Abstract
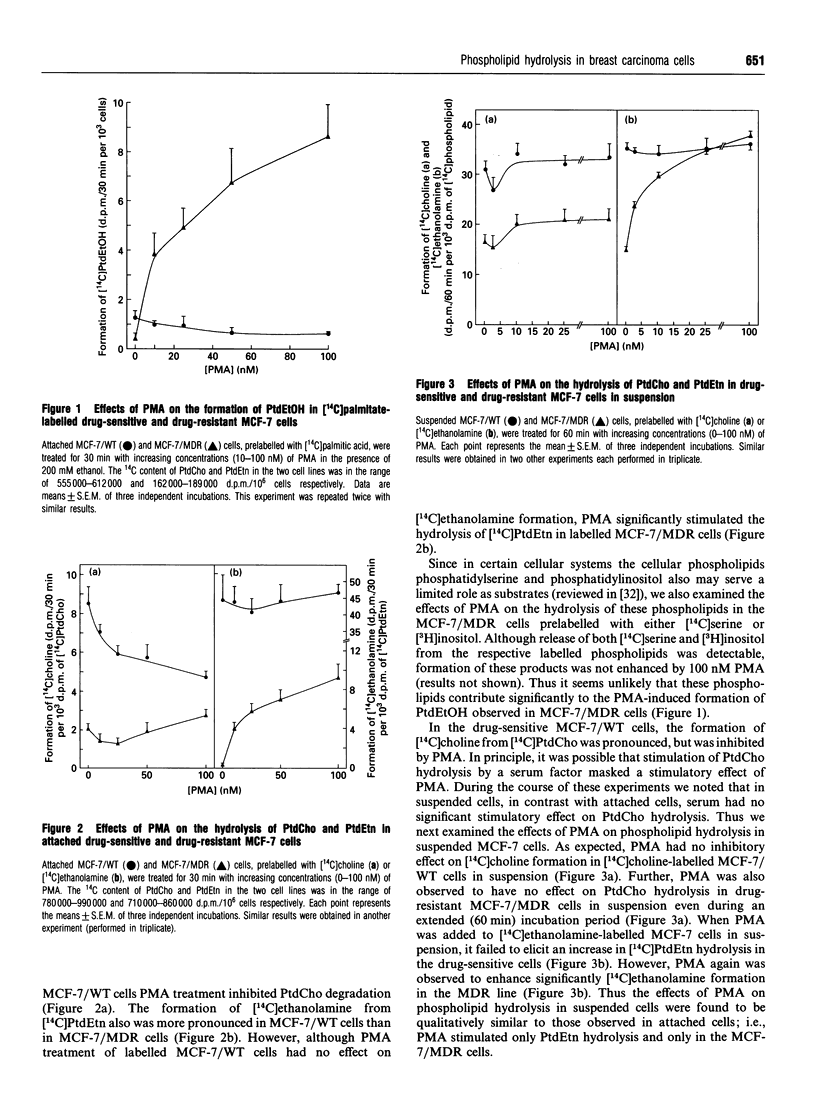
The phospholipase D (PLD)-mediated synthesis of phosphatidylethanol (PtdEtOH) and the hydrolysis of phosphatidylethanolamine (PtdEtn) and phosphatidylcholine (PtdCho) were examined in drug-sensitive and multidrug-resistant lines of MCF-7 human breast carcinoma cells. In drug-sensitive (MCF-7/WT) cells, the protein kinase C (PKC) activator phorbol 12-myristate 13-acetate (PMA) failed to enhance either the synthesis of PtdEtOH or the hydrolysis of either phospholipid. In the drug-resistant (MCF-7/MDR) cells, 100 nM PMA greatly enhanced both the synthesis of PtdEtOH (approximately 21-fold) and the hydrolysis of PtdEtn (approximately 29-fold), but had no effect on the hydrolysis of PtdCho. The PLD activators sphingosine and H2O2 were found to elicit only a slight (1.28-1.4-fold) stimulatory effect on PtdCho hydrolysis in both the MCF-7/WT and MCF-7/MDR cell types, and had only a small effect on PtdEtn hydrolysis in the MCF-7/WT cells as well. However, these agents significantly (approximately 2.6-3.5-fold) stimulated PtdEtn hydrolysis in the MCF-7/MDR cells. These data indicate that MCF-7/MDR cells contain a PtdEtn-specific PLD activity which can be selectively stimulated by PMA, sphingosine and H2O2.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aquino A., Warren B. S., Omichinski J., Hartman K. D., Glazer R. I. Protein kinase C-gamma is present in adriamycin resistant HL-60 leukemia cells. Biochem Biophys Res Commun. 1990 Jan 30;166(2):723–728. doi: 10.1016/0006-291x(90)90869-o. [DOI] [PubMed] [Google Scholar]
- Billah M. M., Anthes J. C. The regulation and cellular functions of phosphatidylcholine hydrolysis. Biochem J. 1990 Jul 15;269(2):281–291. doi: 10.1042/bj2690281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobe G. C., Sachs C. W., Khan W. A., Fabbro D., Stabel S., Wetsel W. C., Obeid L. M., Fine R. L., Hannun Y. A. Selective regulation of expression of protein kinase C (PKC) isoenzymes in multidrug-resistant MCF-7 cells. Functional significance of enhanced expression of PKC alpha. J Biol Chem. 1993 Jan 5;268(1):658–664. [PubMed] [Google Scholar]
- Brown H. A., Gutowski S., Moomaw C. R., Slaughter C., Sternweis P. C. ADP-ribosylation factor, a small GTP-dependent regulatory protein, stimulates phospholipase D activity. Cell. 1993 Dec 17;75(6):1137–1144. doi: 10.1016/0092-8674(93)90323-i. [DOI] [PubMed] [Google Scholar]
- Cook S. J., Wakelam M. J. Analysis of the water-soluble products of phosphatidylcholine breakdown by ion-exchange chromatography. Bombesin and TPA (12-O-tetradecanoylphorbol 13-acetate) stimulate choline generation in Swiss 3T3 cells by a common mechanism. Biochem J. 1989 Oct 15;263(2):581–587. doi: 10.1042/bj2630581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doige C. A., Yu X., Sharom F. J. The effects of lipids and detergents on ATPase-active P-glycoprotein. Biochim Biophys Acta. 1993 Feb 23;1146(1):65–72. doi: 10.1016/0005-2736(93)90339-2. [DOI] [PubMed] [Google Scholar]
