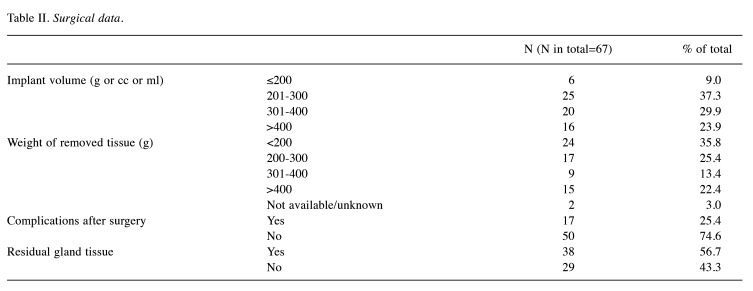
Study design. This cohort study consists of 67 cases that were analyzed retrospectively and anonymously. All patients underwent oncological or prophylactic subcutaneous mastectomy with implant reconstruction at the Breast Cancer Center of the Women’s Hospital of the University of Cologne (Cologne, Germany) between 2009 and 2018. All patients underwent an MRI scan before and six months after surgery to look for residual glandular tissue. The numbers are low as routine MRI examinations before and after surgery have only recently become standard practice. 37.31% (n=25) of patients had bilateral breast surgery. These were counted as two different cases. A summary of patient characteristics is provided in Table I and Table II.
Table I. Patient characteristics.
Table II. Surgical data.
For this study, the implant volume in ml was assumed to be equal to the implant size in cc, which corresponds to the tissue removed in grams. The average implant volume was 325.07 (g, cc, ml) and the average weight of tissue removed was 301.08 g. Although the histology sometimes contained more than one type of carcinoma, only the dominant histology was counted for in Table I. If more than one subtype was dominant, it was counted as ‘mixed histology’. Carcinoma was often graded as G2 and G3, only one sample was graded as G1. Most women had no involved lymph nodes and complications after surgery were rare. Complications included wound healing problems, secondary bleeding, metastasis, or capsular contracture. At follow-up MRI, 56.72% of the sample still had residual glandular parenchyma, although this was always described as a small amount in the radiology reports. To complete the data, the receptor status is listed in Table I. The postoperative reports were followed up until 2021.
MRI examinations. In the preoperative MRI, the pathological breast tissue and the total breast tissue were measured with a margin of 5 mm from the skin using Philips IntelliSpace® (Philips GmbH Market DACH, Hamburg, Germany). This allowed an estimation of the removed tissue while maintaining a 5 mm soft tissue margin around the implant.
During surgery, the weight of tissue removed, and the required implant size were recorded. In the postoperative MRI, the remaining glandular parenchyma was assessed, as this was an important quality feature for the study. It was assessed if and how much glandular parenchyma remained after surgery and if there was a correlation with the volume ratio of the ablated tissue and the pre-scan MRI. If there was less than 10% glandular tissue left or no tissue left at all, no additional intervention was necessary. If more than 10% of glandular tissue remained, revision surgery was required. It should be noted that the radiological assessment of residual glandular tissue is subject to subjective variations and is often given in vague categories such as "little" or "not relevant" in the associated reports.
Statistical analysis. As this was a pilot study, the statistical analysis was initially based on descriptive criteria. The required data was collected and analyzed in Excel by calculating correlation and standard deviation using Pearson’s correlation coefficient. Supplementary diagrams were created to simplify the data analysis.
Results
Usefulness of pre- and postoperative MRI in surgical planning and implant choice. We first compared the weight of the removed tissue with the gland parenchyma measured on preoperative MRI and then with the implant volume determined intraoperatively. Since a linear correlation can be assumed here, a Pearson’s coefficient of determination was calculated.
