Abstract
Background:
Evidence-based timeliness benchmarks have been established to assess quality of breast cancer care, as delays in treatment are associated with poor clinical outcomes. However, few studies have evaluated how current breast cancer care meets these benchmarks and what factors may delay the timely initiation of treatment.
Patients and Methods:
Demographic and disease characteristics of 377 newly diagnosed patients with breast cancer who initiated treatment at Tufts Medical Center (2009–2015) were extracted from electronic medical records. Time from diagnosis to initial surgery and time from diagnosis to initiation of hormone therapy were estimated with Kaplan-Meier curves. Multivariable regression analysis was used to identify factors associated with treatment delays. Thematic analysis was performed to categorize reasons for delay.
Results:
Of 319 patients who had surgery recommended as the first treatment, 248 (78%) met the 45-day benchmark (median, 28 days; 25th-75th %, 19–43). After adjusting for potential confounders, multivariable regression analysis revealed that negative hormone receptor status (odds ratio, 3.48; 95% confidence interval, 1.44–8.43) and mastectomy (odds ratio, 4.07; 95% confidence interval, 2.10–8.06) were significantly associated with delays in surgery. Delays were mostly owing to clinical complexity or logistical/financial reasons. Of 241 patients eligible for hormone therapy initiation, 232 (96%) met the 1-year benchmark (median, 147 days; 25th-75th %, 79–217).
Conclusion:
Most patients met timeliness guidelines for surgery and initiation of hormone therapy, although risk factors for delay were identified. Knowledge of reasons for breast cancer treatment delay, including clinical complexity and logistical/financial issues, may allow targeting interventions for patients at greatest risk of care delays.
Keywords: Breast cancer, Treatment initiation, Timeliness benchmark, Surgery, Hormone therapy
Introduction
Breast cancer is the most common cancer in women, and it is the second leading cause of cancer-related death in women in the United States (US).1 Although breast cancer has the highest incidence of all cancers affecting women, in recent years, its incidence and mortality rates have declined,2,3 in part owing to increased awareness and education, better screening technology for early detection, improvements in evidence-based treatment regimens, and the decreased general use of hormone replacement therapy.3–6
In spite of overall improved survival rates, not all patients have benefited equally from these advances. Multiple studies have documented breast cancer disparities by socioeconomic and race/ethnic groups.7–10 Delayed initiation of effective treatment is known to be associated with poor clinical outcomes, such as higher recurrence and mortality rates.11–13 A multinational analysis showed an average patient-related delay time of 4.7 weeks from initial diagnosis to treatment initiation, with delays being associated with patients’ feelings of distrust and disregard.14 Delays in treatment initiation may be one of the reasons for disparities in breast cancer.12,15,16 Moreover, a cohort study of 6622 women with stage I to III breast cancer across 9 National Comprehensive Cancer Network centers showed that delays in initiation of chemotherapy increased monotonically over the study period from 10.8 weeks in 2003 to 13.3 weeks in 2009.10 Thus, the effects of delayed treatment are an increasingly important consideration.8,10
Many efforts have been made to eliminate delay-related breast cancer disparities, particularly for early stage disease, including establishment of quality benchmarks by several disease-specific consortia and government agencies, such as the National Quality Forum (NQF)17–20 and the Michigan Breast Oncology Quality Initiative (MiBOQI).21,22 Although the timeliness benchmarks have been established for years, few studies have evaluated how current breast cancer care meets these benchmarks. Furthermore, factors associated with meeting the timeliness benchmarks also remain unclear.
Given the high frequency of surgery and hormone therapy use in the study population, we evaluated 2 timeliness benchmarks, the 45-day time-to-surgery benchmark from diagnosis established by MiBOQI21,22 and the 1-year time-to-hormone therapy from diagnosis by NQF.17–20 Given that breast cancer survival is in part dependent upon the time from diagnosis to treatment, efforts to expedite initiation of breast cancer care have great clinical value. Understanding which specific factors at the patient level that may lead to delayed treatment is crucial in informing the development and implementation of thoughtful interventions targeted at optimizing the timely initiation of breast cancer treatment.
Patients and Methods
Study Population and Data Collection
This retrospective single-center cohort study identified 377 incident cases from the Tufts Medical Center (MC) Tumor Registry of stage I to IV breast cancer among patients diagnosed at Tufts MC from 2009 through 2015. Patients with either recurrent ipsilateral breast cancer or those diagnosed elsewhere were excluded. Patient demographics and disease characteristics were retrieved from the electronic medical record. The biopsy date that led to a definitive diagnosis of new breast cancer (“diagnostic biopsy”) was recorded as the diagnosis date. Clinical notes (inclusive of medical, surgical, radiation therapy) were reviewed for up to 1 year from the diagnosis date. Using study-specific abstraction forms, trained study staff dually abstracted all data. Clinical variables (eg, comorbidity status, staging, incident vs. recurrent disease) were confirmed by study oncologists (K.E., J.K.E., S.K.P.). All data were entered into the web-based clinical data management platform Research Electronic Data Capture (REDCap). The study was approved by the Tufts MC Institutional Review Board.
