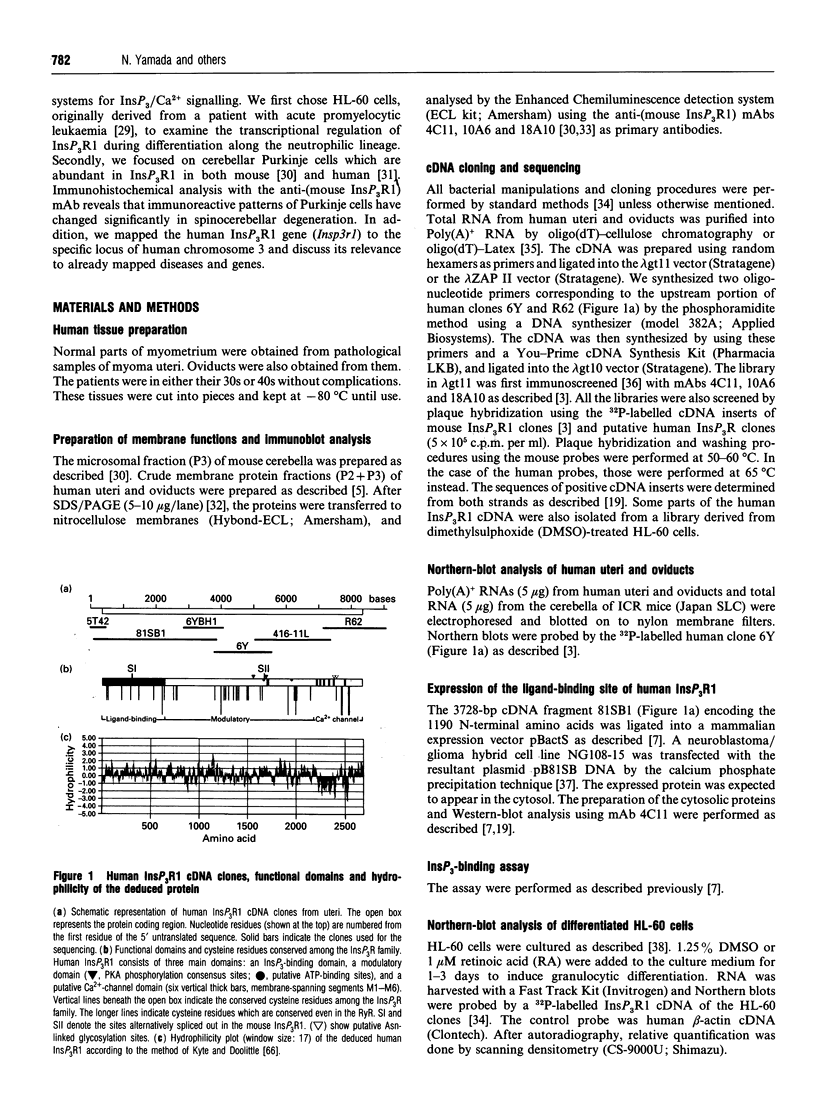
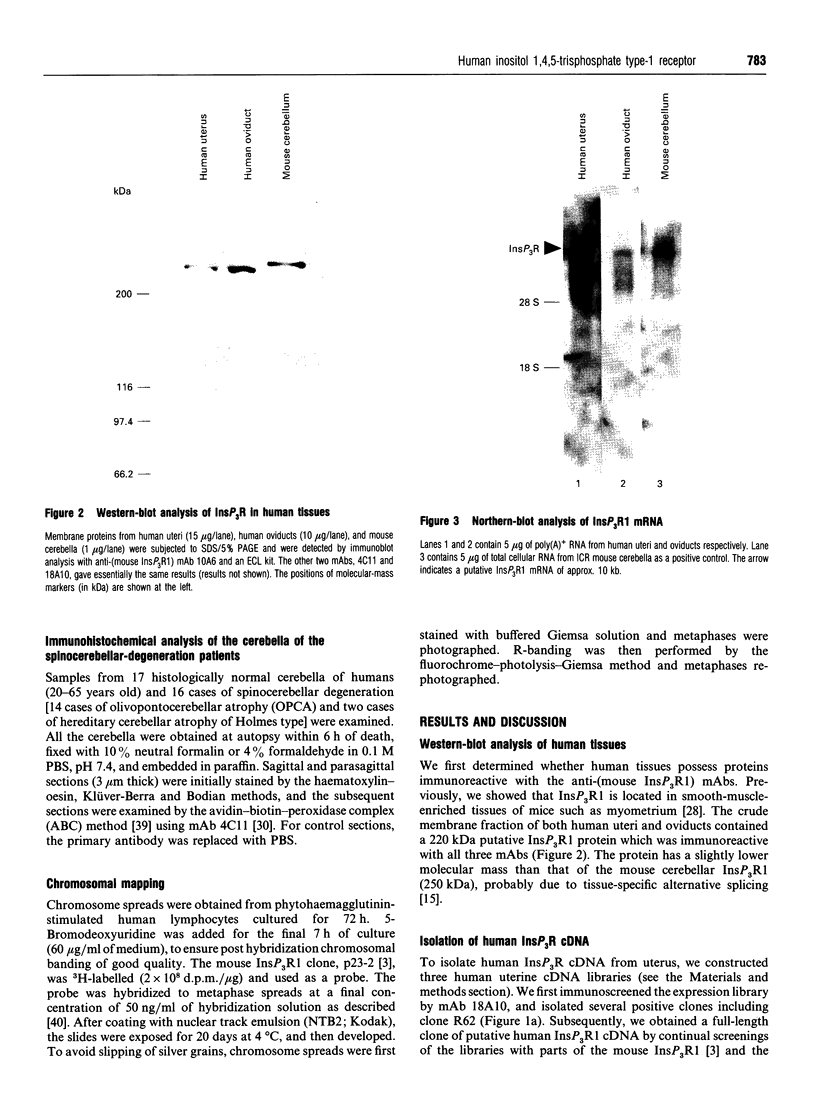
Abstract
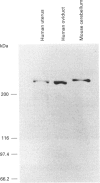
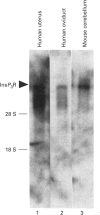
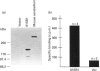
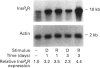
We have isolated cDNA clones encoding an inositol 1,4,5-trisphosphate receptor type 1 (InsP3R1) from human uteri and a leukaemic cell line, HL-60. Northern-blot analysis showed that approx. 10 kb of InsP3R1 mRNA is expressed in human uteri, oviducts and HL-60 cells. The predicted amino acid sequence of human InsP3R1 (2695 amino acids) has 99% identity with that of the mouse SI-/SII- splicing counterpart. Western-blot analysis with anti-(mouse InsP3R1) antibodies showed that InsP3R1 protein of human uteri and oviducts of approx 220 kDa is immunostained. Northern-blot analysis of HL-60 cell differentiation along the neutrophilic lineage induced by retinoic acid or dimethylsulphoxide showed an accompanying enhanced expression of InsP3R1 mRNA. Immunohistochemical analysis of the cerebella of spinocerebellar degeneration patients showed a variable loss of Purkinje cells with an altered pattern of immunostaining. The InsP3R1 gene (Insp3r1) was localized to the 3P25-26 region of human chromosome 3. The data presented here clearly show that InsP3R1 exists widely in human tissues and may play critical roles in various kinds of cellular functions.
