Key Points
-
•
Commercial CAR T-cell therapies including CARs with CD28 domain may be given safely as outpatient without intense home monitoring.
-
•
Low-grade CRS can be managed in the outpatient setting with a well-structured system.
Visual Abstract
Abstract
Recent studies demonstrating the feasibility of outpatient chimeric antigen receptor (CAR)–modified T-cell therapy administration are either restricted to CARs with 41BB costimulatory domains or use intensive at-home monitoring. We report outcomes of outpatient administration of all commercially available CD19- and B-cell maturation antigen (BCMA)–directed CAR T-cell therapy using a strategy of no remote at-home monitoring and an early cytokine release syndrome (CRS) intervention strategy. Patients with hematologic malignancies who received CAR T-cell therapy in the outpatient setting during 2022 to 2023 were included. Patients were seen daily in the cancer center day hospital for the first 7 to 10 days and then twice weekly through day 30. The primary end point was to determine 3-, 7-, and 30-day post–CAR T-cell infusion hospitalizations. Early CRS intervention involved administering tocilizumab as an outpatient for grade ≥1 CRS. Fifty-eight patients received outpatient CAR T-cell infusion (33 myeloma, 24 lymphoma, and 1 acute lymphoblastic leukemia). Of these, 17 (41%), 16 (38%), and 9 patients (21%) were admitted between days 0 to 3, 4 to 7, and 8 to 30 after CAR T-cell infusion, respectively. The most common reason for admission was CAR T-cell–related toxicities (33/42). Hospitalization was prevented in 15 of 35 patients who received tocilizumab for CRS as an outpatient. The nonrelapse mortality rates were 1.7% at 1 month and 3.4% at 6 months. In conclusion, we demonstrate that the administration of commercial CAR T-cell therapies in an outpatient setting is safe and feasible without intensive remote monitoring using an early CRS intervention strategy.
Introduction
Chimeric antigen receptor (CAR)–modified T-cell therapy has significantly altered the management of hematologic malignancies.1, 2, 3, 4 Initially approved for the treatment of relapsed or refractory acute lymphoblastic leukemia (ALL) in 2017, CAR T-cell therapy has since been approved for multiple B-cell malignancies including relapsed or refractory large B-cell lymphoma (LBCL), multiple myeloma (MM), follicular lymphoma, and mantle cell lymphoma (MCL).1,3,5, 6, 7, 8, 9, 10, 11, 12 Given the risk of potentially life-threatening adverse events including cytokine release syndrome (CRS) and immune effector cell–associated neurotoxicity syndrome (ICANS), CAR T-cell therapy was administered in the inpatient setting in the initial clinical trials, and the same pattern followed in the real world. The duration of inpatient stay varies from 1 to ≥4 weeks depending on institutional practices, product features, and patient characteristics. This leads to potentially higher health care costs and inpatient resource utilization and may limit access to this life-saving therapy.13,14 Furthermore, prolonged hospitalization can lead to physical deconditioning, increased risk of nosocomial infections, anxiety, and depression in patients, affecting their quality of life.15
Given the shortcomings of inpatient management of CAR T-cell therapy, emerging data have demonstrated the safety and feasibility of outpatient administration of CAR T-cell therapy.16,17 However, studies reporting outpatient management of CAR T-cell therapy often use a very rigorous monitoring system for patients, including the use of remote patient monitoring devices, after-hour patient checks by the health care team including phone calls, as well as an initial inpatient monitoring period.18,19 This in turn makes outpatient administration logistically complex and may lead to increased patient anxiety, affecting their quality of life.20 On the contrary, due to the higher rates of immune-related adverse events associated with CD28 domain–containing CAR T-cell products, such as axicabtagene ciloleucel (axi-cel), outpatient administration of commercial CAR T-cell therapy has generally been limited to products with 41BB costimulatory domain, such as lisocabtagene maraleucel and tisagenlecleucel, or use complex home monitoring systems for CRS monitoring.18,19 In addition, unlike lymphoma, data for outpatient administration of anti-BCMA CAR T-cell therapy for MM, particularly ciltacabtagene autoleucel (cilta-cel), are limited.19 We report our experience of outpatient management of commercially available CAR T-cell therapy products, using a strategy of no remote monitoring and early intervention for CRS.
Methods
We performed a retrospective review of patients who received commercially available CAR T-cell therapy in the outpatient setting. The study was approved by the institutional review board. The strategy of outpatient CAR T-cell therapy administration was deployed in January 2022 in our center. All adult patients with hematologic malignancies receiving a commercial CAR T-cell therapy product from January 2022 onward were required to receive treatment in the outpatient setting with the following exceptions: presence of active central nervous system disease, ongoing hemodialysis, lack of caregiver support, and a diagnosis of ALL (which were later included from June 2023 onward). Patients were required to stay within 45 minutes of the cancer center, along with an adult caregiver.
Procedure
Patients were seen daily (including weekend days) in the cancer center day hospital for the first 7 to 14 days after infusion and then twice weekly through day 30 in the general hematology outpatient clinics. No remote monitoring of vital signs or patient symptoms was performed via electronic devices or after-hour patient check-ins (eg, telephone calls). Lymphodepletion and CAR T cells were administered in an outpatient infusion center (day hospital), where the patients were evaluated daily by a treating physician and/or an advanced practice provider (APP) and transplant nursing staff. In addition, our hospital has a 24-hour clinic, an outpatient unit staffed with nurses, an APP, and an on-call physician operating 24 hours a day, which was used for after-hours (5 pm-7 AM) patient concerns. The day hospital and 24-hour clinic are equipped to administer outpatient antibiotics, tocilizumab, chemotherapy drugs, blood products, and immunosuppressive agents and are functional on weekends and holidays.