Variables
Demographics included date at diagnosis, gender, race, language, marital status, street address, and health insurance coverage. The median household income by census tract was obtained from the US Census Bureau’s American Fact Finder23 using zip code. Family support was defined as any clinic note documenting presence of a family member on at least 1 occasion during the 1 year of follow-up. Insurance plans were obtained from the administrative database and then categorized into “Private only” and “Any subsidized plan” (including any Medicare, Medicaid, and/or any health insurance exchange product).
Disease and clinical characteristics including the date of diagnosis, hormone receptor status (ie, estrogen and/or progesterone), human epidermal growth factor receptor 2 (HER2) status, family history of breast cancer, and prior history of breast cancer treatment were abstracted from clinical notes. “Multiple biopsies” was defined as receipt of more than 1 biopsy prior to the final determination of the cancer diagnosis. In contrast, the “Diagnostic biopsy” was defined as the first histologic evidence of cancer. Tumor stage was recorded in anatomic stages (I-IV) based on the American Joint Committee on Cancer breast cancer staging systems, 7th edition,24 in use at the time of the study. Hormone receptor status for the estrogen receptor (ER), progesterone receptor (PR), and HER2 were recorded in the medical record, based on American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP) guidelines and dichotomized as “positive for ER and/or positive for PR” or “negative for ER and PR.”25,26 HER2 status was recorded as positive or negative. Triple-negative breast cancer was defined as negative ER, PR, and HER2. Family history of breast cancer was defined as any first and/or second-degree relatives with a prior breast cancer diagnosis. Comorbidity at the time of diagnosis was abstracted, and the modified Charlson Comorbidity score was calculated according to the Charlson Comorbidity Index.27–29 The comorbidity status was dichotomized into: no comorbidity (score = 0) and any comorbidity (score = 1–8) for analysis.
Treatment information was collected by recommended modality (ie, surgery, chemotherapy, radiation, hormone therapy). For each modality, data were collected by date of initiation, specific treatment features (eg, surgery type, chemotherapy drugs and regimens, HER2-targeted therapy, radiation field and dose, and hormone therapy).
Primary Outcome
Evaluable cases for surgery and hormone therapy benchmarks were identified, and the proportion of patients who met each benchmark was calculated. Thematic analysis was conducted to ascertain reasons for delay in extreme cases by reviewing the individuals with the top quartile (n = 18) of surgery delay (not meeting the 45-day time-to-surgery benchmark) and all cases (n = 9) with hormone therapy delay (not meeting the 1-year benchmark). Reasons for delays were categorized into clinical issues (eg, clinical complexity and multiple biopsies), personal issues (eg, insurance change, gap in medication coverage, seeking a second opinion, treatment indecision, family-related issues [eg, within-family disagreement about preferred course], and missing appointments), or an undocumented reason.
Statistical Analysis
Patient demographics and disease characteristics were summarized with descriptive statistics. Continuous variables were summarized with means (SD) or medians (25th-75th percentiles), and binary variables were summarized as total number and percentage (n, %). The Student t test compared mean age, and the Mann-Whitney test compared medians of household income between the groups who reached and who did not reach the benchmark. The Fisher exact test compared race/ethnicity and tumor stage, and the X2 test compared all other categorical variables.
The timeliness benchmark for surgery measures initiation of surgery within 45 days of the initial diagnosis for patients with surgery recommended as the first treatment.21,22 The benchmark of adjuvant hormone treatment measures initiation of hormone therapy within 1 year of diagnosis in stage I to III hormone receptor-positive patients 18 years of age and older. The total number (N) and the percentage of evaluable cases meeting and not meeting each benchmark (%) were reported. Kaplan-Meier survival analysis and median day (25th- 75th percentiles) were used to report time-to treatment initiation.
Univariable analysis and multivariable logistic regression were conducted to identify factor(s) associated with meeting the 45-day time-to surgery benchmark. The following variables were included in the multivariable model regardless of univariable screening: age at time of diagnosis, insurance, family history of breast cancer, prior history of breast cancer treatment, and comorbidity. Variables with a P-value of less than .2 from univariable screening were added to the multivariable regression model. Model goodness-of-fit was estimated using c-statistics. Odds ratios (ORs) and 95% confidence intervals (CIs) were estimated. Two-sided statistical tests were performed in R (Version 3.5.0, https://www.r-project.org/) with alpha = 0.05.