Full text
PDF



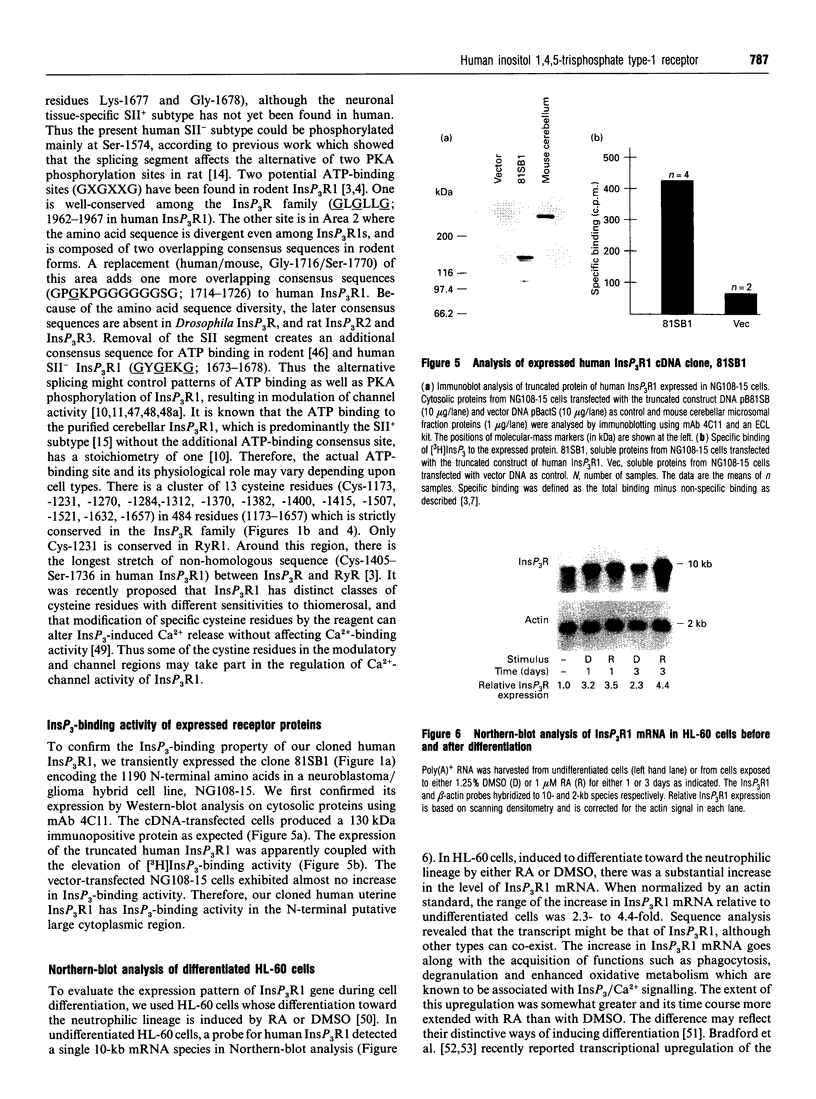
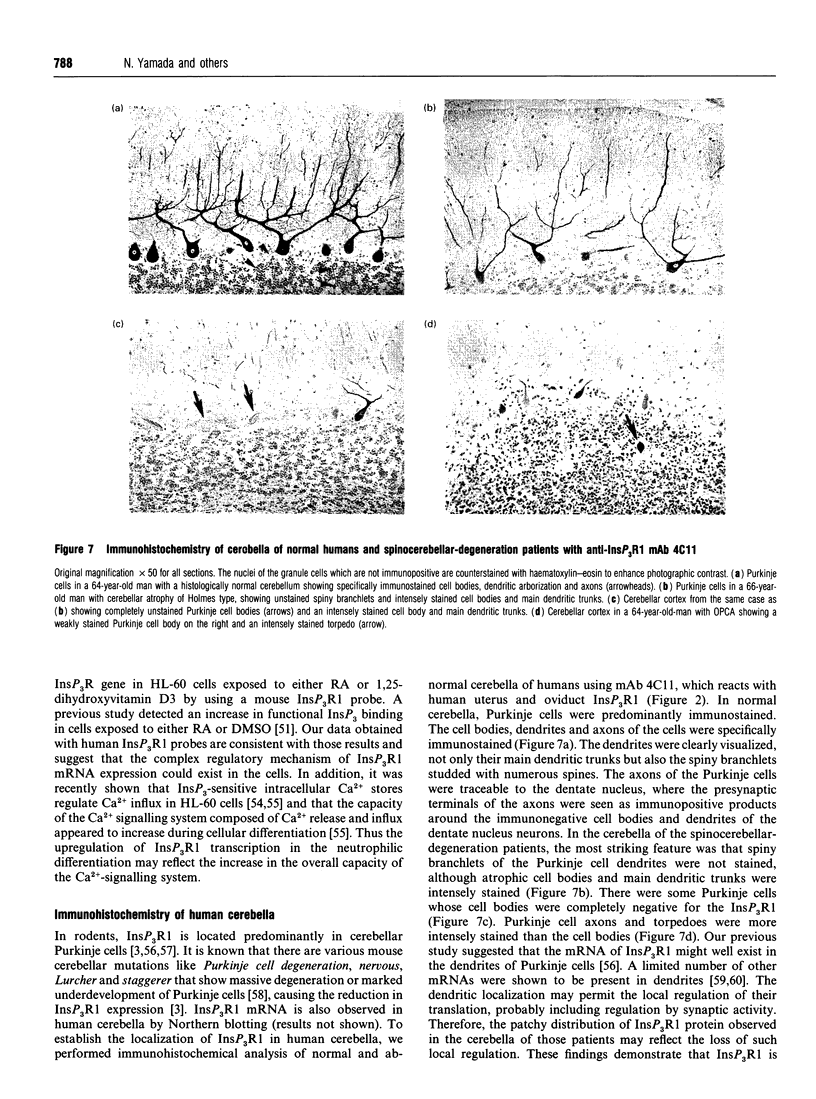
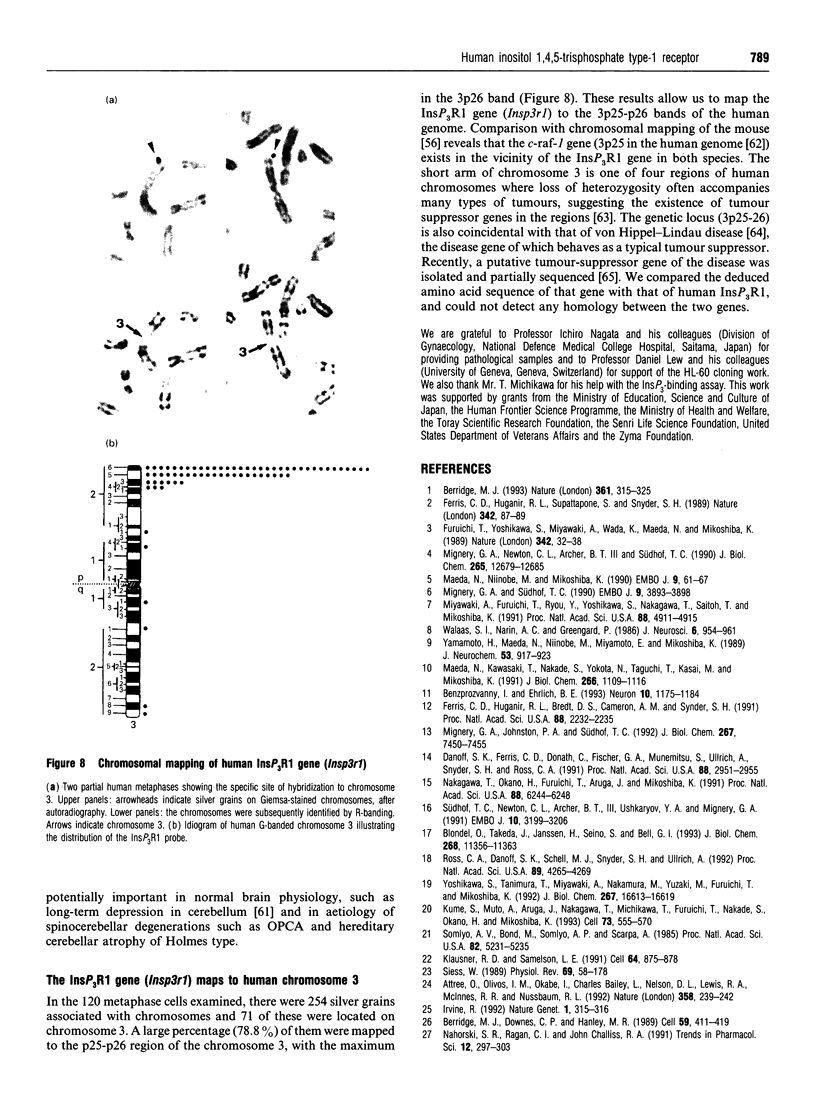

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alonso-Torre S. R., Alvarez J., Montero M., Sanchez A., García-Sancho J. Control of Ca2+ entry into HL60 and U937 human leukaemia cells by the filling state of the intracellular Ca2+ stores. Biochem J. 1993 Feb 1;289(Pt 3):761–766. doi: 10.1042/bj2890761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attree O., Olivos I. M., Okabe I., Bailey L. C., Nelson D. L., Lewis R. A., McInnes R. R., Nussbaum R. L. The Lowe's oculocerebrorenal syndrome gene encodes a protein highly homologous to inositol polyphosphate-5-phosphatase. Nature. 1992 Jul 16;358(6383):239–242. doi: 10.1038/358239a0. [DOI] [PubMed] [Google Scholar]
- Bachvarova R. F. A maternal tail of poly(A): the long and the short of it. Cell. 1992 Jun 12;69(6):895–897. doi: 10.1016/0092-8674(92)90606-d. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Downes C. P., Hanley M. R. Neural and developmental actions of lithium: a unifying hypothesis. Cell. 1989 Nov 3;59(3):411–419. doi: 10.1016/0092-8674(89)90026-3. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and calcium signalling. Nature. 1993 Jan 28;361(6410):315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I., Ehrlich B. E. ATP modulates the function of inositol 1,4,5-trisphosphate-gated channels at two sites. Neuron. 1993 Jun;10(6):1175–1184. doi: 10.1016/0896-6273(93)90065-y. [DOI] [PubMed] [Google Scholar]
- Blondel O., Takeda J., Janssen H., Seino S., Bell G. I. Sequence and functional characterization of a third inositol trisphosphate receptor subtype, IP3R-3, expressed in pancreatic islets, kidney, gastrointestinal tract, and other tissues. J Biol Chem. 1993 May 25;268(15):11356–11363. [PubMed] [Google Scholar]
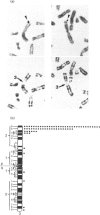
- Bonner T., O'Brien S. J., Nash W. G., Rapp U. R., Morton C. C., Leder P. The human homologs of the raf (mil) oncogene are located on human chromosomes 3 and 4. Science. 1984 Jan 6;223(4631):71–74. doi: 10.1126/science.6691137. [DOI] [PubMed] [Google Scholar]