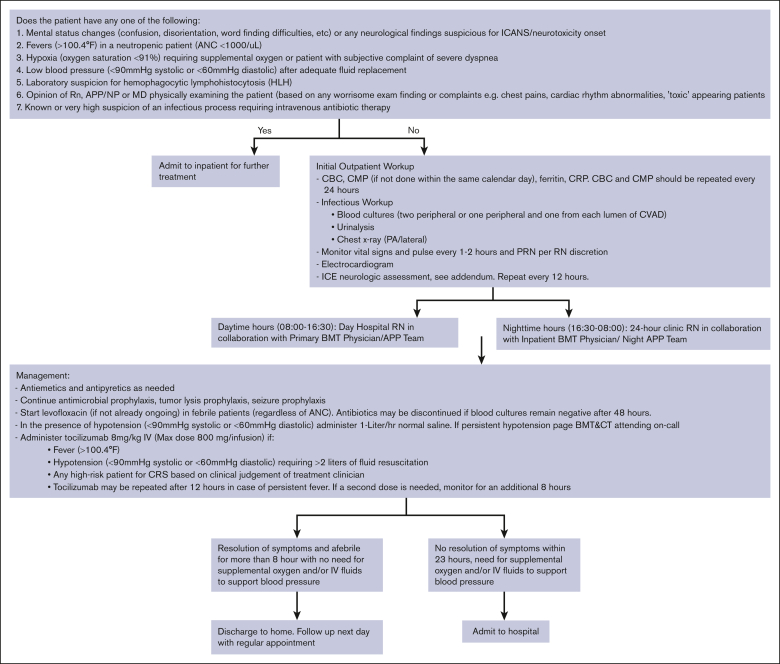
Early CRS intervention involved administering tocilizumab in the outpatient setting for grade ≥1 CRS (Figure 1). Tocilizumab could be administered in the day hospital during normal working hours (7 AM to 5 pm) or in the cancer center’s 24-hour clinic during nighttime (5 pm-7 AM). Patients were admitted if they developed >1 grade CRS, ICANS, neutropenic fevers, suspicion 114 of immune effector cell associated Hemophagocytic Lymphohistiocytosis-like syndrome (IEC115 HS) or evidence of hemodynamic compromise. Patients with CRS but none of the above symptoms received up to 2 doses of tocilizumab as outpatients (day-hospital/24-hour clinic) and were then monitored for a maximum of 8 hours after the last dose. If the symptoms resolved, patients were discharged home with routine follow-up as per their schedule. Patients with persistent fever despite 2 doses of tocilizumab were admitted to the hospital.
Figure 1.
Management of CRS as outpatient. ANC, absolute neutrophil count; BMT, blood and marrow transplant; CBC, complete blood count; CMP, complete metabolic profile; CRP, C-reactive protein; PRN, as needed.
Statistical analysis
Descriptive statistics were used for baseline patient and treatment characteristics, and data for toxicity, efficacy, and resource utilization were summarized using frequency tables. The primary end point of the current analysis was to assess the inpatient admission rates within 3, 7, and 30 days after outpatient CAR T-cell administration. CRS and ICANS were graded according to the American Society for Transplantation and Cellular Therapy consensus criteria.21 Univariate analysis was done using χ2 and Fisher exact tests for categorical variables. Kaplan-Meier methods and log-rank tests were applied for progression-free survival (PFS) and overall survival (OS) for all patients. PFS was defined as the time from CAR T-cell infusion to the earliest of disease relapse, progression, or death. OS was defined as the time from CAR T-cell infusion to death from any cause.
Results
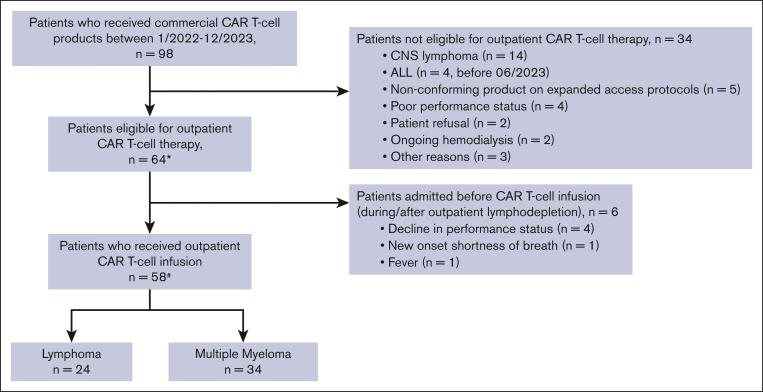
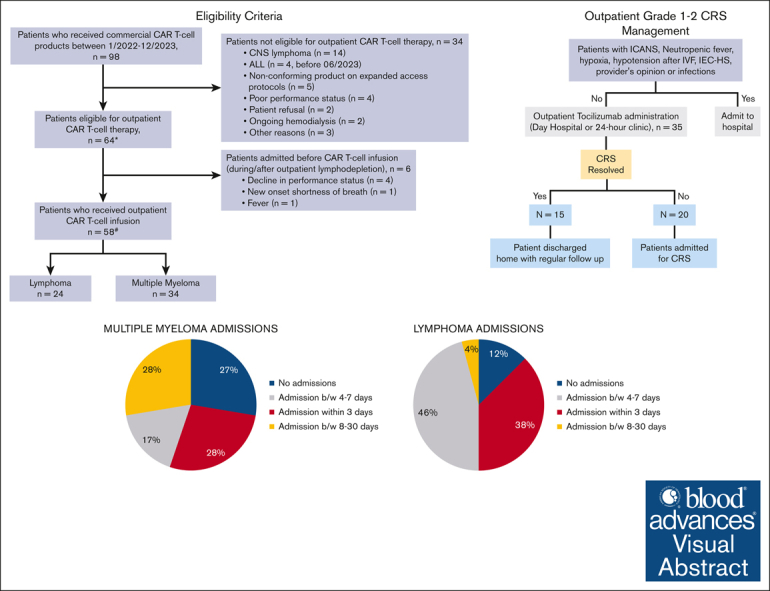
Of the 98 patients who received commercial CAR T-cell therapy between January 2022 to December 2023, a total of 64 patients were eligible to receive outpatient CAR T-cell infusion (Figure 2). Among the 34 patients who received planned inpatient CAR T-cell therapy, the reasons included active central nervous system lymphoma (n = 14), ALL (n = 4; administered before June 2023), recipients of a nonconforming product on expanded access protocols (n = 5), poor performance status (n = 4), patient refusal (n = 2), ongoing hemodialysis (n = 2), and other reasons (n = 3). Of the 64 patients meeting the criteria for outpatient administration, 28 patients lived locally within 45 minutes of the hospital, whereas 26 used an on-campus hospital guest house (Kathy’s house), and 10 used hotels or resided with family in the area. Fifty-eight of these 64 patients received the CAR T-cell infusion as outpatients, whereas 6 patients started lymphodepletion on outpatient basis but had to be admitted and received CAR T-cell infusion inpatient (Figure 2). Reasons for admission included a decline in performance status (n = 4), new onset shortness of breath (n = 1), and fevers (n = 1).