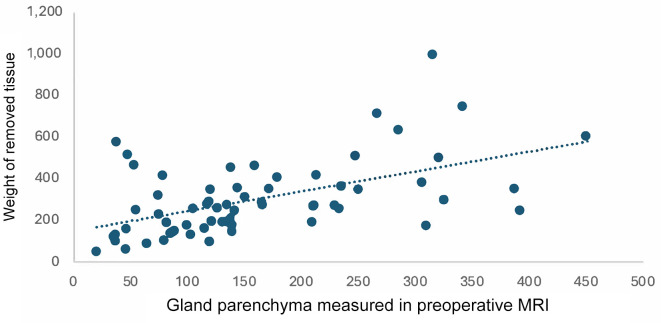
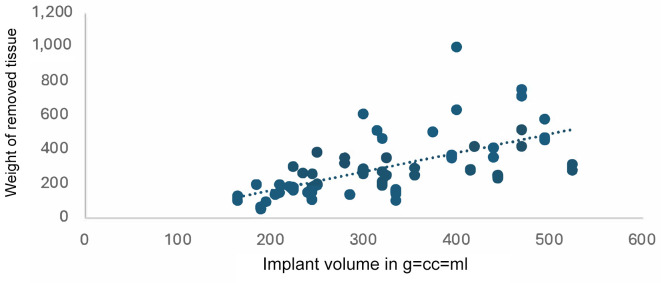
In both cases we found a positive correlation. Two diagrams were created to illustrate this (Figure 1 and Figure 2): the first shows the gland parenchyma measured on the preoperative MRI as a function of the weight of tissue removed (Figure 1) and the second shows the implant volume as a function of the weight of tissue removed (Figure 2). Extremely large and small volumes were removed from the diagrams (n=8 for Figure 1 and n=5 for Figure 2).
Figure 1.
Gland parenchyma measured in the preoperative MRI correlated with the weight of removed tissue. r=0.53 using Pearson’s correlation.
Figure 2.
Implant volume depends on the weight of removed tissue. r=0.61 using Pearson’s correlation.
For both sets of data, the correlation was calculated using data with removed tissue ≤300 g and >300 g. It was noticeable that the correlation for the data ≤300 g was significantly stronger than for the data >300 g in both comparisons.
For the glandular parenchyma measured in the MRI and the weight of the removed tissue, the Pearson correlation was r=0.53 overall (Figure 1). A distinction can be made here between values ≤300 g with r=0.57 and values >300 g (r=0.37). Therefore, the correlation between the weight of the removed tissue and the glandular parenchyma measured preoperatively in the MRI is lower for a weight of more than 300 g than for a weight of less than 300 g. For the correlation between the weight of the removed tissue and the implant volume, Pearson’s correlation coefficient was r=0.61. According to Cohen (1988), this is defined as a strong correlation (15). Again, values for the volume of tissue removed ≤300 g show a higher correlation (r=0.57) than values >300 g (r=0.18).
Overall, there is a correlation between the intraoperatively removed tissue and the preoperatively measured glandular parenchyma. Also, the implant volume seems to correlate to the removed tissue, which is not surprising as this can be used as reference for the intraoperative choice of implant volume. The prediction of implant volume using preoperative MRI is possible but may be more accurate for removed tissue less than 300 g than for larger volumes.
Evaluation of residual tumor and residual gland tissue. Sufficient glandular parenchyma was removed in all surgeries. Overall, residual glandular parenchyma was found in 39 of the 67 patients in the sample. In 30 of these, only very little residual parenchyma was found. Significant residual parenchyma was found in 9 cases (13.4%) but none of the patients in the cohort required follow-up resection during the study period due to more than 10% residual glandular tissue. There was also no association between the indication for surgery (oncological vs. prophylactic) and residual glandular parenchyma. In one oncology patient further surgery was necessary to remove a local recurrence, although no residual glandular tissue was mentioned in the pathology report of this case. Importantly statistical analysis showed no correlation between cases with residual glandular parenchyma on postoperative MRI and the amount of tissue removed compared to preoperative MRI
The Malter et al. correlation equitation. In a previous study Malter et al. established a mathematical function (y=34.71×0.39) to predict the required implant volume after subcutaneous mastectomy when data on the removed tissue are available (14). The formula was applied to our specimen. The arithmetic mean of the ratio of calculated to actual implant volume was found to be 0.99. This confirms a strong correlation.
