Abstract
There is increasing interest in the role of reactive oxygen radicals in the hepatotoxicity associated with ethanol consumption. Reactive oxygen intermediates interact with DNA and can cause single-strand breaks of supercoiled DNA. Experiments were carried out to evaluate the utility of this system as a sensitive assay for the detection of potent oxidants generated by rat liver microsomes isolated from pair-fed control rats and rats treated chronically with ethanol. DNA strand cleavage was assayed by monitoring the migration of the supercoiled and open circular forms in agarose. Microsomes catalysed DNA strand breakage with either NADPH or NADH as cofactors; iron was required to catalyse the reaction and various ferric complexes were effective in promoting the reaction. DNA strand cleavage was prevented by catalase, superoxide dismutase, GSH and hydroxyl-radical-scavenging agents, suggesting that a hydroxyl-radical-like species was the oxidant responsible for the breakage. This assay system proved to be much more sensitive in detecting hydroxyl radicals than are other methods, such as e.s.r. spectroscopy or oxidation of chemical scavenging agents with respect to the amount of microsomal protein and the nature and concentration of the iron catalyst required. Microsomes from ethanol-treated rats were more reactive than control microsomes in catalysing the DNA strand cleavage with either NADPH or NADH; increased catalytic activity was observed with various ferric complexes and was sensitive to the above antioxidants. Compared with preimmune IgG, anti-(cytochrome P4502E1) IgG had no effect on DNA strand cleavage by the control microsomes, but completely prevented the NADPH- and the NADH-dependent increased activity found with microsomes from the ethanol-treated rats. Inhibitors of cytochrome P4502E1, such as diethyl dithiocarbamate and tryptamine, also lowered the extent of increase of DNA strand cleavage produced by microsomes from the ethanol-treated rats. These results indicate that DNA strand cleavage is a very sensitive assay for detecting the production of hydroxyl radicals by microsomes and to demonstrate increased activity by microsomes after chronic ethanol treatment. This increased activity with NADPH and NADH is due, at least in part, to induction of cytochrome P4502E1.
Full text
PDF


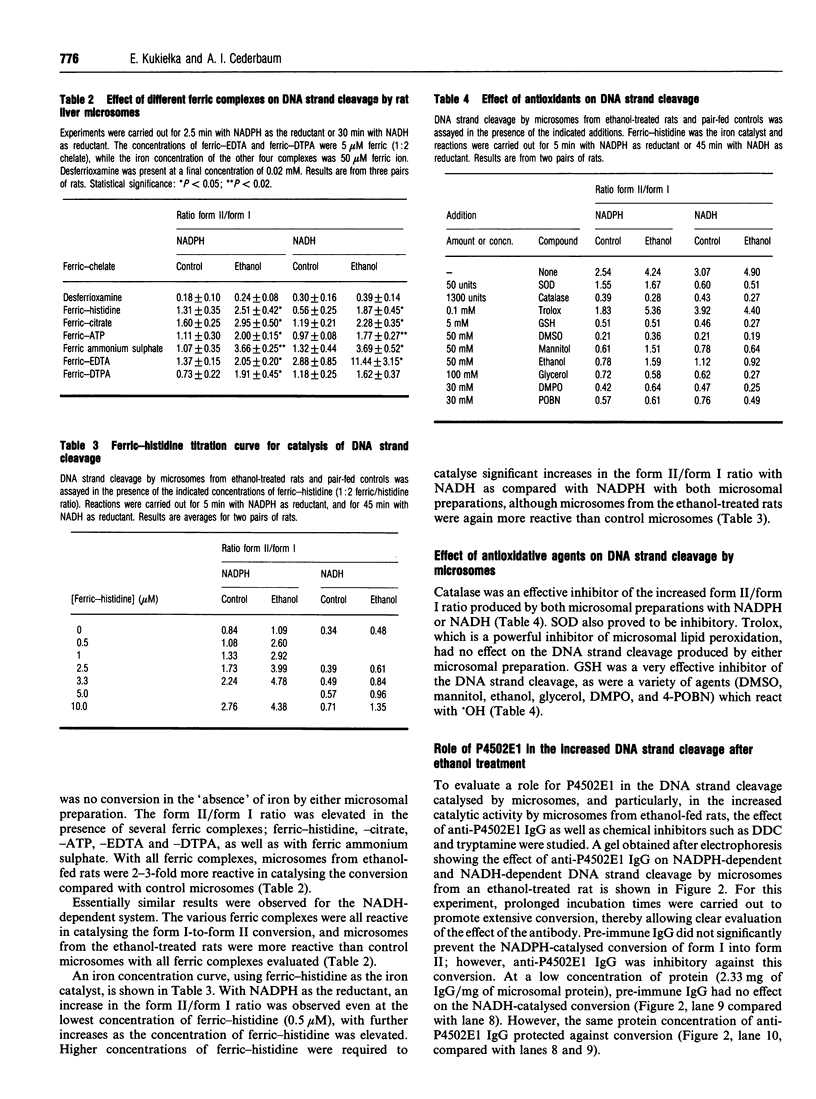
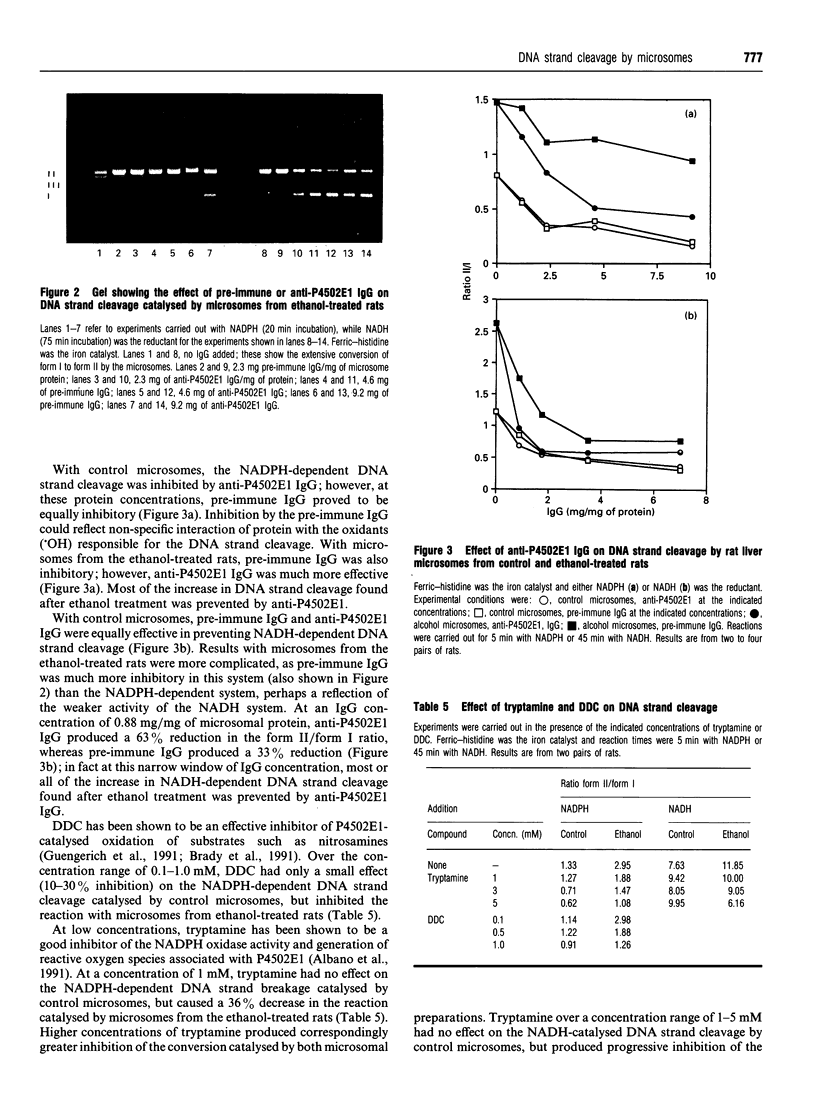