- Dong Z. Y., Ward N. E., Fan D., Gupta K. P., O'Brian C. A. In vitro model for intrinsic drug resistance: effects of protein kinase C activators on the chemosensitivity of cultured human colon cancer cells. Mol Pharmacol. 1991 Apr;39(4):563–569. [PubMed] [Google Scholar]
- Dunlop M., Metz S. A. A phospholipase D-like mechanism in pancreatic islet cells: stimulation by calcium ionophore, phorbol ester and sodium fluoride. Biochem Biophys Res Commun. 1989 Sep 15;163(2):922–928. doi: 10.1016/0006-291x(89)92310-3. [DOI] [PubMed] [Google Scholar]
- Escriba P. V., Ferrer-Montiel A. V., Ferragut J. A., Gonzalez-Ros J. M. Role of membrane lipids in the interaction of daunomycin with plasma membranes from tumor cells: implications in drug-resistance phenomena. Biochemistry. 1990 Aug 7;29(31):7275–7282. doi: 10.1021/bi00483a017. [DOI] [PubMed] [Google Scholar]
- Exton J. H. Signaling through phosphatidylcholine breakdown. J Biol Chem. 1990 Jan 5;265(1):1–4. [PubMed] [Google Scholar]
- Ferguson P. J., Cheng Y. C. Transient protection of cultured human cells against antitumor agents by 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 1987 Jan 15;47(2):433–441. [PubMed] [Google Scholar]
- Fine R. L., Patel J., Chabner B. A. Phorbol esters induce multidrug resistance in human breast cancer cells. Proc Natl Acad Sci U S A. 1988 Jan;85(2):582–586. doi: 10.1073/pnas.85.2.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisk H. A., Kano-Sueoka T. Effect of membrane phosphatidylethanolamine-deficiency/phosphatidylcholine-excess on the metabolism of phosphatidylcholine and phosphatidylethanolamine. J Cell Physiol. 1992 Dec;153(3):589–595. doi: 10.1002/jcp.1041530321. [DOI] [PubMed] [Google Scholar]
- Fukami K., Takenawa T. Phosphatidic acid that accumulates in platelet-derived growth factor-stimulated Balb/c 3T3 cells is a potential mitogenic signal. J Biol Chem. 1992 Jun 5;267(16):10988–10993. [PubMed] [Google Scholar]
- García-Segura L. M., Ferragut J. A., Ferrer-Montiel A. V., Escriba P. V., Gonzalez-Ros J. M. Ultrastructural alterations in plasma membranes from drug-resistant P388 murine leukemia cells. Biochim Biophys Acta. 1990 Nov 2;1029(1):191–195. doi: 10.1016/0005-2736(90)90454-v. [DOI] [PubMed] [Google Scholar]
- García-Segura L. M., Soto F., Planells-Cases R., Gonzalez-Ros J. M., Ferragut J. A. Verapamil reverses the ultrastructural alterations in the plasma membrane induced by drug resistance. FEBS Lett. 1992 Dec 21;314(3):404–408. doi: 10.1016/0014-5793(92)81515-n. [DOI] [PubMed] [Google Scholar]
- Gottesman M. M., Pastan I. The multidrug transporter, a double-edged sword. J Biol Chem. 1988 Sep 5;263(25):12163–12166. [PubMed] [Google Scholar]
- Harrris W. E., Bursten S. L. Lipid A stimulates phospholipase D activity in rat mesangial cells via a G-protein. Biochem J. 1992 Feb 1;281(Pt 3):675–682. doi: 10.1042/bj2810675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hii C. S., Edwards Y. S., Murray A. W. Phorbol ester-stimulated hydrolysis of phosphatidylcholine and phosphatidylethanolamine by phospholipase D in HeLa cells. Evidence that the basal turnover of phosphoglycerides does not involve phospholipase D. J Biol Chem. 1991 Oct 25;266(30):20238–20243. [PubMed] [Google Scholar]
