Abstract
Study design
Retrospective cohort study.
Introduction
Malpractice claims analysis is performed by several specialties to improve quality of patient care and to identify areas where physicians can improve their practice to mitigate the incidence of committing malpractice. The Food and Drug Administration has flagged over 80,000 injuries caused by spinal cord stimulator (SCS), making them the 3rd most flagged medical device. This study analyzed malpractice claims due to SCS by querying two legal databases widely used in medicolegal research.
Methods
Westlaw Edge and VerdictSearch were queried for malpractice cases filed between the years 2000 and 2022 using the keywords “spinal cord stimulator.” Case inclusion criteria was defined as a plaintiff's basis of litigation resting on a claim of medical malpractice due to SCS. Additional data collected included date of case hearing, plaintiff sex and age, defendant specialty, verdict ruling, location of the filed claim, payment or settlement amount, and sustained injuries.
Result
Of the 1773 reviewed cases, 45 cases were included and categorized as battery or implantable pulse generator malfunction (35.56 %), lead complications (28.89 %), surgical complications (20.00 %), and miscellaneous (15.56 %). Four (8.89 %) cases resulted in settlement, 11 (24.44 %) in a plaintiff verdict, and 30 (68.00 %) resulted in a defendant verdict. Claims filed due to infection related to SCS were more likely to result in a defendant verdict (p = .047), whereas claims filed due to neurological deficit were more likely to result in a plaintiff verdict (p = .020). The average settlement amount for the 4 cases is $1,975,309.61.
Conclusion
Our findings suggest obtaining adequate neuroimaging preoperatively with MRIs, disclosing neurological risks specifically paralysis on informed consent, and evaluating radiography intraoperative and postoperatively with anterior-posterior (AP) and lateral x-ray films to ensure proper SCS placement are practices that may mitigate malpractice due to SCS. Battery defects and lead complications were the most common grounds for SCS-related malpractice claims.
Keywords: Spinal cord stimulator, Dorsal column stimulator, Malpractice, Lawsuit, Westlaw, VerdictSearch
1. Introduction
Worldwide, there are nearly 34,000 patients who receive a spinal cord stimulator (SCS) implant annually, which is expected to represent a market size of $2.8 billion by the year 2025 [1,2]. As chronic pain issues continue to affect nearly 20 % of patients in the United States, the indications and outcomes of spinal cord stimulators must be scrutinized to guide proper treatment options [3]. Though SCS implants are considered safe, reversible outpatient procedures, recent reviews have reported a 30 %–40 % complication rate that includes hardware malfunction, infection, and increased pain [4]. Furthermore, the Food and Drug Administration has flagged over 80,000 injuries caused by SCS since 2008, making them the 3rd most flagged medical device [5].
Malpractice litigation costs the nation more than $55 billion annually [6]. Malpractice claims analyses are performed by several medical specialties to improve quality of patient care, to identify areas where physicians can improve their practice to mitigate the incidence of committing malpractice, and to better understand patients’ values regarding a certain practice or procedure [[7], [8], [9], [10], [11]]. As neurosurgery and orthopedic surgery represent the 1st and 4th most litigious specialties, respectively, the importance of analyzing malpractice claims due to SCS cannot be understated [12]. Of note, pain medicine specialist litigation rates were not included in the study. Given that pain medicine, neurosurgery, and orthopedic surgery are the most frequent utilizers of SCS, our study aimed to identify factors that result in legal disputes regarding SCS. To the authors knowledge, the literature has yet to evaluate malpractice claims due to SCS. Thus, the aim of our study was to analyze and compare malpractice lawsuits pertaining to SCS by querying Westlaw Edge and VerdictSearch, two well-established legal databases widely used in medicolegal research.
2. Materials and methods
2.1. Data source
Westlaw Edge (Thomson Reuters, Eagan, MN) and VerdictSearch (ALM Media Properties, LLC, New York, NY) were queried for medical malpractice cases filed between the years 2000 and 2022. VerdictSearch is a database of more than 250,000 claims evaluated in the United States of America federal as well as state court systems, while Westlaw is a consolidation of over 40,000 smaller legal databases. VerdictSearch includes cases from all categories of litigation with the exception of criminal law, while Westlaw is fully comprehensive in its catalog with domestic as well as international cases captured in its repository. However, Westlaw and VerdictSearch are not necessarily all-inclusive, and cases settled outside of the judicial system or without formal registration may not be included. Nonetheless, these databases are still considered to be leading commercial providers for legal research within the professional legal community and have been extensively used for legal research in several medical and surgical specialties including orthopaedics. While the majority of studies review cases from one database or the other, we opted to review both databases and exclude duplicate claims to ensure a more comprehensive analysis was achieved.