Results
Patient Characteristics
Patient characteristics are summarized in Table 1. The majority of patients were white (262; 69%) and English-speakers (321; 85%). More than one-half (211; 56%) of patients had subsidized insurance, which included 30% Medicare, 15% Medicaid, and 11% dual Medicare and Medicaid coverage. The majority (356; 94%) of patients had early stage (stage I-III) breast cancer. Three hundred fifteen (84%) patients were ER-positive and/or PR-positive, indicating possible recommendation of hormone therapy. Forty-five (12%) patients were HER2-positive. One hundred thirty-one (35%) patients had at least 1 documented comorbidity at the time of diagnosis. The majority (322; 85%) of patients had surgery, and 34 (9%) of patients had neoadjuvant chemotherapy as the first treatment regimen.
Table 1.
Patient demographic, disease and clinical characteristics
| Patient characteristic | n(%) |
|---|---|
| Total | 377a |
| Age in years, mean(SD), y | 61 (13) |
| Race/ethnicity | |
| White | 262 (69) |
| Chinese origin | 48 (13) |
| Black/African-American | 40 (11) |
| Hispanic/Latino | 11 (3) |
| Others | 14 (4) |
| Language | |
| English | 321 (85) |
| All others | 56 (15) |
| Census tract median household income, median (25th, 75th percentiles),$ | 71,367 (57,360 – 92,093) |
| Insurance | |
| Private only | 153 (41) |
| Any subsidizedb | 211 (56) |
| Married / Partnered | 211 (56) |
| Tumor stage | |
| I | 216 (57) |
| II | 98 (26) |
| III | 42 (11) |
| IV | 21 (6) |
| HER2-positive | 45 (12) |
| ER- and/or PR-positive | 315 (84) |
| Triple-negative | 45 (12) |
| Had multiple biopsies | 53 (14) |
| Had family history of breast cancer | 164 (44) |
| Had prior history of breast cancer treatment | 35 (9) |
| Had any comorbidity (Charlson score 1–8) | 131 (35) |
| Modality of first treatment | |
| No initiation of treatment | 10 (3) |
| Neoadjuvant chemotherapy | 34 (9) |
| Surgery | 322 (85) |
| Hormone therapy | 10 (3) |
Abbreviations: ER = estrogen receptor; HER2 = human epidermal growth factor receptor 2; PR = progesterone receptor; SD = standard deviation.
All newly diagnosed stage I to IV breast cancer patients presented at Tufts MC from 2009 through 2015.
”Any subsidized” includes any Medicare, MassHealth (MA Medicaid), and/or any health insurance exchange product.
Timeliness Benchmarks for Surgery and Hormone Therapy
Among the 319 patients with stage I to III disease evaluable for the 45-day surgery benchmark, 248 (78%) met the benchmark, with a median time from diagnosis to surgery of 28 days (25th-75th percentile, 19–43 days) (Figure 1A). Among 241 (241/377; 64%) patients evaluable for the 1-year hormone therapy benchmark, 96% (232/241) met the benchmark, with a median of 147 days (25th-75th percentile, 79–217 days) (Figure 1B).
Figure 1.
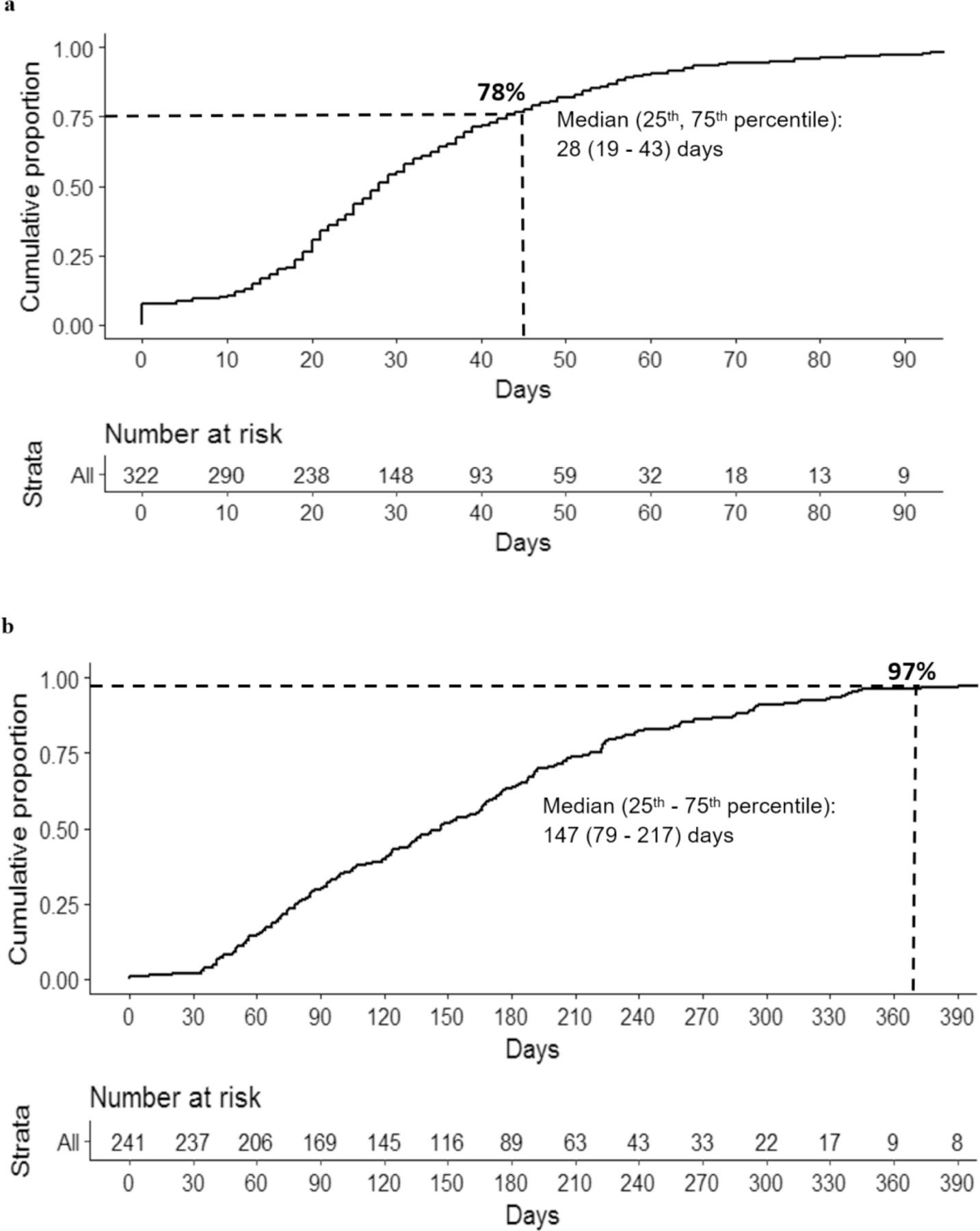