- Horwitz J., Davis L. L. The substrate specificity of brain microsomal phospholipase D. Biochem J. 1993 Nov 1;295(Pt 3):793–798. doi: 10.1042/bj2950793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juranka P. F., Zastawny R. L., Ling V. P-glycoprotein: multidrug-resistance and a superfamily of membrane-associated transport proteins. FASEB J. 1989 Dec;3(14):2583–2592. doi: 10.1096/fasebj.3.14.2574119. [DOI] [PubMed] [Google Scholar]
- Kester M. Platelet-activating factor stimulates phosphatidic acid formation in cultured rat mesangial cells: roles of phospholipase D, diglyceride kinase, and de novo phospholipid synthesis. J Cell Physiol. 1993 Aug;156(2):317–325. doi: 10.1002/jcp.1041560214. [DOI] [PubMed] [Google Scholar]
- Kester M., Simonson M. S., McDermott R. G., Baldi E., Dunn M. J. Endothelin stimulates phosphatidic acid formation in cultured rat mesangial cells: role of a protein kinase C-regulated phospholipase D. J Cell Physiol. 1992 Mar;150(3):578–585. doi: 10.1002/jcp.1041500319. [DOI] [PubMed] [Google Scholar]
- Kester M., Simonson M. S., Mené P., Sedor J. R. Interleukin-1 generates transmembrane signals from phospholipids through novel pathways in cultured rat mesangial cells. J Clin Invest. 1989 Feb;83(2):718–723. doi: 10.1172/JCI113937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsky S. C., Loader J. E., Benedict S. H. Phorbol ester activation of phospholipase D in human monocytes but not peripheral blood lymphocytes. Biochem Biophys Res Commun. 1989 Jul 31;162(2):788–793. doi: 10.1016/0006-291x(89)92379-6. [DOI] [PubMed] [Google Scholar]
- Kiss Z., Anderson W. B. ATP stimulates the hydrolysis of phosphatidylethanolamine in NIH 3T3 cells. Potentiating effects of guanosine triphosphates and sphingosine. J Biol Chem. 1990 May 5;265(13):7345–7350. [PubMed] [Google Scholar]
- Kiss Z., Anderson W. B. Phorbol ester stimulates the hydrolysis of phosphatidylethanolamine in leukemic HL-60, NIH 3T3, and baby hamster kidney cells. J Biol Chem. 1989 Jan 25;264(3):1483–1487. [PubMed] [Google Scholar]
- Kiss Z., Deli E. Regulation of phospholipase D by sphingosine involves both protein kinase C-dependent and -independent mechanisms in NIH 3T3 fibroblasts. Biochem J. 1992 Dec 15;288(Pt 3):853–858. doi: 10.1042/bj2880853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss Z. Differential effects of platelet-derived growth factor, serum and bombesin on phospholipase D-mediated hydrolysis of phosphatidylethanolamine in NIH 3T3 fibroblasts. Biochem J. 1992 Jul 1;285(Pt 1):229–233. doi: 10.1042/bj2850229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss Z. Effects of phorbol ester on phospholipid metabolism. Prog Lipid Res. 1990;29(3):141–166. doi: 10.1016/0163-7827(90)90001-2. [DOI] [PubMed] [Google Scholar]