- Bradford P. G., Autieri M. Increased expression of the inositol 1,4,5-trisphosphate receptor in human leukaemic (HL-60) cells differentiated with retinoic acid or dimethyl sulphoxide. Biochem J. 1991 Nov 15;280(Pt 1):205–210. doi: 10.1042/bj2800205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford P. G., Jin Y., Hui P. 1,25-dihydroxyvitamin D3 enhances the transcription and expression of the inositol trisphosphate receptor gene in HL-60 cells. Mol Pharmacol. 1993 Aug;44(2):292–297. [PubMed] [Google Scholar]
- Bradford P. G., Wang X., Jin Y., Hui P. Transcriptional regulation and increased functional expression of the inositol trisphosphate receptor in retinoic acid-treated HL-60 cells. J Biol Chem. 1992 Oct 15;267(29):20959–20964. [PubMed] [Google Scholar]
- Bruckenstein D. A., Lein P. J., Higgins D., Fremeau R. T., Jr Distinct spatial localization of specific mRNAs in cultured sympathetic neurons. Neuron. 1990 Dec;5(6):809–819. doi: 10.1016/0896-6273(90)90340-l. [DOI] [PubMed] [Google Scholar]
- Collins S. J., Gallo R. C., Gallagher R. E. Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature. 1977 Nov 24;270(5635):347–349. doi: 10.1038/270347a0. [DOI] [PubMed] [Google Scholar]
- Collins S. J. The HL-60 promyelocytic leukemia cell line: proliferation, differentiation, and cellular oncogene expression. Blood. 1987 Nov;70(5):1233–1244. [PubMed] [Google Scholar]
- Danoff S. K., Ferris C. D., Donath C., Fischer G. A., Munemitsu S., Ullrich A., Snyder S. H., Ross C. A. Inositol 1,4,5-trisphosphate receptors: distinct neuronal and nonneuronal forms derived by alternative splicing differ in phosphorylation. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2951–2955. doi: 10.1073/pnas.88.7.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaurex N., Lew D. P., Krause K. H. Cyclopiazonic acid depletes intracellular Ca2+ stores and activates an influx pathway for divalent cations in HL-60 cells. J Biol Chem. 1992 Feb 5;267(4):2318–2324. [PubMed] [Google Scholar]