Figure 2.
Eligibility and outpatient administration of CAR T-cell therapy. ∗Included in safety (CRS/ICANS rates) and efficacy analyses. #Included in hospitalization rates analyses. CNS, central nervous system.
Table 1 summarizes the baseline characteristics of all patients intended for outpatient administration and those who actually received cell infusion as outpatient. The median age in all patients was 62 years (range, 21-82); 40% (n = 28) were female, and 87% (n = 56) were Caucasians. There were 32 patients with MM, 27 with LBCL (including 3 double-hit, 4 Richter transformation, and 4 transformed from follicular lymphoma), and 1 each with Burkitt lymphoma, MCL, ALL, and plasma cell leukemia. The median prior lines of therapy were 4 (range, 1-12), whereas 38 patients (9 LBCL and 29 MM) had a prior autologous hematopoietic cell transplant and 11 (10 MM and 1 LBCL) had a prior allogeneic hematopoietic cell transplant. Only 7 patients received dexamethasone for CRS prophylaxis, all with LBCL, following US Food and Drug Administration amendment to axi-cel prescribing information on 31 January 2022, per treating physician discretion. None of the patients received anakinra for prophylaxis. For reporting outcomes, patients are grouped into B-cell lymphoma (LBCL, Burkitt lymphoma, and MCL), MM (MM and plasma cell leukemia), and ALL.
Table 1.
Baseline characteristics of patients receiving outpatient CAR T-cell therapy
| Baseline characteristics | Patients with outpatient cell infusion (n = 58) | All patients intended for outpatient administration (n = 64) |
|---|---|---|
| Median age (range), y | 62 (21-82) | 62 (21-82) |
| Female | 47% (27) | 44% (28) |
| Race | ||
| Caucasian | 86.2% (50) | 87.6% (56) |
| African American | 5.2% (3) | 5% (3) |
| Hispanic | 3.4% (2) | 3.1% (2) |
| Asian | 3.4% (2) | 3.1% (2) |
| Other | 1.7% (1) | 1.6% (1) |
| Histology | ||
| Aggressive B-cell lymphoma | 39.7% (23) | 44% (28) |
| MCL | 1.7% (1) | 1.5% (1) |
| Myeloma | 56.9% (33) | 53% (34) |
| ALL | 1.7% (1) | 1.5% (1) |
| Median prior lines of therapy (range) | 4 (1-12) | 3 (1-12) |
| Previous auto-HCT | ||
| Aggressive B-cell lymphoma | 35% (8/23) | 36% (10/28) |
| MM | 91% (30/33) | 91% (31/34) |
| Previous allo-HCT | ||
| Aggressive B-cell lymphoma | 4.3% (1/23) | 3.6% (1/28) |
| MM | 27.3% (9/33) | 29.4% (10/34) |
| Bridging therapy | ||
| Aggressive B-cell lymphoma | 39% (9/23) | 43% (12/28) |
| MM | 33% (11/33) | 35% (12/34) |
| Median LDH before lymphodepletion | 205 (112-1228) | 205 (112-1228) |
| Type of CAR | ||
| Axi-cel | 17 | 22 |
| Tisa-cel | 1 | 1 |
| Liso-cel | 5 | 5 |
| Brexu-cel | 2 | 2 |
| Cilta-cel | 21 | 24 |
| Ide-cel | 9 | 10 |
| Lymphodepletion | ||
| Flu-Cy | 50% (29/58) | 52% (33/64) |
| Bendamustine | 50% (29/58) | 48% (31/64) |
Allo-HCT, allogeneic hematopoietic cell transplant; auto-HCT, autologous hematopoietic cell transplant; Brexu-cel, brexucabtagene autoleucel; Ide-cel, idecabtagene vicleucel; LDH, lactate dehydrogenase; Liso-cel, lisocabtagene maraleucel; Tisa-cel, tisagenlecleucel.
Hospital admissions
Among the 58 patients who received CAR T-cell infusion as outpatients, 42 patients (72%) were admitted to the hospital between days 0 to 30 (Table 2), within a median of 4 days (range, 0-22) after CAR T-cell infusion. The median length of hospitalization was 4 days (range, 1-14). Among those who were admitted, 17 patients (40%) were admitted within 72 hours, 16 (38%) between 4 and 7 days, and 9 (22%) between 8 and 30 days. One patient required intensive care unit admission due to grade 4 ICANS.
Table 2.