a. Kaplan Meier curve summarizes the timeliness of care of the 45-day time-to-surgery benchmark. The number of patients who had not received surgery over time is presented at the bottom of the figure. At day 45, 77% of patients who had surgery as the first recommended treatment received surgery. b. Kaplan Meier curve summarizes the timeliness of care of the 1-year (yr) time-to-hormone therapy benchmark. Median day (25th- 75th %). The number of patients who had not started hormone therapy over time is presented at the bottom of the figure. At 1 yr, 97% of patients who were stage I-III and hormone receptor-positive started the hormone therapy.
Factors Associated With Not Meeting the 45-day Surgery Benchmark
Characteristics of the patients who met versus did not meet the time to surgery benchmark are summarized in Table 2. Univariable analysis showed that younger age, living in a community with lower median household income, negative hormone receptor status, presence of comorbidity at diagnosis, and recommendation of mastectomy surgery type were significantly associated with not meeting the 45-day time to surgery benchmark. Multivariable regression (Table 3) showed that after adjusting for age, median household income, insurance, HER2 status, family history, prior breast cancer treatment, and comorbidity, negative hormone receptor status (OR, 3.48; 95%CI, 1.44–8.43) and mastectomy surgery-type (OR, 4.07; 95% CI, 2.10–8.06) were significantly associated with not meeting the 45-day-surgery benchmark (c-statistic = 0.7756).
Table 2.
Comparison of factors associated with not meeting the 45-day surgery benchmark.
| Patient characteristic (n=322a) | > 45 days (N= 71, 22%), n (%) |
≤ 45 days (N= 250, 78%), n (%) |
P value |
|---|---|---|---|
| Age, mean (SD), y | 59 (14) | 62 (13) | .039 e |
| Race/ethnicity | .221d | ||
| White | 47 (67) | 178 (72) | |
| Chinese origin | 8 (11) | 30 (12) | |
| Black/African-American | 7 (10) | 28 (11) | |
| Others | 9 (13) | 11 (4) | |
| Language | .715c | ||
| English | 62 (87) | 210 (85) | |
| All others | 9 (13) | 38 (15) | |
| Census tract median household income, median (25th-75th percentiles), $ | 64,675 (50,934–79,492) | 77,845 (60,589–94,891) | 0.003 f |
| Insurance | .903c | ||
| Private only | 28 (39) | 101 (41) | |
| Any subsidizedb | 42 (59) | 141 (57) | |
| Married / Partnered | 43 (61) | 135 (54) | .435c |
| Tumor stage | .303d | ||
| I | 42 (59) | 170 (69) | |
| II | 22 (31) | 60 (24) | |
| III | 7 (10) | 18 (7) | |
| ER- and/or PR-positive | 55 (77) | 224 (91) | .005 c |
| HER2-positive | 10 (14) | 25 (10) | .475c |
| Had multiple biopsies | 12 (18) | 28 (12) | .275c |
| Had family history of breast cancer | 26 (39) | 115 (47) | .283c |
| Had prior history of breast cancer treatment | 9 (13) | 23 (9) | .502c |
| Had any comorbidity (Charlson score 1–8) | 33 (46) | 80 (33) | .043 c |
| Type of surgery | <.001 c | ||
| Lumpectomy | 33 (46) | 185 (75) | |
| Mastectomy | 37 (53) | 60 (24) |
Bold denotes P < .05.