- Ferris C. D., Huganir R. L., Bredt D. S., Cameron A. M., Snyder S. H. Inositol trisphosphate receptor: phosphorylation by protein kinase C and calcium calmodulin-dependent protein kinases in reconstituted lipid vesicles. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2232–2235. doi: 10.1073/pnas.88.6.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris C. D., Huganir R. L., Snyder S. H. Calcium flux mediated by purified inositol 1,4,5-trisphosphate receptor in reconstituted lipid vesicles is allosterically regulated by adenine nucleotides. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2147–2151. doi: 10.1073/pnas.87.6.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris C. D., Huganir R. L., Supattapone S., Snyder S. H. Purified inositol 1,4,5-trisphosphate receptor mediates calcium flux in reconstituted lipid vesicles. Nature. 1989 Nov 2;342(6245):87–89. doi: 10.1038/342087a0. [DOI] [PubMed] [Google Scholar]
- Ferris C. D., Snyder S. H. Inositol 1,4,5-trisphosphate-activated calcium channels. Annu Rev Physiol. 1992;54:469–488. doi: 10.1146/annurev.ph.54.030192.002345. [DOI] [PubMed] [Google Scholar]
- Fujii J., Otsu K., Zorzato F., de Leon S., Khanna V. K., Weiler J. E., O'Brien P. J., MacLennan D. H. Identification of a mutation in porcine ryanodine receptor associated with malignant hyperthermia. Science. 1991 Jul 26;253(5018):448–451. doi: 10.1126/science.1862346. [DOI] [PubMed] [Google Scholar]
- Furuichi T., Shiota C., Mikoshiba K. Distribution of inositol 1,4,5-trisphosphate receptor mRNA in mouse tissues. FEBS Lett. 1990 Jul 2;267(1):85–88. doi: 10.1016/0014-5793(90)80294-s. [DOI] [PubMed] [Google Scholar]
- Furuichi T., Simon-Chazottes D., Fujino I., Yamada N., Hasegawa M., Miyawaki A., Yoshikawa S., Guénet J. L., Mikoshiba K. Widespread expression of inositol 1,4,5-trisphosphate receptor type 1 gene (Insp3r1) in the mouse central nervous system. Receptors Channels. 1993;1(1):11–24. [PubMed] [Google Scholar]
- Furuichi T., Yoshikawa S., Miyawaki A., Wada K., Maeda N., Mikoshiba K. Primary structure and functional expression of the inositol 1,4,5-trisphosphate-binding protein P400. Nature. 1989 Nov 2;342(6245):32–38. doi: 10.1038/342032a0. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Irvine R. Second messengers and Lowe syndrome. Nat Genet. 1992 Aug;1(5):315–316. doi: 10.1038/ng0892-315. [DOI] [PubMed] [Google Scholar]