2.2. Data gathering
Querying Westlaw and VerdictSearch using the keywords “spinal cord stimulator” and “dorsal column stimulator” our search yielded 1383 and 390 results, respectively. Cases were reviewed and classified by four independent reviewers (SK, EW, KA, & DB) based on the grievance(s) levied by the plaintiff. Discrepancies between reviewers were resolved by a fifth reviewer (WC). Cases were then deemed for inclusion based on whether or not the grievance(s) was directly related to spinal cord stimulator. Inclusion criteria for case relevance were defined as a plaintiff's basis of litigation resting on a claim of medical malpractice due to spinal cord stimulator. Data collection was performed using Microsoft Excel version 16.58 (Microsoft Corporation, 2022, Redmond, WA, USA). Additional data collected included date of case hearing, plaintiff sex and age, defendant specialty, verdict ruling, location of the filed claim, payment or settlement amount, and sustained injuries.
2.3. Statistical analysis
SPSS version 28 (IBM Corporation, 2021, Armonk, NY, USA) was utilized for all statistical analyses with statistical significance defined as p < .05. Descriptive statistics utilized means and standard deviations (SD) for case and demographic data. To assess for homoscedasticity, we utilized homogeneity of variance tests and regression residual plots. We used Q-Q plots and Kolmogorov-Smirnov tests to assess for normality of data. To assess correlations among demographic and case data, Pearson's correlation tests were constructed. To assess differences based on the plaintiff's sex and age, we used independent sample t-tests with Levene's test for equality of variances. We used Pearson's Chi-squared test to identify differences for categorical variables. Case differences based on defendant specialty were analyzed using one-way analysis of variance (ANOVA) with Bonferroni and Tukey corrections.
3. Results
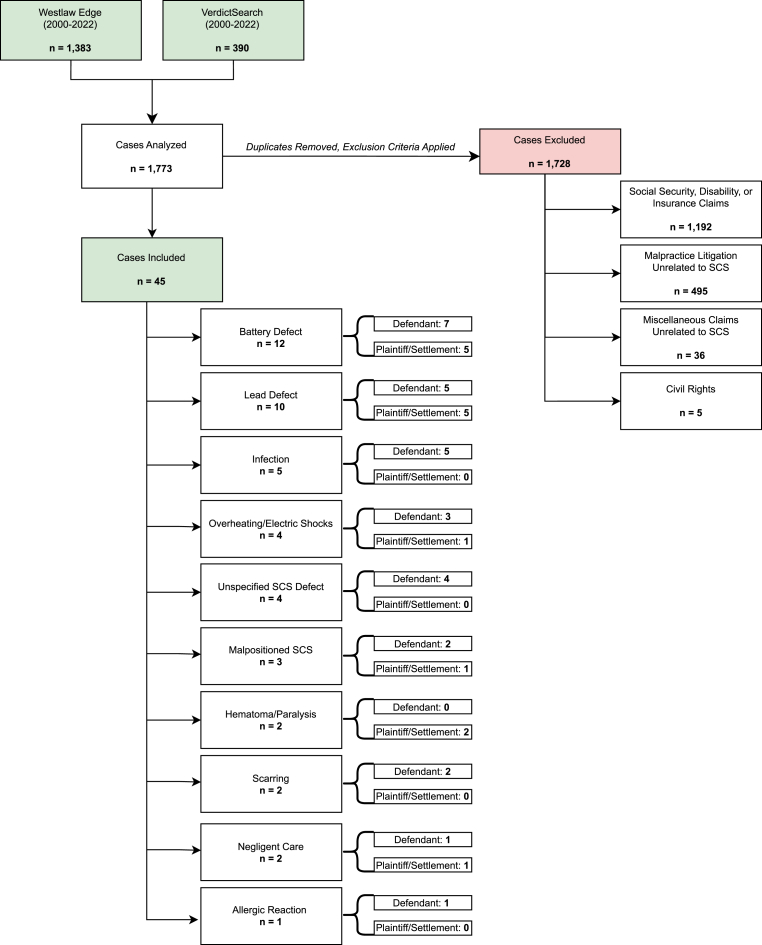
A total of 1773 cases were reviewed from the Westlaw and VerdictSearch databases. Of these, 1728 were excluded as their basis of litigation was not specifically due to SCS (see Fig. 1). Of the remaining 45 cases, 16 (35.56 %) were due to battery or implantable pulse generator (IPG) malfunction, 13 (28.89 %) were due to lead complications, 5 (11.11 %) were due to infection, 4 (8.89 %) were due to unspecified SCS defect, 3 (6.67 %) were due to malpositioned SCS, 2 (4.44 %) were due to hematoma and paralysis, 2 (4.44 %) were due to scarring, 2 (4.44 %) were due to negligence - one from improper preoperative planning and one unspecified, and 1 (2.22 %) was due to allergic reaction to SCS (see Table 1). Claims filed due to infection related to SCS were more likely to result in a defendant verdict (p = .047), whereas claims filed due to neurological deficit were more likely to result in a plaintiff verdict (p = .020) (see Table 2).