Outcomes of patients receiving outpatient CAR T-cell therapy
| Outcomes | Patients with outpatient cell infusion (n = 58) | All patients intended for outpatient administration (n = 64) |
|---|---|---|
| ORR∗ | ||
| LYM | 74% (17/23; CR = 12; PR = 5) | 71% (20/28; CR = 13; PR = 7) |
| MM | 75% (24/32; CR = 15; VGPR/PR = 9) | 73% (24/33; CR = 15; VGPR/PR = 9) |
| Median PFS | ||
| LYM | 4.6 mo | 6.2 |
| MM | 12.8 mo | 14.2 |
| Median OS | ||
| LYM | 9.14 mo | 9.14 |
| MM | NR | NR |
| CRS∗ (All grade 1-2) |
All patients (n = 58) 79% (46) |
LYM (n = 24) 83% (20) |
MM (n = 33) 76% (25) |
All patients (n = 64) 80% (51) |
LYM (n = 29) 83% (24) |
MM (n = 34) 76% (26) |
|---|---|---|---|---|---|---|
| ICANS | 28% (16) | 45% (11) | 15% (5) | 31% (20) | 48% (14) | 18% (6) |
| Grade 1-2 | 15% (9) | 8% (5) | 12% (4) | 14% (9) | 17% (5) | 12% (4) |
| Grade 3-4 | 12% (7) | 25% (6) | 3% (1) | 22% (11) | 31% (9) | 6% (2) |
| 30-d admissions∗ | 72% (42) | 87% (21) | 64% (21) | |||
| Days 0-3 | 34% (17) | 37% (9) | 32% (8) | |||
| Days 4-7 | 44% (16) | 46% (11) | 37% (5) | |||
| Days 8-30 | 19% (9) | 4% (1) | 26% (8) | |||
| First dose tocilizumab | ||||||
| Outpatient | 60% (35) | |||||
| Inpatient | 15% (9) | |||||
LYM, lymphoma; VGPR, very good partial response.
The single patient with ALL had grade 1 CRS and was admitted on day 4 due to bacteremia with a CR on day 28.
Among patients with B-cell lymphoma, 21 of 24 patients (87%) were hospitalized within 30 days, including 9 who were admitted within 72 hours, 11 admitted between 4 and 7 days, and 1 admitted between 8 and 30 days. The median length of hospitalization was 5 days (range, 2-14). Of note, all patients who received dexamethasone prophylaxis were admitted. The rate of admission in patients with MM was 64% (21/33), including 8 who were admitted within 72 hours, 5 admitted between 4 and 7 days, and 8 admitted between 8 and 30 days, whereas the median length of hospitalization was 3.5 days (range, 1-13). The single patient with ALL was admitted on day 4 due to bacteremia.
The rates of 30-day admission varied among different CAR T-cell products. The rate of admission in patients receiving axi-cel, the most common product used for lymphoma in our cohort, was 82% (14/17), and the median time to admission was 4 days (range, 1-13) after CAR infusion. The rate of admission in patients receiving cilta-cel, the most common product used for MM, was 58% (14/24), and the median time to admission was 8 days (range, 3-22) after CAR infusion. All patients who received dexamethasone prophylaxis were admitted.
The most common reason for admission was CAR T-cell–related toxicities in 32 of 42 patients: CRS in 20 patients (48%), ICANS in 3 patients (7%), and both CRS and ICANS in 9 patients (21%). Other reasons for admission included disease progression (3/42), infections (3/42), gastrointestinal symptoms (3/42), and pain (1/42).
CRS/ICANS incidence and management
Grade 1 to 2 CRS developed in 51 patients, including 24 of 29 patients (83%) with B-cell lymphomas, 26 of 34 patients (76%) with MM, and 1 of 1 patient (100%) with ALL (Table 2). There were no cases of grade ≥3 CRS. All patients with CRS received tocilizumab.
Among the patients who received tocilizumab for CRS (n = 48), 35 received the first dose of tocilizumab in the outpatient setting. Fifteen of these 35 patients did not require admission for CRS, whereas 3 patients were admitted for CRS after 48 hours of initial outpatient management. CRS in these patients was managed by using tocilizumab in the outpatient setting, preventing the need for hospitalization. Among patients developing CRS and receiving the first dose of tocilizumab outpatient (n = 35), the number needed to treat (with outpatient tocilizumab) to prevent 1 additional hospitalization was 2.3. Figure 1 summarizes the outpatient management of CRS.
Twenty patients developed ICANS, including 14 patients with lymphoma and 6 with MM. Among these, 9 were grade 1 to 2 (5 with lymphoma and 4 with MM), whereas 11 patients had grade 3 to 4 ICANS (9 with lymphoma and 2 with MM). All patients with ICANS were treated in the inpatient setting.
Among the 7 patients who received dexamethasone for CRS/ICANS prophylaxis, 6 patients developed CRS (all grade 1-2), whereas 5 had ICANS (grade 1-2, n = 2; grade 3, n = 3). There were no differences in rates of CRS (86% vs 82%; P > .99) and ICANS (71.4% vs 40.9%; P = .2) in patients who received dexamethasone compared with those who did not.
Outcomes
Among all patients who were intended for outpatient CAR T-cell therapy (n = 64), 28 patients with lymphoma and 33 patients with MM were evaluable for response. The overall response rate (ORR) in patients with lymphoma was 71% (complete response [CR] = 13 [CR rate = 46%]; partial response [PR] = 7), whereas the ORR in patients with MM was 72% (24/33; CR = 15 [CR rate = 45%]; very good partial response/PR = 9). The 1 patient with ALL had a CR at day 28. Table 2 shows the outcomes of all patients intended for outpatient infusion and patients who received the cell infusion as outpatient.