- Ito M. Long-term depression. Annu Rev Neurosci. 1989;12:85–102. doi: 10.1146/annurev.ne.12.030189.000505. [DOI] [PubMed] [Google Scholar]
- Klausner R. D., Samelson L. E. T cell antigen receptor activation pathways: the tyrosine kinase connection. Cell. 1991 Mar 8;64(5):875–878. doi: 10.1016/0092-8674(91)90310-u. [DOI] [PubMed] [Google Scholar]
- Kleiman R., Banker G., Steward O. Differential subcellular localization of particular mRNAs in hippocampal neurons in culture. Neuron. 1990 Dec;5(6):821–830. doi: 10.1016/0896-6273(90)90341-c. [DOI] [PubMed] [Google Scholar]
- Knudson A. G., Jr Genetics of human cancer. Annu Rev Genet. 1986;20:231–251. doi: 10.1146/annurev.ge.20.120186.001311. [DOI] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Kume S., Muto A., Aruga J., Nakagawa T., Michikawa T., Furuichi T., Nakade S., Okano H., Mikoshiba K. The Xenopus IP3 receptor: structure, function, and localization in oocytes and eggs. Cell. 1993 May 7;73(3):555–570. doi: 10.1016/0092-8674(93)90142-d. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Latif F., Tory K., Gnarra J., Yao M., Duh F. M., Orcutt M. L., Stackhouse T., Kuzmin I., Modi W., Geil L. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science. 1993 May 28;260(5112):1317–1320. doi: 10.1126/science.8493574. [DOI] [PubMed] [Google Scholar]
- Maeda N., Kawasaki T., Nakade S., Yokota N., Taguchi T., Kasai M., Mikoshiba K. Structural and functional characterization of inositol 1,4,5-trisphosphate receptor channel from mouse cerebellum. J Biol Chem. 1991 Jan 15;266(2):1109–1116. [PubMed] [Google Scholar]
- Maeda N., Niinobe M., Inoue Y., Mikoshiba K. Developmental expression and intracellular location of P400 protein characteristic of Purkinje cells in the mouse cerebellum. Dev Biol. 1989 May;133(1):67–76. doi: 10.1016/0012-1606(89)90297-2. [DOI] [PubMed] [Google Scholar]
- Maeda N., Niinobe M., Mikoshiba K. A cerebellar Purkinje cell marker P400 protein is an inositol 1,4,5-trisphosphate (InsP3) receptor protein. Purification and characterization of InsP3 receptor complex. EMBO J. 1990 Jan;9(1):61–67. doi: 10.1002/j.1460-2075.1990.tb08080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda N., Niinobe M., Nakahira K., Mikoshiba K. Purification and characterization of P400 protein, a glycoprotein characteristic of Purkinje cell, from mouse cerebellum. J Neurochem. 1988 Dec;51(6):1724–1730. doi: 10.1111/j.1471-4159.1988.tb01151.x. [DOI] [PubMed] [Google Scholar]