Fig. 1.
This flow sheet illustrates the inclusion and exclusion criteria for malpractice claims from Westlaw Edge and VerdictSearch databases.
Table 1.
Categorization of included cases per basis of litigation.
| Basis of Litigation (n = 45) | Category Description |
|---|---|
| Alleged SCS/IPG malfunction (16) Short battery life (8) Nonfunctional battery charging (2) Unspecified battery defect (2) Overheating (2) Electrical shocks (2) |
Basis of litigation was injury due to unforeseen SCS or IPG malfunction |
| Alleged lead complications (13) Improper lead attachment (4) Broken leads (3) Malpositioned SCS (3) Unspecified lead defect (3) |
Basis of litigation was injury due to alleged lead migration and complications |
| Alleged SCS surgical complication (9) Infection (5) Hematoma/paralysis (2) Scarring (2) |
Basis of litigation was injury due to SCS surgical complication |
| Miscellaneous/unknown (7) Unspecified SCS defect (4) Damage due to negligence (1) Improper pre-operative planning (1) Allergy (1) |
Basis of litigation was negative outcome due to implantation into stenotic spinal canal, negligent patient care, and patient allergy to SCS |
Table 1: This table outlines the basis of litigation for the included cases in this study. Alleged SCS/IPG malfunction, lead complications, surgical complications, and miscellaneous were the four categories the cases were divided into.
Table 2.
Case reasons per settlement, defendant, or plaintiff ruling.
| Reason for Malpractice Claim | Defendant Verdict n (%) | Plaintiff Verdict or Settlement n (%) | p |
|---|---|---|---|
| Scarring | 2 (%) | 0 (%) | .306 |
| No Scarring | 28 (%) | 15 (%) | |
| Overheating/electric shocks | 3 (%) | 1 (%) | .711 |
| No overheating/electric shocks | 27 (%) | 14 (%) | |
| Nerve damage/paralysis | 1 (%) | 1 (%) | .609 |
| No nerve damage/paralysis | 29 (%) | 14 (%) | |
| Malpositioned SCS | 2 (%) | 1 (%) | 1.000 |
| No malpositioned SCS | 28 (%) | 14 (%) | |
| Lead defect | 5 (11.1 %) | 5 (11.1 %) | .205 |
| No lead defect | 25 (55.6 %) | 10 (22.2 %) | |
| Infection | 5 (%) | 0 (%) | .047 |
| No infection | 25 (%) | 15 (%) | |
| Hematoma/Paralysis | 0 (0 %) | 2 (4.4 %) | .020 |
| No hematoma/paralysis | 30 (%) | 13 (%) | |
| Battery Defect | 7 (%) | 5 (%) | .237 |
| No battery defect | 23 (%) | 10 (%) | |
| Allergic Reaction | 1 (%) | 0 (%) | .313 |
| No allergic reaction | 29 (%) | 15 (%) | |
| Unspecified Problem | 4 (%) | 0 (%) | .138 |
| No Unspecified Problem | 26 (%) | 15 (%) |
Table 2: Claims filed due to infection related to SCS were more likely to result in a defendant verdict (p = .047), whereas claims filed due to neurological deficit were more likely to result in a plaintiff verdict (p = .020).