Discussion
Due to these observations, we can state that pre- and postoperative MRI scans can indeed be useful for surgical planning as the gland parenchyma calculated on MRI correlates with the required implant volume for breast reconstruction. This correlation is greater for below 300 ml than for above 300 ml gland parenchyma which should be taken into account.
The equation set up by Malter et al. in the paper "Correlation analysis of resected breast tissue and implant volume after mastectomy and its association with breast density" is also applicable in this context (14). Other studies also support the correlation between removed tissue weight and implant volume (16).
Our results show that preoperative MRI is helpful to avoid residual tissue based on the calculation of gland volume. As recurrence rates after NSM are generally known to be low, it has been controversial whether preoperative MRI scans can reduce re-excision rates to a statistically significant level (12,17-19). Since none of the patients in this study required a second operation, it can be assumed for this cohort that preoperative MRI was beneficial in this context. Additionally, the incidence of residual gland parenchyma in this study was lower (58.2%) than stated in a previous study (77.8%) (20).
Nevertheless, it must be said that in the present study more tissue was often removed than was predicted by the preoperative MRI and that there was not a strong correlation between the relation between predicted and removed tissue and the presence of residual gland.
Significantly associated factors, such as the distinction between pre- and post-menopausal women and correlating breast density, were not considered separately in this study although it is known, that breast density influences the weight of removed tissue (14,16) and these factors can limit the quality of MRI scans for prediction of removed tissue. Studies indicate that premenopausal women with high breast density may mostly benefit from MRI for surgical planning (21). Contrary to our findings, another study found that preoperative MRI was not associated with a better surgical outcome. Further analysis showed that MRI had no effect on the number of positive margins or the number of reoperations (22). Unlike our study, they only looked at women with DCIS (23). Other studies found that positive surgical margins decreased in women with invasive lobular cancer (24). In addition, there are other factors that affect the outcome of surgery that were not considered in this trial: Delayed time to surgery due to an MRI might be associated with increased mortality (22,23). Another risk associated with preoperative MRI is overdiagnosing (24).
Due to these previous observations pre-operative MRI is not yet a standard procedure and is only recommended in exceptional circumstances (25).
Conclusion
The present study shows that it is possible to measure and estimate the required size of the breast implant preoperatively using MRI, which could lead to savings in time and material during the surgery, thus reducing costs and risks. According to our findings, the use of MRI before and after surgery is beneficial in the regard of surgical planning and avoiding residual tissue in mastectomy and should be considered more often.
Conflicts of Interest
There are no conflicts of interest to be declared in relation to this study.
Authors’ Contributions
RT: literature review, data analysis, manuscript writing. HF: data analysis, manuscript writing, editing. JH: conception, editing. MW: conception, editing, data acquisition. WM: conception, editing, data acquisition. BK: conception, editing, data acquisition. CE: conception, editing, planning.