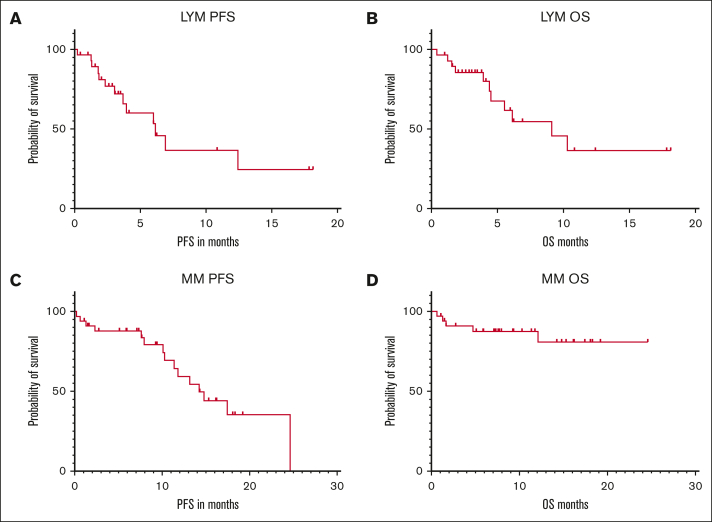
After a median follow-up of 6 months, the median PFS and OS in all patients with lymphoma were 6.15 months and 9.14 months, whereas the 6-month PFS and OS were 53% and 61%, respectively (Fig. 3A-B). The median PFS and OS in all patients with MM were 14.2 months and not reached (NR), whereas the 6-month PFS and OS were 88% and 88%, respectively (Fig. 3C-D).
Figure 3.
Outpatient CAR-T outcomes. (A) PFS of patients with lymphoma. (B) OS of patients with lymphoma. (C) PFS of patients with MM. (D) OS of patients with MM. LYM, lymphoma.
The rates of 1-month and 6-month non-relapse mortality (NRM) in patients with lymphoma were 1.7% and 3.4%, respectively. The cause of 1-month and 6-month NRM was refractory ICANS.
The 1-month and 6-month NRM in patients with MM were both 0. After a median follow-up of 6 months, 28 patients relapsed. There were 16 deaths, including 11 patients with lymphoma and 5 with MM. The most common cause of death was progressive disease, which occurred in 14 patients (lymphoma, n = 9; MM, n = 5), followed by ICANS (lymphoma, n = 2).
Impact of lymphodepletion
Thirty-three patients received fludarabine and cyclophosphamide (Flu-Cy), whereas 31 received bendamustine lymphodepletion. Although, not statistically significant, the rates of CRS (68% vs 88%; P = .07) and ICANS (23% vs 39%; P = .18) were lower in patients who received bendamustine than those who received Flu-Cy. Similarly, rates of admission were lower in patients who received bendamustine, although not statistically significant (62% vs 86%; P = .07). The median length of hospitalization in patients who received Flu-Cy vs bendamustine is 5 days (range, 2-14) vs 3 days (range, 2-10), respectively.
In patients with lymphoma, the rates of hospital admission were significantly lower in patients who received bendamustine than those who received Flu-Cy (33% vs 100%; P = .001). The rates of CRS (71% vs 87%; P = .4) and ICANS (29% vs 67%; P = .06) in patients with lymphoma were lower in bendamustine, although not statistically significant. There was no difference in response rates (69% vs 85%; P = .6), PFS (NR vs 6 months; P = .75) or OS (NR vs 6 months; P = .84). The median length of hospitalization was longer in the Flu-Cy (6.5 days; range, 2-14) than the bendamustine group (2.5 days; range, 2-8).
In patients with MM, the rates of CRS (65% vs 88%; P = .22), ICANS (18% vs 18%; P > .99), and hospital admissions (59% vs 64%; P > .99) were not statistically different between the 2 groups. There was no difference in response rates (71% vs 86%; P = .3) and OS (NR for both cohorts; P = .36), whereas PFS was higher in patients who received Flu-Cy than those who received bendamustine (24.6 vs 10.3 months; P = .01). There was no difference in the median length of stay between the Flu-Cy (3.5 days; range, 1-13) and bendamustine (3.5 days; range, 2-10) groups.
Impact of bridging therapy
Twenty-four patients (37.5%) required bridging therapy before CAR T-cell therapy, including 12 patients with lymphoma and 12 with MM. In patients with lymphoma, the rates of CRS (92% vs 71%; P = .35), ICANS (50% vs 47%; P > .99), and hospital admissions (89% vs 80%; P > .99) were not different among the patients who received bridging therapy compared with those who did not. Similarly, there were no differences in the rates of CRS (67% vs 82%; P = .5), ICANS (23% vs 14%; P = .65), and hospital admissions (83% vs 55%; P = .14) among patients with MM who received bridging therapy compared with those who did not.
Impact of inpatient vs outpatient administration
To compare the outpatient vs inpatient administration of CAR T-cell therapy, we included a historical control receiving planned inpatient CAR T-cell therapy in the 2 years (2020-2021) preceding our outpatient program (n = 23; baseline characteristics shown in supplemental Table 2).
The median length of hospitalization for patients in the historical control was 18 days (range, 6-89). During the first 30 days after CAR T-cell therapy, the historical control used an average of 20.6 days per patient in the hospital compared with a per patient average of 6.2 days in the outpatient cohort (average hospital days saved per patient of 14.4 days in the first month after therapy).
In patients with lymphoma (n = 21), the ORR was 62% (CR = 11; PR = 2). The PFS and OS rates at 6 months were 43% and 59%, respectively. The median PFS and OS were 1.3 months and 13 months, respectively, which were not statistically significant from the median PFS (6 months; P = .4) and OS (9 months; P = .4) of patients who received outpatient CAR T-cell therapy. The 1-month and 6-month NRM were both 14.3% in the inpatient CAR T-cell therapy group. None of the patients with MM (n = 2) had a response to CAR T-cell therapy.
Discussion
Although several recent studies have reported the feasibility of outpatient CAR T-cell therapy, these studies, such as our cohort, were generally small but were often either limited to the 41BB-containing CAR T cells or had applied intense home monitoring strategies.17,18,22, 23, 24 We report our experience of outpatient administration of all commercially available CAR T-cell products including the CD28-containing axi-cel and both anti-BCMA–containing CAR T-cell therapies, cilta-cel and idecabtagene vicleucel, without instituting an intense remote monitoring system. This is also, to our knowledge, the first report of management of low-grade CRS in the outpatient setting, thus preventing admission in select patients.