Four (8.89 %) cases resulted in settlement, 11 (24.44 %) a plaintiff verdict, and 30 (68.00 %) resulted in a defendant verdict. Fifteen (33.33 %) cases involved a pain management specialist, 10 (22.00 %) involved a neurosurgeon, 2 (4.44 %) involved an orthopedic surgeon, and 18 (40.00 %) involved an unspecified physician. Among pain specialists, 6 cases resulted from battery defects, 4 from lead defects, 1 from infection, from scarring, 1 from neurological deficits, and 2 unspecified grievances. Among neurosurgery, 2 cases arose from battery defects, 2 from malpositioning of SCS, 2 from lead defects, 2 from hematoma, and 2 from infection. Among orthopedics, 1 case arose from allegations of scarring and 1 from neurological deficits. 29 (64.44 %) of cases were filed against the SCS manufacturer and 16 (35.56 %) were filed against the physician. Of the 29 cases filed against the SCS manufacturer, 20 (68.97 %) resulted in a defendant verdict and 9 (31.03 %) resulted in a plaintiff or settlement verdict. Of the 16 cases filed against the physician, 10 (62.50 %) cases resulted in a defendant verdict and 6 (37.5 %) resulted in a plaintiff or settlement verdict. Case outcomes (settlement, plaintiff, or defendant ruling) did not statistically vary based on whether or not the physician was a pain management specialist or surgeon (p = .814). The average settlement amount for the 4 cases is $1,975,309.61 (see Table 3).
Table 3.
Description of malpractice cases due to SCS.
| Number of Cases per Outcome | ||
|---|---|---|
| Outcome | n | n% |
| Defendant | 30 | 66.67 % |
| Plaintiff | 10 | 22.22 % |
| Settlement | 5 | 11.11 % |
| Number of Cases per Specialty of Practitioner | ||
|---|---|---|
| Overall Cases per Specialty | ||
| Specialty | n | n% |
| Neurosurgeon | 10 | 22.00 % |
| Orthopedic Surgeon | 2 | 4.44 % |
| Pain management | 15 | 33.33 % |
| Unspecified | 18 | 40.00 % |
| Neurosurgeon (n = 10) | ||
|---|---|---|
| Outcome | n | n% |
| Defendant verdict | 6 | 60.00 % |
| Settlement | 3 | 30.00 % |
| $4,500,000 | ||
| $1,550,000 | ||
| $800,000 | ||
| Plaintiff verdict | 1 | 10.00 % |
| Orthopedic Surgeon (n = 2) | ||
| Defendant verdict | 1 | 50.00 % |
| Settlement | 1 | 50.00 % |
| $750,000 | ||
| Pain Management (n = 15) | ||
| Defendant verdict | 13 | 86.67 % |
| Plaintiff verdict | 2 | 13.33 % |
| Unspecified (n = 18) | ||
|---|---|---|
| Outcome | n | n% |
| Defendant verdict | 10 | 55.56 % |
| Plaintiff verdict | 7 | 38.89 % |
| Settlement | 1 | 5.55 % |
| $301,238.45 | ||
Table 3: Discusses overall outcomes (defendant/plaintiff/settlement verdict with the physician as defendant), followed by outcomes of the lawsuits stratified by specialties involved as defendants. The average settlement amount for the 4 cases is $1,975,309.61.
Regarding battery issues, battery type and life were specified in 6 and 3 cases respectively, and the remaining cases did not specify either. Among these 6 cases, there were 5 rechargeable batteries and 1 non-rechargeable. For the rechargeable batteries, the 3 of the devices were from St. Jude, 1 was from Medtronics, and 1 was unspecified. For the one cited non-rechargeable battery, the manufacturer was Medtronics. In the three rechargeable battery cases with a mentioned battery lifespan, the average life span was about 8 months. Of these, the only case that resulted in a plaintiff verdict also described “painful and powerful shock” associated with the battery.