Abbreviations: ER = estrogen receptor; HER2 = human epidermal growth factor receptor 2; PR = progesterone receptor; SD = standard deviation.
Stage I to III patients who had surgery recommended as the first treatment and received surgery.
”Any subsidized” includes any Medicare, MassHealth (MA Medicaid), and/or any health insurance exchange product.
X2 test.
Fisher exact test.
t test.
Mann-Whitney test.
Table 3.
Risk factors associated with not meeting the 45-day surgery benchmark.
| Patient characteristic (n=319) | Univariable analysis, OR (95% CI) | Adjusted, OR (95% CI) |
|---|---|---|
| Age (10-y increment) | 0.80 (0.65 – 0.98) | 0.78 (0.59 – 1.03) |
| Race | ||
| White | Ref | |
| Chinese origin | 1.01 (0.41 – 2.26) | |
| Black/African-American | 0.95 (0.36 – 2.20) | |
| Others | 2.76 (1.01 – 7.20) | |
| Language | ||
| English | Ref | |
| Non-English | 0.80 (0.35 – 1.68) | |
| Median household income by census tract, $ | ||
| $92,326 - $180,972 | Ref | Ref |
| $73,785 - $92,326 | 1.13 (0.45 – 2.90) | 0.86 (0.28 – 2.56) |
| $58,120 - $73,785 | 2.57 (1.14 – 6.13) | 2.14 (0.84 – 5.71) |
| $13,488 - $58,120 | 3.10 (1.40 – 7.35) | 2.51 (0.98 – 6.85) |
| Insurance | ||
| Private only | Ref | Ref |
| Any subsidizeda | 1.07 (0.63 – 1.86) | 0.92 (0.44 – 1.92) |
| Marital status | ||
| Currently married/partnered | Ref | |
| Single/widowed/divorced/separated | 0.71 (0.47 – 1.06) | |
| Tumor stage | ||
| I | Ref | |
| II | 1.48 (0.81 – 2.67) | |
| III | 1.57 (0.58 – 3.87) | |
| Hormone receptor positivity | ||
| ER- and/or PR-positive | Ref | Ref |
| ER- and/or PR-negative | 2.83 (1.38 – 5.70) | 3.48 (1.44 – 8.43) |
| HER2 positivity | ||
| Positive | Ref | Ref |
| Negative | 1.45 (0.63 – 3.10) | 1.03 (0.38 – 2.62) |
| Multiple biopsy | ||
| No | Ref | |
| Yes | 1.62 (0.75 – 3.32) | |
| Family history of breast cancer | ||
| No | Ref | Ref |
| Yes | 0.71 (0.41 – 1.23) | 0.58 (0.29 – 1.14) |
| Prior history of breast cancer treatment | ||
| No | Ref | Ref |
| Yes | 1.45 (0.61 – 3.21) | 1.18 (0.37 – 3.38) |
| Charlson Comorbidity Score | ||
| 0 | Ref | Ref |
| 1–8 | 1.80 (1.05 – 3.09) | 1.77 (0.89 – 3.50) |
| Surgery type | ||
| Lumpectomy | Ref | Ref |
| Mastectomy | 3.46 (1.99 – 6.04) | 4.07 (2.010 – 8.06) |
Bold denotes P < .05.
Abbreviations: CI = confidence interval; ER = estrogen receptor; HER2 = human epidermal growth factor receptor 2; OR = odds ratio; PR = progesterone receptor; Ref = reference; SD = standard deviation.
Any subsidized includes any Medicare, MassHealth (MA Medicaid), and/or any exchange product.
Factors Associated With Not Meeting the 1-year Hormone Therapy Benchmark
Characteristics of the patients who met versus did not meet the benchmark of hormone therapy are summarized in Supplemental Table 1 (in the online version). Younger age, lower census track median household income, and stage III tumor stage were significantly associated with not meeting the hormone therapy benchmark in univariable analysis. However, given the small number of patients not meeting the 1-year benchmark (n = 9), adjusted analyses were not performed.
Thematic Analysis for Reasons of Delay
Within the top quartile of delay, there were 18 cases with a time to surgery in excess of 70 days (range, 70–186 days), and 9 cases with hormone therapy delays, ranging from 368 to 492 days. The individual reasons for delay are summarized in Table 4. Clinical complexity accounted for 7 of the 18 surgery delays in excess of 70 days and 3 of 9 hormone therapy delays. Other non-clinical reasons included missed appointments, insurance change, multiple biopsies, seeking a second opinion, treatment indecision, and within family disagreement about preferred treatment course.
