Dear editor,
Chronic pelvic pain (CPP), defined as a noncyclic pain perceived to originate from the pelvis and lasting more than 6 months, is a debilitating disease affecting 25 % of the world’s female population [1]. The medical impact of CPP is impressive, with the condition accounting for 10 % of gynecology visits, 40 % of laparoscopies, and 12 % of hysterectomies despite no clear gynecologic association in 80 % of patients [2]. This condition similarly presents an economic burden, estimated to cost $5.8 billion in 2020 [3]. Appropriate treatment is paramount, though CPP remains a challenge for providers. Multimodal management with analgesics, neuropathic medications, physical therapies, psychiatric therapies, nerve blocks, and/or trigger point or botulinum toxin injections have provided a promising and acceptable solution for a large proportion of patients [4]. Oftentimes, however, conservative management is inadequate in the treatment of this condition.
Spinal cord stimulation (SCS), in which electrical pulses are delivered through leads in the dorsal epidural space, is an alternate treatment for chronic pain that provides a safe, reliable, and precisely localized method of pain control [5]. Open-loop spinal cord stimulation (OLSCS) relies on frequent device adjustments via external remote to maintain a steady level of neural activation. Closed-loop spinal cord stimulation (CLSCS) measures neural output and self-modulates electrical input from a single device, thus creating a “closed-loop” of stimulation. This system allows for precise, real-time adjustment that might provide better pain relief than OLSCS [6] and might reduce opioid burden while improving quality of life [5].
Limited but existing evidence detailing the use of OLSCS for CPP is encouraging. In 2006, Kapural et al. described the placement of OLSCS leads at T11-L1 in six patients with refractory visceral pelvic pain, with postoperative pain reduction, improvement in pain disability index and decreasing opioid use among participants [7]. In 2013, Hunter et al. described the use of OLSCS in six patients, with lead placement at both mid-thoracic levels and the conus medullaris yielding good symptom control [8]. In a more recent and prospective cohort, Tate et al. realized a mean of greater than 70 % pain relief over 12 months in a predominantly female group with lead placement between T8 and T12 [9]. To our knowledge, and with informed patient consent, we offer the first report of closed-loop spinal cord stimulation for chronic pelvic pain.
A 73-year-old woman with past medical history including two uncomplicated vaginal deliveries presented to clinic for evaluation of chronic atraumatic pelvic pain. The patient reported a constant, burning pain located superficially about the vagina and perineum, rating it 1–2/10 in severity at rest, with sitting and light touch causing daily flares to 10/10 on the Visual Analog Scale (VAS, from 0, no pain, to 10, worst pain). She denied associated incontinence and lower extremity weakness. The patient had previously received specialty care from urology and gynecology but had failed to establish a urologic or gynecologic source of this pain and had failed to realize significant improvement with pudendal nerve block. Despite a medication regimen of gabapentin 800 mg three times daily, duloxetine 60 mg daily, and amantadine 100 mg twice daily in addition to as-needed oral acetaminophen and transdermal lidocaine, she was unable to tolerate undergarments and was thus unable to maintain her five times weekly exercise regimen, to attend religious services, or to be intimate with her husband. Her condition also caused her to take extended leave from work.
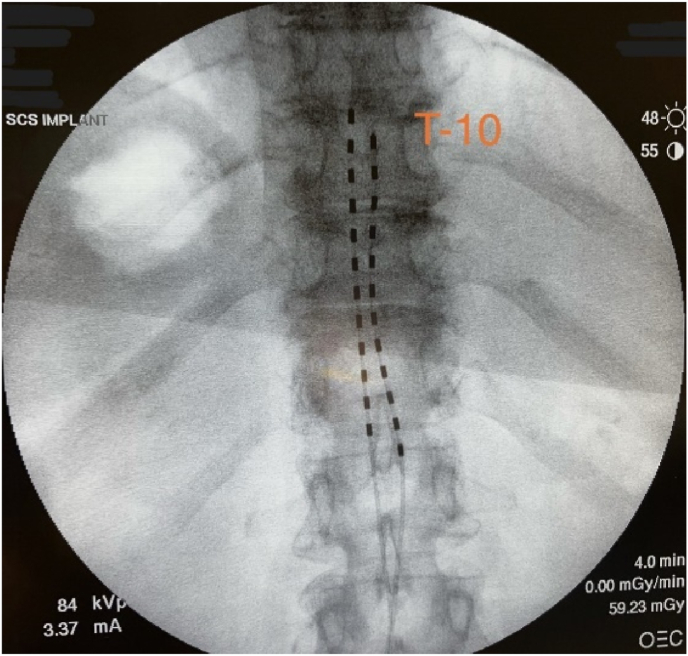
With a primary diagnosis of vulvodynia, the patient underwent a successful trial and then implantation of a closed-loop spinal cord stimulator seventeen months after symptom onset. Two leads were introduced into the dorsal epidural space through an interlaminar approach at T12-L1 and were advanced to the level of T9-T10 under fluoroscopic guidance (Fig. 1). Intraoperative mapping with open loop stimulation ensured bilateral regional coverage, and the leads were then anchored to the interspinous ligament before being attached to the pulse generator, located at the left flank.