Regarding lead defects in these cases, there was one instance where a defective lead led to revision surgery which resulted in an epidural hematoma that caused paraplegia for the patient below T6. The case did not specify where the lead was placed. After the initial SCS trial period, no x-ray was taken to determine if lead migration accounted for the absent pain coverage to his left leg, and the patient was consequently given an $800,000 settlement. Another settlement of $301,238.45 was awarded to a patient whose SCS became infected after the leads punctured the plaintiff's skin nine days after the implantation stage and were subsequently reinserted after being exposed to air. No further description of the mechanism of injury was provided in the legal documents.
Regarding hematoma formation, the first of these cases involved a spinal canal hematoma at the base of the lumbar spine that formed after the removal of a failed SCS, resulting in complete paralysis below the waist for the plaintiff. The lead type and description about whether this was the trial or implantation stage was not given. Another case details a spinal cord hematoma that was not identified despite the plaintiff reporting he did not have weight-bearing capabilities immediately following the surgery. Even after a successful trial stage, this patient required 2 surgeries for the implantation phase due to misplaced SCS placement during the first procedure. Although the patient expressed their inability to ambulate, the neurosurgeon's note detailed the patient was able to bear weight and he was discharged with a missed hematoma. The two plaintiffs were given settlement amounts of $4,500,000 and $1,550,000 respectively.
Regarding cases that cited “negligence”, lack of preoperative imaging in one patient led to incomplete paralysis at the thoracic level and below after the implantation of a paddle lead SCC. The surgeon failed to conduct CT myelogram and/or MRI at the thoracic level to diagnose a preexisting thoracic spinal stenosis. Despite resistance during initial placement, the surgeon continued with the operation. Specifically, the surgeon failed to ensure appropriate fit of the implant prior to SCC placement. The location of the SCS implant, information about whether this was the trial or implantation stage, and the method used to ultimately diagnose the thoracic stenosis was not discussed. This patient developed paralysis and was awarded $750,000.
Six (13.33 %) cases were filed in Georgia, 4 (8.89 %) in Delaware, 4 (8.89 %) in Louisiana, 4 (8.89 %) in Ohio, 4 (8.89 %) in Texas, 3 (6.67 %) in California, 3 (6.67 %) in Florida, 3 (6.67 %) in Indiana, 2 (4.44 %) in Michigan, 2 (4.44 %) in Mississippi, 2 (4.44 %) in New York, 2 (4.44 %) in Oklahoma, 2 (4.44 %) in Virginia, 1 (2.22 %) in Illinois, 1 (2.22 %) in Oregon, 1 (2.22 %) in Pennsylvania, and 1 (2.22 %) in Utah. Due to insufficient power for subgroup analysis based on state of filed claim, we did not analyze case outcomes based on state location.
Case outcomes (settlement, plaintiff or defendant ruling) did not statistically vary based on the SCS manufacturer type—St. Jude, Boston Scientific Corp., or Medtronic (see Table 4).
Table 4.
Manufacturer outcomes per settlement, defendant, or plaintiff ruling.
| Manufacturer | Defendant Verdict n (%) | Plaintiff Verdict or Settlement n (%) | p |
|---|---|---|---|
| St. Jude | 9 (81.8 %) | 2 (18.2 %) | .288 |
| Medtronic or Boston Scientific Corp. | 21 (61.8 %) | 13 (38.2 %) | |
| Medtronic | 7 (63.6 %) | 4 (36.4 %) | 1.000 |
| St. Jude or Boston Scientific Corp. | 23 (67.6 %) | 11 (32.4 %) | |
| Boston Scientific Corp. | 5 (55.6 %) | 4 (44.4 %) | .454 |
| Medtronic or St. Jude | 25 (69.4 %) | 11 (30.6 %) |
Table 4: Compares the likelihood of defendant vs. plaintiff/settlement verdicts for all SCS related causes of litigation against the three manufacturers seen in this paper: St. Jude, Medtronic, and Boston Scientific Corp. The P value describes whether one manufacturer had statistically significant different defendant/plaintiff/settlement versus the other two manufacturers. Case outcomes (settlement, plaintiff or defendant ruling) did not statistically vary based on the SCS manufacturer type—St. Jude, Boston Scientific Corp., or Medtronic.