- Kiss Z. Possible phospholipid precursor for phosphatidylserine in rat heart. Eur J Biochem. 1976 Aug 16;67(2):557–561. doi: 10.1111/j.1432-1033.1976.tb10721.x. [DOI] [PubMed] [Google Scholar]
- Kiss Z., Rapp U. R., Pettit G. R., Anderson W. B. Phorbol ester and bryostatin differentially regulate the hydrolysis of phosphatidylethanolamine in Ha-ras- and raf-oncogene-transformed NIH 3T3 cells. Biochem J. 1991 Jun 1;276(Pt 2):505–509. doi: 10.1042/bj2760505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss Z. The long-term combined stimulatory effects of ethanol and phorbol ester on phosphatidylethanolamine hydrolysis are mediated by a phospholipase C and prevented by overexpressed alpha-protein kinase C in fibroblasts. Eur J Biochem. 1992 Oct 1;209(1):467–473. doi: 10.1111/j.1432-1033.1992.tb17311.x. [DOI] [PubMed] [Google Scholar]
- Kiss Z. The zinc chelator 1,10-phenanthroline enhances the stimulatory effects of protein kinase C activators and staurosporine, but not sphingosine and H2O2, on phospholipase D activity in NIH 3T3 fibroblasts. Biochem J. 1994 Feb 15;298(Pt 1):93–98. doi: 10.1042/bj2980093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavie Y., Liscovitch M. Activation of phospholipase D by sphingoid bases in NG108-15 neural-derived cells. J Biol Chem. 1990 Mar 5;265(7):3868–3872. [PubMed] [Google Scholar]
- Lee S. A., Karaszkiewicz J. W., Anderson W. B. Elevated level of nuclear protein kinase C in multidrug-resistant MCF-7 human breast carcinoma cells. Cancer Res. 1992 Jul 1;52(13):3750–3759. [PubMed] [Google Scholar]
- Liscovitch M., Amsterdam A. Gonadotropin-releasing hormone activates phospholipase D in ovarian granulosa cells. Possible role in signal transduction. J Biol Chem. 1989 Jul 15;264(20):11762–11767. [PubMed] [Google Scholar]
- Mizunuma M., Tanaka S., Kudo R., Kanoh H. Phospholipase D activity of human amnion cells stimulated with phorbol ester and bradykinin. Biochim Biophys Acta. 1993 Jun 12;1168(2):213–219. [PubMed] [Google Scholar]
- Montaudon D., Vrignaud P., Londos-Gagliardi D., Robert J. Fluorescence anisotropy of cell membranes of doxorubicin-sensitive and -resistant rodent tumoral cells. Cancer Res. 1986 Nov;46(11):5602–5605. [PubMed] [Google Scholar]
- Moolenaar W. H., Kruijer W., Tilly B. C., Verlaan I., Bierman A. J., de Laat S. W. Growth factor-like action of phosphatidic acid. Nature. 1986 Sep 11;323(6084):171–173. doi: 10.1038/323171a0. [DOI] [PubMed] [Google Scholar]
- Natarajan V., Taher M. M., Roehm B., Parinandi N. L., Schmid H. H., Kiss Z., Garcia J. G. Activation of endothelial cell phospholipase D by hydrogen peroxide and fatty acid hydroperoxide. J Biol Chem. 1993 Jan 15;268(2):930–937. [PubMed] [Google Scholar]
- O'Brian C. A., Fan D., Ward N. E., Seid C., Fidler I. J. Level of protein kinase C activity correlates directly with resistance to adriamycin in murine fibrosarcoma cells. FEBS Lett. 1989 Mar 27;246(1-2):78–82. doi: 10.1016/0014-5793(89)80257-1. [DOI] [PubMed] [Google Scholar]