Fig. 1.
Intraoperative lead placement.
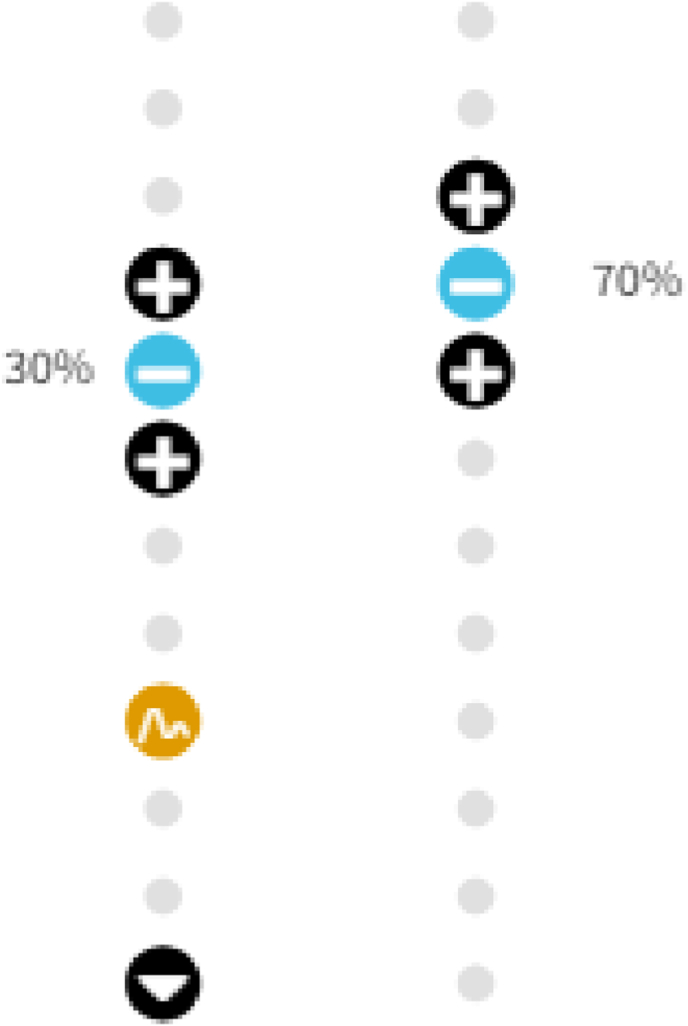
The patient’s device was programmed postoperatively (Fig. 2), with the active electrodes at the level of the T10-T11 vertebral bodies, the ECAP recording electrode at the T12 vertebral body, and the reference electrode at the inferior margin of the T12 vertebral body. A frequency of 60Hz and pulse width 270μs was utilized, and her evoked compound action potential (ECAP) therapeutic window was established between 1μV and 91μV.
Fig. 2.
Patient-preferred program.
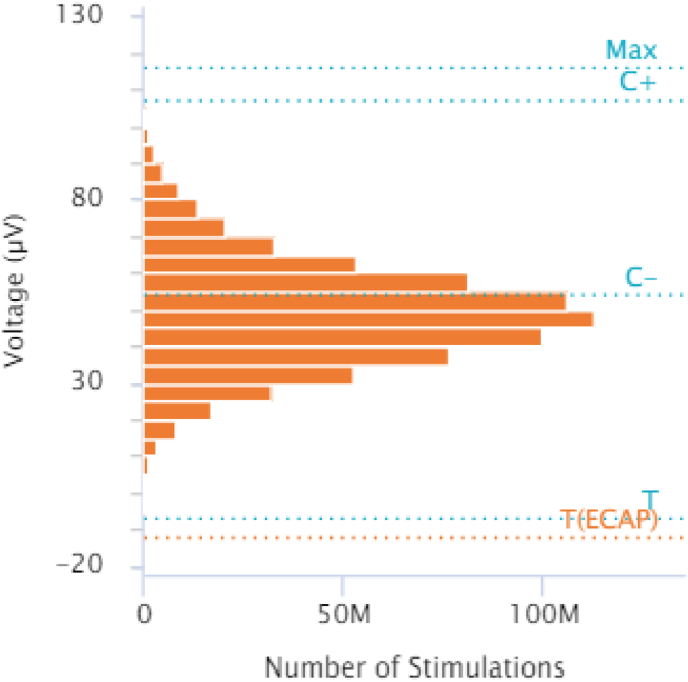
Within seven weeks of implantation, the patient’s pelvic pain had disappeared, with VAS score 0/10. She was again comfortably wearing undergarments, attending religious service, gently exercising, and shopping. At nine months postoperatively the patient reported that she remained free of pelvic pain, with VAS score 0/10. She remained on her preoperative dose of duloxetine 60 mg daily, had reduced her gabapentin dose to 800 mg once nightly, and had stopped taking amantadine. She was using transdermal lidocaine less than once daily as needed for battery site discomfort due to a paucity of subcutaneous tissue and was fully reintegrated into community activity with a return to work and regular exercise. Device reports at nine months postoperatively revealed over one billion closed-loop adjustments had been made, with a mean measured dose of 74.4μV, suggesting that the system had been maintaining the patient near her ECAP target dose of 75.4 μV (Fig. 3). The patient reported subjectively that she was “doing great” and “couldn’t be better.”