4. Discussion
This study analyzed the reasons for malpractice claims filed due to SCS. Claims of battery defects were the most frequent basis for litigation (26.7 %), of which 66.7 % were specifically due to a short battery life. As non-rechargeable pulse generators typically have a battery life of 2–5 years, patients filed lawsuits when their device required multiple surgeries within 1 year [13]. Regarding battery manufacturers, we did not find a difference in the case outcome based on whether St. Jude, Medtronic, or Boston Scientific Corp. was the battery manufacturer. 41.6 % of patients with battery defects encountered painful electrical shocks originating from the site of the SCS implant. The FDA published a report in 2008 describing increased incidence of inadequate pain control and battery charging problems as the two most common patient and device reported problems respectively and urged care providers to trial stimulation for 3–7 days looking for 50 % pain reduction [5].
A previous study found that lead migration was the most cited complication for SCS (23 %) [14]. In our study, alleged lead defects incited litigation in 22.2 % of cases. Although we found no significant difference in incidence between paddle and percutaneous lead migration, studies have documented that percutaneous leads tend to have increased migration rates [15]. As SCS lead defects represent 50 % of the cases with settlement or plaintiff verdict, it is clear that informing patients of lead risks preoperatively is crucial to avoid litigation.
The SCS trial also serves as an important checkpoint for monitoring leads, as obtaining X-ray data intraoperatively and post-trial can check for lead migration and optimal lead placement [16]. Osborne et al. prospective study demonstrated that in addition to intraoperative X-ray imaging, imaging at the end of the trial period can detect lead migration due to patient's transferring after surgery and resumption of daily activity. Thus, post-operative imaging can help with adjusting lead placement for the implantation stage.
In addition to postoperative imaging, our review of these cases identify the lack of preoperative imaging as a potential cause for litigation. It is necessary that physicians follow the Neurostimulation Appropriateness Consensus Committee's recommendations for preoperative MRI or CT to assess for spinal anatomy abnormalities that may increase risk for serious neurologic injury [17]. A recent retrospective study found that preoperative MRIs affected SCS placement and management in 22 % of 160 cases [18].
While the average settlement amount for our cases was $1,975,309.61, the settlement amounts described in literature vary greatly based on physician specialty and surgical procedure. However, to contextualize the settlements of this study, recent reviews of orthopedic surgery and neurosurgery malpractice claims described in the Westlaw legal database the mean settlement value was $1,570,833 and $1,300,000 respectively [19,20]. No data reviewing the average malpractice settlements amount for pain medicine specialists was found in Westlaw.
Although improved surgical techniques and preventative strategies have lowered the SCS infection rate to 3.1 %, infections continue to be a common reason for SCS malpractice claims (11.11 % of claims) [21]. However, our findings demonstrate malpractice claims filed on the grounds of an infection due to SCS are more likely to result in a defendant ruling.
Contrastingly, this study found that cases filed on grounds of SCS resulting in hematoma or paralysis were more likely to result in a settlement ruling. When comparing SCS to non-SCS laminectomies, previous literature has found a 300 % higher risk of SCS-associated hematoma formation despite the smaller nature of the procedure [22]. In our study, hematomas were the basis of litigation for 2 cases, both of which resulted in settlement. These cases demonstrate the importance of obtaining a thorough patient consent disclosing procedural risks and complications.
4.1. Limitations
This study is not without several limitations. While we used two of the leading commercial providers of legal research both within the professional legal as well as medical communities, neither repository captures all filed malpractice claims within the legal system. It is estimated that 72 % of malpractice claims are dropped, denied, or dismissed prior to trial or settlement [23]. Correspondingly, it is possible that some malpractice claims due to SCS were not accessible for analysis in this study as they were not part of formal judicial registration. As a result, we by no means intend to make claims that our study was entirely comprehensive of all malpractice claims due to SCS. Rather, the cases reviewed in our study likely represent only a sampling of all malpractice claims due to SCS. Another limitation of our study is that not all court documents possessed detailed descriptions of the patient medical history. The granularity of detail and medical jargon varied on an unstandardized, case-by-case basis. Furthermore, this study is limited due to its subjective nature, whereby the categorization of the cases reviewed was performed in a qualitative manner, with reviewers classifying cases based on the perceived interpretation of case details. While this subjectivity was, in part, accounted for by the use of multiple independent reviewers, there may be some degree of inherent variation in classification that stems from the study methodology.