Table 4.
Thematic analysis for reasons for delay
| Top 25% surgery delay (n)a | Hormone therapy delay (n)b |
|---|---|
| ● Clinical complexity (8) ● Missed appointments (1) ● Insurance change (2) ● Second opinion (2) ● Treatment indecision (2) ● Undocumented reason (3) |
● Clinical complexity (5) ● Missed appointments (1) ● Gap in medication coverage (1) ● Patient family issue (1) ● Undocumented reason (1) |
Eighteen cases (top 25%) with surgery delays _ 70 days.
Nine cases with hormone therapy delay.
Discussion
Although the majority of patients within the study cohort met pre-selected quality benchmarks, we found that 22% of patients did not meet the 45-day surgery benchmark, with clinical complexity being the most common reason (n ¼ 8; 44%). In the adjusted multivariable analysis, mastectomy had a 3-fold increased odds of delay compared with lumpectomy. A recent study by Golshan et al reported that patients who received a mastectomy with immediate reconstruction had preoperative delays owing to the requirements of coordination between breast and reconstructive surgical teams.30 The impact of additional breast imaging on surgical wait times also suggests that patients requiring additional imaging had statistically longer wait times to first definitive surgery compared with those without additional imaging (28.5 days with vs. 21.4 days without).31
Although our thematic analysis among individual patients with surgery and/or hormone therapy delays suggests that clinical complexity was a common reason for delay, non-clinical issues including insurance, scheduling, treatment indecision, seeking a second opinion, and personal patient/family issues altogether accounted for nearly one-half of all delays. Non-clinical issues may be more modifiable than clinical complexity-related issues, if promptly identified and acted upon. Surgery type (that is, mastectomy) was associated with delays in surgery. Although we did not find age to be a factor in treatment delays in our adjusted analysis, other studies have found it to be associated with breast cancer disparity. A recent study on racial mortality disparity by age cohorts across 10 racially diverse US cities found the most pronounced breast cancer disparities among women younger than 40 years and the smallest disparity among women older than 65 years.32 Prognosis also tends to be worse in women under 40 years than in older women. Breast cancers in younger women are more likely to be fast-growing, higher grade, and hormone receptor negative, and each of these factors makes breast cancer more aggressive and more likely to need chemotherapy.33,34 Moreover, the diagnosis of breast cancer in younger women introduces additional complexity owing to potential loss of fertility and ovarian function with associated reproductive ramifications, as well as body image implications.34
Potential Interventions to Avoid the Delay
Reasons for delay involve both clinical and non-clinical factors, and some are more modifiable than others. A centralized breast/reconstructive surgical initiative with designated coordinators, as proposed by Golshan et al, can significantly reduce time to surgery.30 Another potential intervention to address clinical complexity may include bringing such cases back to a multidisciplinary tumor board for reconsideration, as patients approach timeliness benchmarks (ie, 45 days and 1 year, respectively). By eliciting input from experts from multiple specialties, additional treatment possibilities may also become apparent and possible. A recent study on the efforts being made to reduce time to treatment for newly diagnosed patients with cancer suggests that a multidisciplinary program can reduce time to treatment initiation by 33%.35
In response to delays caused by non-clinical issues, including insurance difficulties, treatment indecision, and personal issues, the prompt and steady involvement of an interdisciplinary support team may help to mitigate such challenges through and/or beyond completion of the planned treatment. Support team members, including patient navigators, social workers, and/or financial coordination specialists, could all help identify and work through potential barriers to care and biopsychosocial aspects surrounding cancer care.36 Interventions targeting these issues have the potential to improve meeting the timeliness benchmarks.
Strengths and Limitations
This retrospective single-center cohort study reviewed all patients with incident breast cancer who presented to Tufts MC from 2009 through 2015. As these benchmarks do not pertain to patients with Stage 0 or Stage IV disease, future studies are needed to evaluate quality care in these subgroups. The study population was drawn from a single academic medical center that is located in an urban community with a sizable Asian immigrant population, which limits generalizability of study results. Further, patients’ acceptance of recommended treatments was ascertained via data abstraction and perspective of the electronic medical record documentation, which may not completely reflect the patients’ point of view.
Nevertheless, this study employed rigorous dual data abstraction and reconciliation techniques. All clinical data were reviewed by study oncologists, and clinical data pertaining to receipt of treatment modalities, including neoadjuvant therapy, surgery, radiation, chemotherapy, and hormone therapy, and were verified using the institutional tumor registry.
Conclusion
Missing the timeliness benchmarks is a potential source of disparities in breast cancer care. This study suggests both clinical and non-clinical factors associated with delays. Identifying patient populations at higher risk of delayed treatment initiation can inform the development of interventions and policies aimed toward improving timely and quality breast cancer care.
Supplementary Material
Clinical Practice Points.