Fig. 3.
Dose-control adherence.
Although prior evidence supports the use of open-loop spinal cord stimulation for chronic pelvic pain, no literature yet details the use of closed-loop spinal cord stimulation for this condition. In our patient, for whom chronic pelvic pain was severe and refractory, closed-loop spinal cord stimulation with lead placement at the level of T9-T10 has managed to alleviate vulvodynia entirely. Her medication burden has been reduced, her activity tolerance has increased, and relief has been sustained to nine months. Further research may help to evaluate the potential for this therapy in the management of chronic pelvic pain.
Declaration of competing interest
Dr. Brian M. Bruel reports personal fees from Abbott, personal fees from Biotronik, personal fees from Boston Scientific, and personal fees from Saluda.
Contributor Information
Daniel R. Briggi, Email: Daniel.briggi@bcm.edu.
Christian T. Vangeison, Email: Christian.vangeison@bcm.edu.
Peter D. Vu, Email: Peter.d.vu@uth.tmc.edu.
Zane Shah, Email: Zane.shah@bcm.edu.
Brian M. Bruel, Email: Brian.m.bruel@uth.tmc.edu.
References
- 1.Latthe P., Latthe M., Say L., Gülmezoglu M., Khan K.S. WHO systematic review of prevalence of chronic pelvic pain: a neglected reproductive health morbidity. BMC Publ Health. 2006 Jul 6;6:177. doi: 10.1186/1471-2458-6-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lamvu G., Carrillo J., Ouyang C., Rapkin A. Chronic pelvic pain in women: a review. JAMA. 2021 Jun 15;325(23):2381–2391. doi: 10.1001/jama.2021.2631. [DOI] [PubMed] [Google Scholar]
- 3.Huang G., Le A.L., Goddard Y., et al. A systematic review of the cost of chronic pelvic pain in women. J Obstet Gynaecol Can. 2022 Mar;44(3):286–293.e3. doi: 10.1016/j.jogc.2021.08.011. [DOI] [PubMed] [Google Scholar]
- 4.Parsons B.A., Baranowski A.P., Berghmans B., et al. Management of chronic primary pelvic pain syndromes. BJU Int. 2022 May;129(5):572–581. doi: 10.1111/bju.15609. [DOI] [PubMed] [Google Scholar]
- 5.Brooker C., Russo M., Cousins M.J., et al. ECAP-controlled closed-loop spinal cord stimulation efficacy and opioid reduction over 24-months: final results of the prospective, multicenter, open-label avalon study. Pain Pract. 2021;21(6):680–691. doi: 10.1111/papr.13008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mekhail N., Levy R.M., Deer T.R., et al. Long-term safety and efficacy of closed-loop spinal cord stimulation to treat chronic back and leg pain (Evoke): a double-blind, randomised, controlled trial. Lancet Neurol. 2020;19(2):123–134. doi: 10.1016/S1474-4422(19)30414-4. [DOI] [PubMed] [Google Scholar]
- 7.Kapural L., Narouze S.N., Janicki T.I., Mekhail N. Spinal cord stimulation is an effective treatment for the chronic intractable visceral pelvic pain. Pain Med. 2006;7(5):440–443. doi: 10.1111/j.1526-4637.2006.00165.x. [DOI] [PubMed] [Google Scholar]
- 8.Hunter Corey, et al. Neuromodulation of pelvic visceral pain: review of the literature and case series of potential Novel targets for treatment. Pain Pract. 2013;13(1):3–17. doi: 10.1111/j.1533-2500.2012.00558.x. [DOI] [PubMed] [Google Scholar]
- 9.Tate J.L., Stauss T., Li S., er al. A prospective, multi-center, clinical trial of a 10-kHz spinal cord stimulation system in the treatment of chronic pelvic pain. Pain Pract. 2021;21(1):45–53. doi: 10.1111/papr.12932. [DOI] [PMC free article] [PubMed] [Google Scholar]