5. Conclusion
Our findings suggest obtaining adequate neuroimaging preoperatively with MRIs, disclosing neurological risks specifically paralysis on informed consent, and evaluating radiography intraoperatively as well as postoperatively with anterior-posterior (AP) and lateral x-ray films to ensure proper SCS placement are practices that may mitigate malpractice due to SCS. Battery defects and lead complications were the most common grounds for SCS-related litigation. Case outcomes did not statistically vary based on whether or not the physician was a pain management specialist or surgeon. Claims due to SCS infection were more likely to result in a defendant verdict, whereas claims filed due to hematoma and resultant paralysis were more likely to result in a plaintiff verdict.
Sources of support
The authors declaration no financial support for the work of this study. The authors have no conflicts of interest to disclose.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
IRB approval was not needed, as only anonymized data was utilized in performing outcomes analysis. As such, identifiable subjects are not involved in this study and the study was conducted in keeping with established ethical considerations in the study of human subjects.
References
- 1.Spinal cord stimulation. 2019. https://www.neuromodulation.com/spinal-cord-stimulation [Google Scholar]
- 2.Wood L. Research and markets: spinal implants and spinal devices market. Global Forecast to 2020: Thoracic, Lumbar, Cervical Fusion, Biologics, Motion Preservation, VCFs, Bone Stimulator. 2015 https://www.businesswire.com/news/home/20151002005230/en/Research-and-Markets-Spinal-Implants-and-Spinal-Devices-Market---Global-Forecast-to-2020-Thoracic-Lumbar-Cervical-Fusion-Biologics-Motion-Preservation-VCFs-Bone-Stimulator; [Google Scholar]
- 3.Dahlhamer J. Prevalence of chronic pain and high-impact chronic pain among adults — United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67 doi: 10.15585/mmwr.mm6736a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeon Y.H. Spinal cord stimulation in pain management: a review. Korean J Pain. 2012;25(3):143–150. doi: 10.3344/kjp.2012.25.3.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Health C for D and R . FDA; 2020. Conduct a trial stimulation period before implanting a spinal cord stimulator (SCS) - letter to health care providers.https://www.fda.gov/medical-devices/letters-health-care-providers/conduct-trial-stimulation-period-implanting-spinal-cord-stimulator-scs-letter-health-care-providers [Google Scholar]
- 6.Mello M.M., Chandra A., Gawande A.A., Studdert D.M. National costs of the medical liability system. Health Aff. 2010;29(9):1569. doi: 10.1377/hlthaff.2009.0807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arif H., Razzouk J., Bohen D., et al. Analysis of the reasons for medical malpractice litigation due to facet injections. Cureus. 2023;15(2) doi: 10.7759/cureus.35015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bouterse A., Razzouk J., Bohen D., Ramos O., Danisa O., Cheng W.K. Analysis of reasons for medical malpractice litigation due to laminectomy. J Neurosurg Spine. 2023;3:1–10. doi: 10.3171/2023.1.SPINE221148. Published online February. [DOI] [PubMed] [Google Scholar]
- 9.Weldon E., Razzouk J., Bohen D., Ramos O., Danisa O., Cheng W. Medical malpractice litigation due to off-label use of bone morphogenetic protein. Spine. December 28, 2022 doi: 10.1097/BRS.0000000000004563. Published online. [DOI] [PubMed] [Google Scholar]
- 10.Prabhu A.V., Quang T.S., Funahashi R., et al. A national WestlawNext database analysis of malpractice litigation in radiation oncology. Fed Pract. 2018;35(Suppl 1):S44–S52. [PMC free article] [PubMed] [Google Scholar]