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albano E., Tomasi A., Persson J. O., Terelius Y., Goria-Gatti L., Ingelman-Sundberg M., Dianzani M. U. Role of ethanol-inducible cytochrome P450 (P450IIE1) in catalysing the free radical activation of aliphatic alcohols. Biochem Pharmacol. 1991 Jun 15;41(12):1895–1902. doi: 10.1016/0006-2952(91)90129-s. [DOI] [PubMed] [Google Scholar]
- Aruoma O. I., Evans P. J., Kaur H., Sutcliffe L., Halliwell B. An evaluation of the antioxidant and potential pro-oxidant properties of food additives and of trolox C, vitamin E and probucol. Free Radic Res Commun. 1990;10(3):143–157. doi: 10.3109/10715769009149883. [DOI] [PubMed] [Google Scholar]
- Aruoma O. I., Halliwell B., Dizdaroglu M. Iron ion-dependent modification of bases in DNA by the superoxide radical-generating system hypoxanthine/xanthine oxidase. J Biol Chem. 1989 Aug 5;264(22):13024–13028. [PubMed] [Google Scholar]
- Aust S. D., Morehouse L. A., Thomas C. E. Role of metals in oxygen radical reactions. J Free Radic Biol Med. 1985;1(1):3–25. doi: 10.1016/0748-5514(85)90025-x. [DOI] [PubMed] [Google Scholar]
- Berlin V., Haseltine W. A. Reduction of adriamycin to a semiquinone-free radical by NADPH cytochrome P-450 reductase produces DNA cleavage in a reaction mediated by molecular oxygen. J Biol Chem. 1981 May 25;256(10):4747–4756. [PubMed] [Google Scholar]
- Bode V. C. Single-strand scissions induced in circular and linear lambda DNA by the presence of dithiothreitol and other reducing agents. J Mol Biol. 1967 May 28;26(1):125–129. doi: 10.1016/0022-2836(67)90266-5. [DOI] [PubMed] [Google Scholar]
- Boveris A., Fraga C. G., Varsavsky A. I., Koch O. R. Increased chemiluminescence and superoxide production in the liver of chronically ethanol-treated rats. Arch Biochem Biophys. 1983 Dec;227(2):534–541. doi: 10.1016/0003-9861(83)90482-4. [DOI] [PubMed] [Google Scholar]
- Brady J. F., Xiao F., Wang M. H., Li Y., Ning S. M., Gapac J. M., Yang C. S. Effects of disulfiram on hepatic P450IIE1, other microsomal enzymes, and hepatotoxicity in rats. Toxicol Appl Pharmacol. 1991 Apr;108(2):366–373. doi: 10.1016/0041-008x(91)90125-x. [DOI] [PubMed] [Google Scholar]
- Brawn K., Fridovich I. DNA strand scission by enzymically generated oxygen radicals. Arch Biochem Biophys. 1981 Feb;206(2):414–419. doi: 10.1016/0003-9861(81)90108-9. [DOI] [PubMed] [Google Scholar]
- Burk R. F. Glutathione-dependent protection by rat liver microsomal protein against lipid peroxidation. Biochim Biophys Acta. 1983 May 4;757(1):21–28. doi: 10.1016/0304-4165(83)90148-4. [DOI] [PubMed] [Google Scholar]