Several factors influence the successful administration of CAR T-cell therapy in the outpatient setting, including an adequate infrastructure (eg, appropriate infusion space), well-trained staff (physicians, APPs, nurses, and pharmacy), the ability to treat patients after work hours, and several patient-related factors (eg, performance status, availability of a caregiver, and ability to reside near the center).25, 26, 27 Therefore, at our center, we established several safeguards and guidelines for the successful outpatient administration of CAR T cells. We have a well-established day hospital capable of outpatient transplant and cell therapy infusions, as well as a 24-hour clinic for after-hours care, staffed by a dedicated oncology nurse, APP, and physician (on call). We have previously reported the feasibility of performing outpatient autologous and allogeneic hematopoietic cell transplantation with reduced-intensity conditioning using the same system.28,29
The feasibility of outpatient administration has been demonstrated in several clinical trials and retrospective studies.22,23,30 Although these CAR T-cell therapies contain the 41BB costimulatory domain, the data for CD28-containing CAR T cells are limited and often use a very intense home monitoring system. The use of remote monitoring systems/devices adds to health care resource utilization, can lead to patient anxiety, and result in excessive reliance on these devices, thus delaying medical care.18,20,31,32 We did not implement such remote monitoring systems and relied on patient and caregiver education as well as on a system that has worked in our outpatient autologous and allogeneic transplant recipients for over a decade.28,29
Another noteworthy point in our study is the outpatient management of grade 1 CRS. Due to this, 15 of the 35 patients who developed CRS and received tocilizumab in the outpatient setting did not require admission for CRS. Our study, albeit small, provides data that low-grade CRS can be managed in the outpatient setting using an early intervention strategy, thus preventing hospital admission and saving inpatient resources for patients with the highest needs.
There are several advantages of outpatient CAR T-cell therapy. With the decrease in hospitalization rates and duration, there is a considerably lower utilization of health care resources and a decreased burden on limited hospital beds. The median hospitalization duration is at least 2 to 3 times shorter in the outpatient than inpatient CAR T-cell administration. Additionally, the health care costs associated with CAR T-cell therapy administration are 2 to 4 times higher in the inpatient than in the outpatient setting, which is mainly driven by the cost of inpatient stay.26 On the contrary, given the complex regulations regarding insurance coverage and copays as well possible need for travel and/or lodging, there may be a higher financial burden on the patients, especially in the United States.27,33 Outpatient administration also requires a need for a 24-hour caregiver who can provide adequate support and has a sufficient understanding of CAR T-cell therapy–related toxicities.
The rate of admission in our study was ∼72%; the most common reason for admission was CRS, whereas the rates and days of hospitalization varied considerably with different CAR T-cell therapy products.17,22 As previously reported in several studies, due to higher rates of immune-related toxicities, the admission rate was considerably higher in axi-cel, with a median day of admission at 4 days after infusion.1,29 Conversely, the median day of admission was day 8 with cilta-cel, consistent with the known late onset of toxicities with cilta-cel.9
In our study, although not statistically significant, the rates of CRS, ICANS, and hospital admissions were lower in patients who received bendamustine than Flu-Cy, especially among patients with lymphoma. This is consistent with prior studies reporting comparable efficacy but lower toxicities rates with bendamustine than Flu-Cy lymphodepletion in patients with lymphoma.34, 35, 36 On the contrary, both the efficacy and toxicity profiles were comparable between bendamustine and Flu-Cy in patients with MM receiving anti-BCMA CART-cell therapy.37 Therefore, this may present a potential to reduce immune toxicities–related admissions in patients who receive outpatient CAR T-cell therapy. On the contrary, although a small number of patients (n = 6) received dexamethasone prophylaxis, this did not affect the rates of any grade CRS, ICANS, and hospital admissions, which is similar to reported data.38
Our study has some limitations including its retrospective nature as well as lack of a prospective comparator, which can create selection bias. Additionally, the follow-up for survival outcomes is relatively short but the primary aim of this study was to report 30-day hospitalizations for which all included patients had adequate follow-up. Our data for outpatient administration for patients with ALL or those who received brexucabtagene autoleucel are also limited (1 each). In addition, our approach of outpatient management of low-grade CRS requires well-trained staff and availability of a 24-hour center where these patients can be monitored without being hospitalized, which may not be possible at other centers.
In conclusion, we demonstrate that CAR T-cell therapy can be successfully administered as outpatient in a suitable setting, which includes patient and caregiver education regarding CAR T-cell–related toxicities, a 24-hour available center for monitoring patients in case of toxicity, and a well-trained nursing staff and physician (including on-call physicians). In addition, carefully selected patients with low-grade CRS may be safely treated as outpatients with early administration of tocilizumab for grade 1 symptoms, preventing hospital admissions.
Conflict-of-interest disclosure: S.A. reports consulting roles with AbbVie, Servier, Incyte, and Daichi Sankyo; research funding from Incyte, Actinium Pharmaceuticals, and AltruBio, Inc. A.D. reports advisory boards and/or consultancy from Bristol Myers Squibb (BMS), Janssen, Pfizer, and Prothena; and institutional research funding from AbbVie, Caelum, Janssen, Novartis, Prothena, and Regeneron. B.D. reports consulting and advisory board roles with Arcellx, Janssen, BMS, Sanofi, Karyopharm, Genentech, and Pfizer; and research support from Janssen, BMS, Sanofi, Carsgen, and Arcellx. T.S.F. reports consulting and/or speaking fees from Adaptive Biotechnologies, ADCT, AstraZeneca, BeiGene, Kite (Gilead), Lilly/Loxo, MorphoSys, Ono Pharmaceuticals, Pharmacyclics (AbbVie), Seagen, and TG Therapeutics. M.H. reports consultancy fees from Incyte Corporation, ADC Therapeutics, Pharmacyclics, Omeros, Genmab, MorphoSys, Kadmon, Kite, Novartis, AbbVie, Legend, Gamida Cell, Seagen, Caribou, CRISPR, and Autolus; and speaker’s bureau fees from Sanofi Genzyme, AstraZeneca, BeiGene, and ADC Therapeutics. G.S.G.M. reports advisory board/consultancy fees from BMS, BeiGene, Pfizer, Gilead/Kite, Cancer Expert Now, Qessential, and Techspert; honoria from Cardinal Health, DAVA Oncology, Aptitude Health, and Curio Science; and speaking bureau fees from Amgen and Rigel. M.P. reports research support from BMS, Kite, Novartis, and Janssen; and consultant fees from Novartis and BMS. N.N.S. reports advisory boards and/or consultancy fees from Gilead-Kite, BMS-Juno, Miltenyi Biomedicine, Lilly Oncology, Incyte, Novartis, Seattle Genetics, Janssen, AbbVie, Cargo, BeiGene, and Galapagos; research funding, travel support, and honoraria from Lilly Oncology, Genentech, and Miltenyi Biomedicine; scientific advisory board and fees from Tundra Therapeutics. The remaining authors declare no competing financial interests.