Evidence-based timeliness benchmarks have been established to assess quality of breast cancer care. This retrospective study analyzed timeliness of breast cancer care of 377 patients presenting to Tufts Medical Center between 2009 and 2015.
Of 319 patients with stage I to III disease who had surgery recommended as the first treatment, 248 (78%) met the 45-day benchmark. After adjusting for age, median household income, insurance, HER2 status, family history, prior breast cancer treatment, and comorbidity, multivariable regression analysis revealed that negative hormone receptor status (ER/PR) and mastectomy were significantly associated with delays in surgery. Delays were mostly owing to clinical complexity or logistical/financial reasons.
Of 241 patients eligible for hormone therapy initiation, 232 (96%) met the 1-year benchmark. Whereas younger age was associated with both delays in surgery and hormone therapy initiation in univariable analysis, it was no longer significantly associated with delays after adjusting for potential confounders.
Thematic analysis for reasons of delays also suggests that both clinical and non-clinical factors are associated with delays in treatment, and some factors may be more modifiable than others. A potential intervention to address clinical complexity may include bringing such cases back to a multi-disciplinary tumor board for reconsideration, as patients approach timeliness benchmarks (ie, at 45 days and 1 year, respectively). In response to delays caused by non-clinical issues, including insurance difficulties, treatment indecision, and personal issues, the prompt and steady involvement of an interdisciplinary support team may help to mitigate such challenges through and/or beyond completion of the planned treatment.
Identifying patient populations at higher risk of delayed treatment initiation can inform the development of interventions and policies aimed toward improving timely and quality breast cancer care.
Acknowledgments
The study was funded in part by the Komen for the Cure Health Disparities Training Grant (Freund and Parsons, Dual PI; Dong, Esham, and Mao, Komen Scholars) and the Tufts Clinical and Translational Science Institute NIH CTSA award UL1TR002544.
Funding sources:
This work was supported in part by the Komen for the Cure Health Disparities Training Grant (Freund and Parsons, Dual PI; Dong, Esham, and Mao, Komen Scholars) and the Tufts Clinical and Translational Science Institute NIH CTSA award [grant number UL1TR002544]. Funding sources had no involvement in the conduct of the research or preparation of article.
Footnote of Abbreviations:
- NCCN
National Comprehensive Cancer Network
- NQF
National Quality Forum
- MiBOQI
Michigan Breast Oncology Quality Initiative
- REDCap
Research Electronic Data Capture
- AJCC
American Joint Committee on Cancer
- ER
estrogen receptor
- PR
progesterone receptor
- CI
confidence intervals
Footnotes
Declarations of interest: none
Disclosure
The authors have stated that they have no conflicts of interest.
Supplemental Data
Supplemental table accompanying this article can be found in the online version at https://doi.org/10.1016/j.clbc.2019.06.009.
References
- 1.DeSantis CE, Ma J, Goding Sauer A, Newman LA, Jemal A. Breast cancer statistics, 2017, racial disparity in mortality by state. CA Cancer J Clin 2017; 67: 439–48. [DOI] [PubMed] [Google Scholar]
- 2.Ravdin PM, Cronin KA, Howlader N, et al. The decrease in breast-cancer incidence in 2003 in the United States. N Engl J Med 2007; 356:1670–4. [DOI] [PubMed] [Google Scholar]
- 3.Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 2016; 66:271–89. [DOI] [PubMed] [Google Scholar]
- 4.Berry DA, Cronin KA, Plevritis SK, et al. , Cancer Intervention and Surveillance Modeling Network (CISNET) Collaborators. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med 2005; 353:1784–92. [DOI] [PubMed] [Google Scholar]
- 5.Coombs NJ, Cronin KA, Taylor RJ, Freedman AN, Boyages J. The impact of changes in hormone therapy on breast cancer incidence in the US population. Cancer Causes Control 2010; 21:83–90. [DOI] [PubMed] [Google Scholar]
- 6.Munoz D, Near AM, van Ravesteyn NT, et al. Effects of screening and systemic adjuvant therapy on ER-specific US breast cancer mortality. J Natl Cancer Inst 2014:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bradley CJ, Given CW, Roberts C. Race, socioeconomic status, and breast cancer treatment and survival. J Natl Cancer Inst 2002; 94:490–6. [DOI] [PubMed] [Google Scholar]
- 8.McLaughlin JM, Anderson RT, Ferketich AK, Seiber EE, Balkrishnan R, Paskett ED. Effect on survival of longer intervals between confirmed diagnosis and treatment initiation among low-income women with breast cancer. J Clin Oncol 2012; 30:4493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chavez-MacGregor M, Clarke CA, Lichtensztajn DY, Giordano SH. Delayed initiation of adjuvant chemotherapy among patients with breast cancer. JAMA Oncol 2016; 2:322–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vandergrift JL, Niland JC, Theriault RL, et al. Time to adjuvant chemotherapy for breast cancer in National Comprehensive Cancer Network institutions. J Natl Cancer Inst 2013; 105:104–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdel-Rahman O Impact of timeliness of adjuvant chemotherapy and radiotherapy on the outcomes of breast cancer; a pooled analysis of three clinical trials. Breast 2018; 38:175–80. [DOI] [PubMed] [Google Scholar]