- Castillo T., Koop D. R., Kamimura S., Triadafilopoulos G., Tsukamoto H. Role of cytochrome P-450 2E1 in ethanol-, carbon tetrachloride- and iron-dependent microsomal lipid peroxidation. Hepatology. 1992 Oct;16(4):992–996. doi: 10.1002/hep.1840160423. [DOI] [PubMed] [Google Scholar]
- Dicker E., Cederbaum A. I. Hydroxyl radical generation by microsomes after chronic ethanol consumption. Alcohol Clin Exp Res. 1987 Jun;11(3):309–314. doi: 10.1111/j.1530-0277.1987.tb01314.x. [DOI] [PubMed] [Google Scholar]
- Dicker E., Cederbaum A. I. Increased NADH-dependent production of reactive oxygen intermediates by microsomes after chronic ethanol consumption: comparisons with NADPH. Arch Biochem Biophys. 1992 Mar;293(2):274–280. doi: 10.1016/0003-9861(92)90395-d. [DOI] [PubMed] [Google Scholar]
- Ekström G., Cronholm T., Ingelman-Sundberg M. Hydroxyl-radical production and ethanol oxidation by liver microsomes isolated from ethanol-treated rats. Biochem J. 1986 Feb 1;233(3):755–761. doi: 10.1042/bj2330755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekström G., Ingelman-Sundberg M. Rat liver microsomal NADPH-supported oxidase activity and lipid peroxidation dependent on ethanol-inducible cytochrome P-450 (P-450IIE1). Biochem Pharmacol. 1989 Apr 15;38(8):1313–1319. doi: 10.1016/0006-2952(89)90338-9. [DOI] [PubMed] [Google Scholar]
- Gorsky L. D., Koop D. R., Coon M. J. On the stoichiometry of the oxidase and monooxygenase reactions catalyzed by liver microsomal cytochrome P-450. Products of oxygen reduction. J Biol Chem. 1984 Jun 10;259(11):6812–6817. [PubMed] [Google Scholar]
- Guengerich F. P., Kim D. H., Iwasaki M. Role of human cytochrome P-450 IIE1 in the oxidation of many low molecular weight cancer suspects. Chem Res Toxicol. 1991 Mar-Apr;4(2):168–179. doi: 10.1021/tx00020a008. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen free radicals and iron in relation to biology and medicine: some problems and concepts. Arch Biochem Biophys. 1986 May 1;246(2):501–514. doi: 10.1016/0003-9861(86)90305-x. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. The importance of free radicals and catalytic metal ions in human diseases. Mol Aspects Med. 1985;8(2):89–193. doi: 10.1016/0098-2997(85)90001-9. [DOI] [PubMed] [Google Scholar]
- Klein S. M., Cohen G., Lieber C. S., Cederbaum A. I. Increased microsomal oxidation of hydroxyl radical scavenging agents and ethanol after chronic consumption of ethanol. Arch Biochem Biophys. 1983 Jun;223(2):425–432. doi: 10.1016/0003-9861(83)90606-9. [DOI] [PubMed] [Google Scholar]