Acknowledgments
Supported by grant 1R50CA275856-01 from the National Cancer Institute to M.H.
Authorship
Contribution: F. Furqan and M.H. conceived and designed the study; F. Furqan, V.B., and M.H. collected and assembled the data; F. Furqan and M.H. were responsible for data analysis; all authors contributed to data interpretation; F. Furqan and M.H. wrote the manuscript and prepared the first draft; and all authors helped revise the manuscript and provided the final approval of manuscript.
Footnotes
Data are available on request from the corresponding author, Mehdi Hamadani (mhamadani@mcw.edu).
The full-text version of this article contains a data supplement.
Supplementary Material
References
- 1.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Munshi NC, Anderson LD, Jr., Shah N, et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med. 2021;384(8):705–716. doi: 10.1056/NEJMoa2024850. [DOI] [PubMed] [Google Scholar]
- 4.Kanate AS, Majhail N, DeFilipp Z, et al. Updated indications for immune effector cell therapy: 2023 guidelines from the American Society for Transplantation and Cellular Therapy. Transplant Cell Ther. 2023;29(10):594–597. doi: 10.1016/j.jtct.2023.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Berdeja JG, Madduri D, Usmani SZ, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet. 2021;398(10297):314–324. doi: 10.1016/S0140-6736(21)00933-8. [DOI] [PubMed] [Google Scholar]
- 6.Fowler NH, Dickinson M, Dreyling M, et al. Tisagenlecleucel in adult relapsed or refractory follicular lymphoma: the phase 2 ELARA trial. Nat Med. 2022;28(2):325–332. doi: 10.1038/s41591-021-01622-0. [DOI] [PubMed] [Google Scholar]
- 7.Wang M, Munoz J, Goy A, et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2020;382(14):1331–1342. doi: 10.1056/NEJMoa1914347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobson CA, Chavez JC, Sehgal AR, et al. Axicabtagene ciloleucel in relapsed or refractory indolent non-Hodgkin lymphoma (ZUMA-5): a single-arm, multicentre, phase 2 trial. Lancet Oncol. 2022;23(1):91–103. doi: 10.1016/S1470-2045(21)00591-X. [DOI] [PubMed] [Google Scholar]
- 9.San-Miguel J, Dhakal B, Yong K, et al. Cilta-cel or standard care in lenalidomide-refractory multiple myeloma. N Engl J Med. 2023;389(4):335–347. doi: 10.1056/NEJMoa2303379. [DOI] [PubMed] [Google Scholar]
- 10.Locke FL, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med. 2022;386(7):640–654. doi: 10.1056/NEJMoa2116133. [DOI] [PubMed] [Google Scholar]
- 11.Kamdar M, Solomon SR, Arnason J, et al. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet. 2022;399(10343):2294–2308. doi: 10.1016/S0140-6736(22)00662-6. [DOI] [PubMed] [Google Scholar]
- 12.Bishop MR, Dickinson M, Purtill D, et al. Second-line tisagenlecleucel or standard care in aggressive B-cell lymphoma. N Engl J Med. 2022;386(7):629–639. doi: 10.1056/NEJMoa2116596. [DOI] [PubMed] [Google Scholar]
- 13.Cliff ERS, Kelkar AH, Russler-Germain DA, et al. High cost of chimeric antigen receptor T-cells: challenges and solutions. Am Soc Clin Oncol Educ Book. 2023;43(43) doi: 10.1200/EDBK_397912. [DOI] [PubMed] [Google Scholar]
- 14.Lyman GH, Nguyen A, Snyder S, Gitlin M, Chung KC. Economic evaluation of chimeric antigen receptor T-cell therapy by site of care among patients with relapsed or refractory large B-cell lymphoma. JAMA Netw Open. 2020;3(4) doi: 10.1001/jamanetworkopen.2020.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rojas-Garcia A, Turner S, Pizzo E, Hudson E, Thomas J, Raine R. Impact and experiences of delayed discharge: a mixed-studies systematic review. Health Expect. 2018;21(1):41–56. doi: 10.1111/hex.12619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bachier CR, Palomba ML, Abramson JS, et al. Outpatient treatment with lisocabtagene maraleucel (liso-cel) in 3 ongoing clinical studies in relapsed/refractory (R/R) large B cell non-Hodgkin lymphoma (NHL), including second-line transplant noneligible (TNE) patients: Transcend NHL 001, Outreach, and PILOT. Biol Blood Marrow Transplant. 2020;26(3):S25–S26. [Google Scholar]
- 17.Borogovac A, Keruakous A, Bycko M, et al. Safety and feasibility of outpatient chimeric antigen receptor (CAR) T-cell therapy: experience from a tertiary care center. Bone Marrow Transplant. 2022;57(6):1025–1027. doi: 10.1038/s41409-022-01664-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dholaria B, Mehraban N, Baer B, et al. Feasibility of outpatient administration of axicabtagene ciloleucel and brexucabtagene autoleucel using telemedicine tools: the Vanderbilt experience. Br J Haematol. 2022;198(6):1073–1075. doi: 10.1111/bjh.18339. [DOI] [PubMed] [Google Scholar]
- 19.McGann M, Davis JA, Gaffney KJ, et al. Real-world experience and optimization of outpatient chimeric antigen receptor T cell therapy. Transplant Cell Ther. 2022;28(9):583–585. doi: 10.1016/j.jtct.2022.06.021. [DOI] [PubMed] [Google Scholar]