References
- 1.Giaquinto AN, Sung H, Miller KD, Kramer JL, Newman LA, Minihan A, Jemal A, Siegel RL. Breast cancer statistics, 2022. CA Cancer J Clin. 2022;72(6):524–541. doi: 10.3322/caac.21754. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad A. Breast cancer statistics: recent trends. Adv Exp Med Biol. 2019;1152:1–7. doi: 10.1007/978-3-030-20301-6_1. [DOI] [PubMed] [Google Scholar]
- 3.Eisemann N, Waldmann A, Katalinic A. Epidemiology of breast cancer - current figures and trends. Geburtshilfe Frauenheilkd. 2013;73(2):130–135. doi: 10.1055/s-0032-1328075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 5.Eichler C, Westerhoff A, Warm M, Hanstein B, Puppe J, Krug B, Malter W. Improving breast conserving surgery using the Faxitron® OR specimen radiography system – a complication analysis, cost evaluation and literature review. Anticancer Res. 2022;42(4):1925–1932. doi: 10.21873/anticanres.15670. [DOI] [PubMed] [Google Scholar]
- 6.Piper M, Peled AW, Sbitany H. Oncoplastic breast surgery: current strategies. Gland Surg. 2015;4(2):154–163. doi: 10.3978/j.issn.2227-684X.2015.03.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bertozzi N, Pesce M, Santi PL, Raposio E. Oncoplastic breast surgery: Comprehensive review. Eur Rev Med Pharmacol Sci. 2017;21(11):2572–2585. [PubMed] [Google Scholar]
- 8.Caruso F, Ferrara M, Castiglione G, Trombetta G, De Meo L, Catanuto G, Carillio G. Nipple sparing subcutaneous mastectomy: Sixty-six months follow-up. Eur J Surg Oncol. 2006;32(9):937–940. doi: 10.1016/j.ejso.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 9.Benediktsson KP, Perbeck L. Survival in breast cancer after nipple-sparing subcutaneous mastectomy and immediate reconstruction with implants: A prospective trial with 13 years median follow-up in 216 patients. Eur J Surg Oncol. 2008;34(2):143–148. doi: 10.1016/j.ejso.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 10.Kaidar-Person O, Offersen BV, Boersma LJ, de Ruysscher D, Tramm T, Kühn T, Gentilini O, Mátrai Z, Poortmans P. A multidisciplinary view of mastectomy and breast reconstruction: Understanding the challenges. Breast. 2021;56:42–52. doi: 10.1016/j.breast.2021.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arnaout A, Catley C, Booth CM, McInnes M, Graham I, Kumar V, Simos D, Van Walraven C, Clemons M. Use of preoperative magnetic resonance imaging for breast cancer. JAMA Oncol. 2015;1(9):1238. doi: 10.1001/jamaoncol.2015.3018. [DOI] [PubMed] [Google Scholar]
- 12.Vos EL, Voogd AC, Verhoef C, Siesling S, Obdeijn IM, Koppert LB. Benefits of preoperative MRI in breast cancer surgery studied in a large population-based cancer registry. Br J Surg. 2015;102(13):1649–1657. doi: 10.1002/bjs.9947. [DOI] [PubMed] [Google Scholar]
- 13.Colwell AS, Christensen JM. Nipple-sparing mastectomy and direct-to-implant breast reconstruction. Plast Reconstr Surg 140(5S Advances in Breast. 2017;Reconstruction):44S–50S. doi: 10.1097/PRS.0000000000003949. [DOI] [PubMed] [Google Scholar]
- 14.Malter W, Bachmann BJ, Krug B, Hellmich M, Zinser M, Mallmann P, Eichler C, Puppe J. Correlation analysis of resected breast tissue and implant volume after mastectomy and its association with breast density. Arch Gynecol Obstet. 2022;305(1):169–177. doi: 10.1007/s00404-021-06128-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eid M, Gollwitzer M, Schmitt M. Statistik und Forschungsmethoden. Basel, Beltz Verlag. 2015:pp. 529. [Google Scholar]
- 16.Wazir U, El Hage Chehade H, Choy C, Kasem A, Mokbel K. A study of the relation between mastectomy specimen weight and volume with implant size in oncoplastic reconstruction. In Vivo. 2019;33(1):125–132. doi: 10.21873/invivo.11448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mann RM, Loo CE, Wobbes T, Bult P, Barentsz JO, Gilhuijs KGA, Boetes C. The impact of preoperative breast MRI on the re-excision rate in invasive lobular carcinoma of the breast. Breast Cancer Res Treat. 2010;119(2):415–422. doi: 10.1007/s10549-009-0616-6. [DOI] [PubMed] [Google Scholar]