- Krebs H. A. The effects of ethanol on the metabolic activities of the liver. Adv Enzyme Regul. 1968;6:467–480. doi: 10.1016/0065-2571(68)90029-0. [DOI] [PubMed] [Google Scholar]
- Krikun G., Cederbaum A. I. Effect of chronic ethanol consumption on microsomal lipid peroxidation. Role of iron and comparison between controls. FEBS Lett. 1986 Nov 24;208(2):292–296. doi: 10.1016/0014-5793(86)81035-3. [DOI] [PubMed] [Google Scholar]
- Kukiełka E., Cederbaum A. I. NADH-dependent microsomal interaction with ferric complexes and production of reactive oxygen intermediates. Arch Biochem Biophys. 1989 Dec;275(2):540–550. doi: 10.1016/0003-9861(89)90400-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lieber C. S., DeCarli L. M. Reduced nicotinamide-adenine dinucleotide phosphate oxidase: activity enhanced by ethanol consumption. Science. 1970 Oct 2;170(3953):78–80. doi: 10.1126/science.170.3953.78. [DOI] [PubMed] [Google Scholar]
- Lieber C. S., DeCarli L. M. The feeding of alcohol in liquid diets: two decades of applications and 1982 update. Alcohol Clin Exp Res. 1982 Fall;6(4):523–531. doi: 10.1111/j.1530-0277.1982.tb05017.x. [DOI] [PubMed] [Google Scholar]
- Minotti G. tert-butyl hydroperoxide-dependent microsomal release of iron and lipid peroxidation. I. Evidence for the reductive release of nonheme, nonferritin iron. Arch Biochem Biophys. 1989 Aug 15;273(1):137–143. doi: 10.1016/0003-9861(89)90171-9. [DOI] [PubMed] [Google Scholar]
- Montgomery M. R., Clark C., Holtzman J. L. Iron species of hepatic microsomes from control and phenobarbital-treated rats. Arch Biochem Biophys. 1974 Jan;160(1):113–118. doi: 10.1016/s0003-9861(74)80015-9. [DOI] [PubMed] [Google Scholar]
- Muindi J., Sinha B. K., Gianni L., Myers C. Thiol-dependent DNA damage produced by anthracycline-iron complexes. The structure-activity relationships and molecular mechanisms. Mol Pharmacol. 1985 Mar;27(3):356–365. [PubMed] [Google Scholar]
- Myers C. E., Muindi J. R., Zweier J., Sinha B. K. 5-Iminodaunomycin. An anthracycline with unique properties. J Biol Chem. 1987 Aug 25;262(24):11571–11577. [PubMed] [Google Scholar]
- Palakodety R. B., Clejan L. A., Krikun G., Feierman D. E., Cederbaum A. I. Characterization and identification of a pyrazole-inducible form of cytochrome P-450. J Biol Chem. 1988 Jan 15;263(2):878–884. [PubMed] [Google Scholar]