- 20.Serrano LP, Maita KC, Avila FR, et al. Benefits and challenges of remote patient monitoring as perceived by health care practitioners: a systematic review. Perm J. 2023;27(4):100–111. doi: 10.7812/TPP/23.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee DW, Santomasso BD, Locke FL, et al. ASTCT Consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25(4):625–638. doi: 10.1016/j.bbmt.2018.12.758. [DOI] [PubMed] [Google Scholar]
- 22.Ahmed N, Wesson W, Mushtaq MU, et al. Patient characteristics and outcomes of outpatient tisagenlecleucel recipients for B cell non-Hodgkin lymphoma. Transplant Cell Ther. 2023;29(7):449.e1–449.e7. doi: 10.1016/j.jtct.2023.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bachier CR, Godwin JE, Andreadis C, et al. Outpatient treatment with lisocabtagene maraleucel (liso-cel) across a variety of clinical sites from three ongoing clinical studies in relapsed/refractory (R/R) large B-cell lymphoma (LBCL) Journal of Clinical Oncology. 2020;38(suppl 15) [Google Scholar]
- 24.Oluwole OO, Dholaria B, Knight TE, et al. Chimeric antigen receptor T-cell therapy in the outpatient setting: an expert panel opinion from the American Society for Transplantation and Cellular Therapy. Transplant Cell Ther. 2024;30(2):131–142. doi: 10.1016/j.jtct.2023.11.008. [DOI] [PubMed] [Google Scholar]
- 25.Alexander M, Culos K, Roddy J, et al. Chimeric antigen receptor T cell therapy: a comprehensive review of clinical efficacy, toxicity, and best practices for outpatient administration. Transplant Cell Ther. 2021;27(7):558–570. doi: 10.1016/j.jtct.2021.01.014. [DOI] [PubMed] [Google Scholar]
- 26.Hansen DK, Liu YH, Ranjan S, et al. The impact of outpatient versus inpatient administration of CAR-T therapies on clinical, economic, and humanistic outcomes in patients with hematological cancer: a systematic literature review. Cancers (Basel) 2023;15(24) doi: 10.3390/cancers15245746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Myers GD, Verneris MR, Goy A, Maziarz RT. Perspectives on outpatient administration of CAR-T cell therapy in aggressive B-cell lymphoma and acute lymphoblastic leukemia. J Immunother Cancer. 2021;9(4) doi: 10.1136/jitc-2020-002056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Graff TM, Singavi AK, Schmidt W, et al. Safety of outpatient autologous hematopoietic cell transplantation for multiple myeloma and lymphoma. Bone Marrow Transplant. 2015;50(7):947–953. doi: 10.1038/bmt.2015.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guru Murthy GS, Hari PN, Szabo A, et al. Outcomes of reduced-intensity conditioning allogeneic hematopoietic cell transplantation performed in the inpatient versus outpatient setting. Biol Blood Marrow Transplant. 2019;25(4):827–833. doi: 10.1016/j.bbmt.2018.12.069. [DOI] [PubMed] [Google Scholar]
- 30.Bansal R, Paludo J, De Menezes Silva Corraes A, et al. Outpatient practice utilization for CAR-T and T cell engager in patients with lymphoma and multiple myeloma. J Clin Oncol. 2023;41(16 suppl):1533. [Google Scholar]
- 31.Fraiche AM, Matlock DD, Gabriel W, Rapley FA, Kramer DB. Patient and provider perspectives on remote monitoring of pacemakers and implantable cardioverter-defibrillators. Am J Cardiol. 2021;149:42–46. doi: 10.1016/j.amjcard.2021.03.023. [DOI] [PubMed] [Google Scholar]
- 32.Sharif F, Rahman A, Tonner E, et al. Can technology optimise the pre-operative pathway for elective hip and knee replacement surgery: a qualitative study. Perioper Med (Lond) 2020;9(1):33. doi: 10.1186/s13741-020-00166-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shah J. 28 April 2019. Achieving sustainable market and patient access: Medicare and Medicaid coverage, reimbursement, and coding issues for CAR-T. Paper presented at: ASGCT Meeting. Washington, DC. [Google Scholar]
- 34.Landego I, Nemirovsky D, Devlin SM, et al. Comparison of bendamustine versus fludarabine/ cyclophosphamide lymphodepletion in adult patients receiving CD19 CAR-T cell therapy. Blood. 2023;142(suppl 1):3510. [Google Scholar]
- 35.Ong SY, Pak S, Mei M, et al. Bendamustine lymphodepletion is a well-tolerated alternative to fludarabine and cyclophosphamide lymphodepletion for axicabtagene ciloleucel therapy for aggressive B-cell lymphoma. Am J Hematol. 2023;98(11):1751–1761. doi: 10.1002/ajh.27069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghilardi G, Chong EA, Svoboda J, et al. Bendamustine is safe and effective for lymphodepletion before tisagenlecleucel in patients with refractory or relapsed large B-cell lymphomas. Ann Oncol. 2022;33(9):916–928. doi: 10.1016/j.annonc.2022.05.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sidana S, Hosoya H, Jensen A, et al. Bendamustine vs. fludarabine/cyclophosphamide lymphodepletion prior to BCMA CAR-T cell therapy in multiple myeloma. Blood Cancer J. 2023;13(1):158. doi: 10.1038/s41408-023-00929-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oluwole OO, Bouabdallah K, Munoz J, et al. Prophylactic corticosteroid use in patients receiving axicabtagene ciloleucel for large B-cell lymphoma. Br J Haematol. 2021;194(4):690–700. doi: 10.1111/bjh.17527. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.