- Pastan I., Gottesman M. Multiple-drug resistance in human cancer. N Engl J Med. 1987 May 28;316(22):1388–1393. doi: 10.1056/NEJM198705283162207. [DOI] [PubMed] [Google Scholar]
- Ramu A., Glaubiger D., Magrath I. T., Joshi A. Plasma membrane lipid structural order in doxorubicin-sensitive and -resistant P388 cells. Cancer Res. 1983 Nov;43(11):5533–5537. [PubMed] [Google Scholar]
- Riordan J. R., Ling V. Genetic and biochemical characterization of multidrug resistance. Pharmacol Ther. 1985;28(1):51–75. doi: 10.1016/0163-7258(85)90082-8. [DOI] [PubMed] [Google Scholar]
- Siegfried J. A., Kennedy K. A., Sartorelli A. C., Tritton T. R. The role of membranes in the mechanism of action of the antineoplastic agent adriamycin. Spin-labeling studies with chronically hypoxic and drug-resistant tumor cells. J Biol Chem. 1983 Jan 10;258(1):339–343. [PubMed] [Google Scholar]
- Sinicrope F. A., Dudeja P. K., Bissonnette B. M., Safa A. R., Brasitus T. A. Modulation of P-glycoprotein-mediated drug transport by alterations in lipid fluidity of rat liver canalicular membrane vesicles. J Biol Chem. 1992 Dec 15;267(35):24995–25002. [PubMed] [Google Scholar]
- Stutchfield J., Cockcroft S. Correlation between secretion and phospholipase D activation in differentiated HL60 cells. Biochem J. 1993 Aug 1;293(Pt 3):649–655. doi: 10.1042/bj2930649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Anthes J. C., Siegel M. I., Egan R. W., Billah M. M. Existence of cytosolic phospholipase D. Identification and comparison with membrane-bound enzyme. J Biol Chem. 1991 Aug 15;266(23):14877–14880. [PubMed] [Google Scholar]
- Wright L. C., Dyne M., Holmes K. T., Mountford C. E. Phospholipid and ether linked phospholipid content alter with cellular resistance to vinblastine. Biochem Biophys Res Commun. 1985 Dec 17;133(2):539–545. doi: 10.1016/0006-291x(85)90940-4. [DOI] [PubMed] [Google Scholar]
- Yu C. L., Tsai M. H., Stacey D. W. Cellular ras activity and phospholipid metabolism. Cell. 1988 Jan 15;52(1):63–71. doi: 10.1016/0092-8674(88)90531-4. [DOI] [PubMed] [Google Scholar]
- Zhou H. L., Chabot-Fletcher M., Foley J. J., Sarau H. M., Tzimas M. N., Winkler J. D., Torphy T. J. Association between leukotriene B4-induced phospholipase D activation and degranulation of human neutrophils. Biochem Pharmacol. 1993 Jul 6;46(1):139–148. doi: 10.1016/0006-2952(93)90358-4. [DOI] [PubMed] [Google Scholar]
- van Corven E. J., Groenink A., Jalink K., Eichholtz T., Moolenaar W. H. Lysophosphatidate-induced cell proliferation: identification and dissection of signaling pathways mediated by G proteins. Cell. 1989 Oct 6;59(1):45–54. doi: 10.1016/0092-8674(89)90868-4. [DOI] [PubMed] [Google Scholar]
- van Corven E. J., van Rijswijk A., Jalink K., van der Bend R. L., van Blitterswijk W. J., Moolenaar W. H. Mitogenic action of lysophosphatidic acid and phosphatidic acid on fibroblasts. Dependence on acyl-chain length and inhibition by suramin. Biochem J. 1992 Jan 1;281(Pt 1):163–169. doi: 10.1042/bj2810163. [DOI] [PMC free article] [PubMed] [Google Scholar]