- Puntarulo S., Cederbaum A. I. Comparison of the ability of ferric complexes to catalyze microsomal chemiluminescence, lipid peroxidation, and hydroxyl radical generation. Arch Biochem Biophys. 1988 Aug 1;264(2):482–491. doi: 10.1016/0003-9861(88)90313-x. [DOI] [PubMed] [Google Scholar]
- Puntarulo S., Cederbaum A. I. Increased NADPH-dependent chemiluminescence by microsomes after chronic ethanol consumption. Arch Biochem Biophys. 1988 Nov 1;266(2):435–445. doi: 10.1016/0003-9861(88)90275-5. [DOI] [PubMed] [Google Scholar]
- Rashba-Step J., Turro N. J., Cederbaum A. I. Increased NADPH- and NADH-dependent production of superoxide and hydroxyl radical by microsomes after chronic ethanol treatment. Arch Biochem Biophys. 1993 Jan;300(1):401–408. doi: 10.1006/abbi.1993.1054. [DOI] [PubMed] [Google Scholar]
- Rumyantseva G. V., Weiner L. M., Frolova E. I., Fedorova O. S. Hydroxyl radical generation and DNA strand scission mediated by natural anticancer and synthetic quinones. FEBS Lett. 1989 Jan 2;242(2):397–400. doi: 10.1016/0014-5793(89)80509-5. [DOI] [PubMed] [Google Scholar]
- Schneider J. E., Browning M. M., Floyd R. A. Ascorbate/iron mediation of hydroxyl free radical damage to PBR322 plasmid DNA. Free Radic Biol Med. 1988;5(5-6):287–295. doi: 10.1016/0891-5849(88)90099-8. [DOI] [PubMed] [Google Scholar]
- Schneider J. E., Browning M. M., Zhu X., Eneff K. L., Floyd R. A. Characterization of hydroxyl free radical mediated damage to plasmid pBR322 DNA. Mutat Res. 1989 Sep;214(1):23–31. doi: 10.1016/0027-5107(89)90194-2. [DOI] [PubMed] [Google Scholar]
- Sinha B. K., Eliot H. M., Kalayanaraman B. Iron-dependent hydroxyl radical formation and DNA damage from a novel metabolite of the clinically active antitumor drug VP-16. FEBS Lett. 1988 Jan 25;227(2):240–244. doi: 10.1016/0014-5793(88)80906-2. [DOI] [PubMed] [Google Scholar]
- Thurman R. G. Induction of hepatic microsomal reduced nicotinamide adenine dinucleotide phosphate-dependent production of hydrogen peroxide by chronic prior treatment with ethanol. Mol Pharmacol. 1973 Sep;9(5):670–675. [PubMed] [Google Scholar]
- Williamson J. R., Scholz R., Browning E. T., Thurman R. G., Fukami M. H. Metabolic effects of ethanol in perfused rat liver. J Biol Chem. 1969 Sep 25;244(18):5044–5054. [PubMed] [Google Scholar]
- Winston G. W., Feierman D. E., Cederbaum A. I. The role of iron chelates in hydroxyl radical production by rat liver microsomes, NADPH-cytochrome P-450 reductase and xanthine oxidase. Arch Biochem Biophys. 1984 Jul;232(1):378–390. doi: 10.1016/0003-9861(84)90553-8. [DOI] [PubMed] [Google Scholar]