- 12.Brawley OW. Health disparities in breast cancer. Obstet Gyn Clin N Am 2013; 40:513–23. [DOI] [PubMed] [Google Scholar]
- 13.Bleicher RJ, Ruth K, Sigurdson ER, et al. Time to surgery and breast cancer survival in the United States. JAMA Oncol 2016; 2:330–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jassem J, Ozmen V, Bacanu F, et al. Delays in diagnosis and treatment of breast cancer: a multinational analysis. Eur J Public Health 2014; 24:761–7. [DOI] [PubMed] [Google Scholar]
- 15.Selove R, Kilbourne B, Fadden MK, et al. Time from screening mammography to biopsy and from biopsy to breast cancer treatment among black and white, women Medicare beneficiaries not participating in a health maintenance organization. Womens Health Issues 2016; 26:642–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gagliato Dde M, Gonzalez-Angulo AM, Lei X, et al. Clinical impact of delaying initiation of adjuvant chemotherapy in patients with breast cancer. J Clin Oncol 2014; 32:735–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Early Breast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005; 365:1687–717. [DOI] [PubMed] [Google Scholar]
- 18.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002; 347:1233–41. [DOI] [PubMed] [Google Scholar]
- 19.Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 2002; 347:1227–32. [DOI] [PubMed] [Google Scholar]
- 20.Castaldi M, Safadjou S, Elrafei T, McNelis J. A multidisciplinary patient navigation program improves compliance with adjuvant breast cancer therapy in a public hospital. Am J Med Qual 2017; 32:406–13. [DOI] [PubMed] [Google Scholar]
- 21.Breslin TM, Caughran J, Pettinga J, et al. Improving breast cancer care through a regional quality collaborative. Surgery 2011; 150:635–40. [DOI] [PubMed] [Google Scholar]
- 22.Smith DR, Caughran J, Kreinbrink JL, et al. Clinical presentation of breast cancer: age, stage, and treatment modalities in a contemporary cohort of Michigan women. J Clin Oncol 2011; 29(27 Suppl):1.21115866 [Google Scholar]
- 23.United States Census Bureau. American FactFinder. Available at: https://factfinder.census.gov/faces/nav/jsf/pages/index.xhtml. Accessed: August 31, 2015. [Google Scholar]
- 24.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th Edition of the AJCC Cancer Staging Manual and the Future of TNM. Ann Surg Oncol 2010; 17:1471–4. [DOI] [PubMed] [Google Scholar]
- 25.Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version). Arch Pathol Lab Med 2010; 134:e48–72. [DOI] [PubMed] [Google Scholar]
- 26.Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 2007; 25:118–45. [DOI] [PubMed] [Google Scholar]
- 27.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992; 45:613–9. [DOI] [PubMed] [Google Scholar]
- 28.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005; 43:1130–9. [DOI] [PubMed] [Google Scholar]
- 29.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–83. [DOI] [PubMed] [Google Scholar]
- 30.Golshan M, Losk K, Mallory MA, et al. Implementation of a breast/reconstruction surgery coordinator to reduce preoperative delays for patients undergoing mastectomy with immediate reconstruction. J Oncol Pract 2016;12:e338–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Golshan M, Losk K, Mallory MA, et al. Variation in additional breast imaging orders and impact on surgical wait times at a comprehensive cancer center. Ann Surg Oncol 2015; 22(Suppl 3):S428–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sighoko D, Hunt BR, Irizarry B, Watson K, Ansell D, Murphy AM. Disparity in breast cancer mortality by age and geography in 10 racially diverse US cities. Cancer Epidemiol 2018; 53:178–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith EC, Ziogas A, Anton-Culver H. Delay in surgical treatment and survival after breast cancer diagnosis in young women by race/ethnicity.JAMASurg 2013; 148:516–23. [DOI] [PubMed] [Google Scholar]
- 34.Taylan E, Oktay KH. Current state and controversies in fertility preservation in women with breast cancer. World J Clin Oncol 2017; 8:241–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khorana A, Bolwell B. Reducing time-to-treatment for newly diagnosed cancer patients. NEJM Catalyst, 2019. Available at: https://catalyst.nejm.org/time-totreatment-cancer-patients/. Accessed: April 15, 2019. [Google Scholar]
- 36.Parsons SK, Fineberg IC, Lin MQ, Singer M, Tang M, Erban JK. Promoting high quality cancer care and equity through disciplinary diversity in team composition. J Oncol Pract 2016; 12:1141–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