- 11.Palaniappan A., Sellke F. An analysis of medical malpractice litigations in coronary artery bypass grafting from 1994-2019. Ann Thorac Surg. 2022;113(2):600–607. doi: 10.1016/j.athoracsur.2021.03.042. [DOI] [PubMed] [Google Scholar]
- 12.Schaffer A.C., Jena A.B., Seabury S.A., Singh H., Chalasani V., Kachalia A. Rates and characteristics of paid malpractice claims among US physicians by specialty, 1992-2014. JAMA Intern Med. 2017;177(5):710–719. doi: 10.1001/jamainternmed.2017.0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hornberger J., Kumar K., Verhulst E., Clark M.A., Hernandez J. Rechargeable spinal cord stimulation versus non-rechargeable system for patients with failed back surgery syndrome: a cost-consequences analysis. Clin J Pain. 2008;24(3):244–252. doi: 10.1097/AJP.0b013e318160216a. [DOI] [PubMed] [Google Scholar]
- 14.Cameron T. Safety and efficacy of spinal cord stimulation for the treatment of chronic pain: a 20-year literature review. J Neurosurg Spine. 2004;100(3):254–267. doi: 10.3171/spi.2004.100.3.0254. [DOI] [PubMed] [Google Scholar]
- 15.Dombovy-Johnson M.L., D'Souza R.S., Ha C.T., Hagedorn J.M. Incidence and risk factors for spinal cord stimulator lead migration with or without loss of efficacy: a retrospective review of 91 consecutive thoracic lead implants. Neuromodulation: Technology at the Neural Interface. 2022;25(5):731–737. doi: 10.1111/ner.13487. [DOI] [PubMed] [Google Scholar]
- 16.Osborne M.D., Ghazi S.M., Palmer S.C., Boone K.M., Sletten C.D., Nottmeier E.W. Spinal cord stimulator—trial lead migration study. Pain Med. 2011;12(2):204–208. doi: 10.1111/j.1526-4637.2010.01019.x. [DOI] [PubMed] [Google Scholar]
- 17.Deer T.R., Lamer T.J., Pope J.E., Falowski S.M., Provenzano D.A., Slavin K., Golovac S., Arle J., Rosenow J.M., Williams K., McRoberts P., Narouze S., Eldabe S., Lad S.P., De Andrés J.A., Buchser E., Rigoard P., Levy R.M., Simpson B., Mekhail N. The neurostimulation appropriateness Consensus committee (NACC) safety guidelines for the reduction of severe neurological injury. Neuromodulation : journal of the International Neuromodulation Society. 2017;20(1):15–30. doi: 10.1111/ner.12564. [DOI] [PubMed] [Google Scholar]
- 18.Best B.J., Porwal M.H., Pahapill P.A. Preoperative thoracic spine magnetic resonance imaging for spinal cord stimulation: should such a recommendation Be an absolute requirement? Neuromodulation. 2022;25(5):758–762. doi: 10.1111/ner.13518. [DOI] [PubMed] [Google Scholar]
- 19.Rynecki N.D., Coban D., Gantz O., Gupta R., Ayyaswami V., Prabhu A.V., Ruskin J., Lin S.S., Beebe K.S. Medical malpractice in orthopedic surgery: a westlaw-based demographic analysis. Orthopedics. 2018;41(5):e615–e620. doi: 10.3928/01477447-20180621-06. [DOI] [PubMed] [Google Scholar]
- 20.Thomas R., Gupta R., Griessenauer C.J., Moore J.M., Adeeb N., Motiei-Langroudi R., Guidal B., Agarwal N., Alterman R.L., Friedlander R.M., Ogilvy C.S., Thomas A.J. Medical malpractice in neurosurgery: a comprehensive analysis. World neurosurgery. 2018;110:e552–e559. doi: 10.1016/j.wneu.2017.11.051. [DOI] [PubMed] [Google Scholar]
- 21.Esquer Garrigos Z., Farid S., Bendel M.A., Sohail M.R. Spinal cord stimulator infection: approach to diagnosis, management, and prevention. Clin Infect Dis. 2020;70(12):2727–2735. doi: 10.1093/cid/ciz994. [DOI] [PubMed] [Google Scholar]
- 22.Moufarrij N.A. Epidural hematomas after the implantation of thoracic paddle spinal cord stimulators. J Neurosurg. 2016;125(4):982–985. doi: 10.3171/2015.8.JNS15396. [DOI] [PubMed] [Google Scholar]
- 23.DePasse J.M., Ruttiman R., Eltorai A.E.M., Palumbo M.A., Daniels A.H. Assessment of malpractice claims due to spinal epidural abscess. J Neurosurg Spine. 2017;27(4):476–480. doi: 10.3171/2016.12.SPINE16814. [DOI] [PubMed] [Google Scholar]