- 18.Houssami N, Turner R, Morrow M. Preoperative magnetic resonance imaging in breast cancer. Ann Surg. 2013;257(2):249–255. doi: 10.1097/SLA.0b013e31827a8d17. [DOI] [PubMed] [Google Scholar]
- 19.Houssami N, Turner R, Macaskill P, Turnbull LW, McCready DR, Tuttle TM, Vapiwala N, Solin LJ. An individual person data meta-analysis of preoperative magnetic resonance imaging and breast cancer recurrence. J Clin Oncol. 2014;32(5):392–401. doi: 10.1200/JCO.2013.52.7515. [DOI] [PubMed] [Google Scholar]
- 20.Park KU, Tozbikian GH, Ferry D, Tsung A, Chetta M, Schulz S, Skoracki R. Residual breast tissue after robot-assisted nipple sparing mastectomy. Breast. 2021;55:25–29. doi: 10.1016/j.breast.2020.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Debald M, Abramian A, Nemes L, Dobler M, Kaiser C, Keyver-Paik MD, Leutner C, Holler T, Braun M, Kuhl C, Kuhn W, Schild HH. Who may benefit from preoperative breast MRI? A single-center analysis of 1102 consecutive patients with primary breast cancer. Breast Cancer Res Treat. 2015;153(3):531–537. doi: 10.1007/s10549-015-3556-3. [DOI] [PubMed] [Google Scholar]
- 22.Houssami N, Turner RM, Morrow M. Meta-analysis of pre-operative magnetic resonance imaging (MRI) and surgical treatment for breast cancer. Breast Cancer Res Treat. 2017;165(2):273–283. doi: 10.1007/s10549-017-4324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fancellu A, Turner RM, Dixon JM, Pinna A, Cottu P, Houssami N. Meta-analysis of the effect of preoperative breast MRI on the surgical management of ductal carcinoma in situ. Br J Surg. 2015;102(8):883–893. doi: 10.1002/bjs.9797. [DOI] [PubMed] [Google Scholar]
- 24.Lobbes MB, Vriens IJ, van Bommel AC, Nieuwenhuijzen GA, Smidt ML, Boersma LJ, van Dalen T, Smorenburg C, Struikmans H, Siesling S, Voogd AC, Tjan-Heijnen VC. Breast MRI increases the number of mastectomies for ductal cancers, but decreases them for lobular cancers. Breast Cancer Res Treat. 2017;162(2):353–364. doi: 10.1007/s10549-017-4117-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wöckel A, Festl J, Stüber T, Brust K, Krockenberger M, Heuschmann PU, Jírů-Hillmann S, Albert US, Budach W, Follmann M, Janni W, Kopp I, Kreienberg R, Kühn T, Langer T, Nothacker M, Scharl A, Schreer I, Link H, Engel J, Fehm T, Weis J, Welt A, Steckelberg A, Feyer P, König K, Hahne A, Baumgartner T, Kreipe HH, Knoefel WT, Denkinger M, Brucker S, Lüftner D, Kubisch C, Gerlach C, Lebeau A, Siedentopf F, Petersen C, Bartsch HH, Schulz-Wendtland R, Hahn M, Hanf V, Müller-Schimpfle M, Henscher U, Roncarati R, Katalinic A, Heitmann C, Honegger C, Paradies K, Bjelic-Radisic V, Degenhardt F, Wenz F, Rick O, Hölzel D, Zaiss M, Kemper G, Budach V, Denkert C, Gerber B, Tesch H, Hirsmüller S, Sinn HP, Dunst J, Münstedt K, Bick U, Fallenberg E, Tholen R, Hung R, Baumann F, Beckmann MW, Blohmer J, Fasching P, Lux MP, Harbeck N, Hadji P, Hauner H, Heywang-Köbrunner S, Huober J, Hübner J, Jackisch C, Loibl S, Lück HJ, von Minckwitz G, Möbus V, Müller V, Nöthlings U, Schmidt M, Schmutzler R, Schneeweiss A, Schütz F, Stickeler E, Thomssen C, Untch M, Wesselmann S, Bücker A, Buck A, Stangl S. Interdisciplinary screening, diagnosis, therapy and follow-up of breast cancer. Guideline of the DGGG and the DKG (S3-level, AWMF registry number 032/045OL, December 2017) - Part 2 with recommendations for the therapy of primary, recurrent and advanced breast cancer. Geburtshilfe Frauenheilkd. 2018;78(11):1056–1088. doi: 10.1055/a-0646-4630. [DOI] [PMC free article] [PubMed] [Google Scholar]