- Mattei M. G., Philip N., Passage E., Moisan J. P., Mandel J. L., Mattei J. F. DNA probe localization at 18p113 band by in situ hybridization and identification of a small supernumerary chromosome. Hum Genet. 1985;69(3):268–271. doi: 10.1007/BF00293038. [DOI] [PubMed] [Google Scholar]
- Michikawa T., Hamanaka H., Otsu H., Yamamoto A., Miyawaki A., Furuichi T., Tashiro Y., Mikoshiba K. Transmembrane topology and sites of N-glycosylation of inositol 1,4,5-trisphosphate receptor. J Biol Chem. 1994 Mar 25;269(12):9184–9189. [PubMed] [Google Scholar]
- Mignery G. A., Johnston P. A., Südhof T. C. Mechanism of Ca2+ inhibition of inositol 1,4,5-trisphosphate (InsP3) binding to the cerebellar InsP3 receptor. J Biol Chem. 1992 Apr 15;267(11):7450–7455. [PubMed] [Google Scholar]
- Mignery G. A., Newton C. L., Archer B. T., 3rd, Südhof T. C. Structure and expression of the rat inositol 1,4,5-trisphosphate receptor. J Biol Chem. 1990 Jul 25;265(21):12679–12685. [PubMed] [Google Scholar]
- Mignery G. A., Südhof T. C. The ligand binding site and transduction mechanism in the inositol-1,4,5-triphosphate receptor. EMBO J. 1990 Dec;9(12):3893–3898. doi: 10.1002/j.1460-2075.1990.tb07609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki A., Furuichi T., Ryou Y., Yoshikawa S., Nakagawa T., Saitoh T., Mikoshiba K. Structure-function relationships of the mouse inositol 1,4,5-trisphosphate receptor. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4911–4915. doi: 10.1073/pnas.88.11.4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahorski S. R., Ragan C. I., Challiss R. A. Lithium and the phosphoinositide cycle: an example of uncompetitive inhibition and its pharmacological consequences. Trends Pharmacol Sci. 1991 Aug;12(8):297–303. doi: 10.1016/0165-6147(91)90581-c. [DOI] [PubMed] [Google Scholar]
- Nakade S., Rhee S. K., Hamanaka H., Mikoshiba K. Cyclic AMP-dependent phosphorylation of an immunoaffinity-purified homotetrameric inositol 1,4,5-trisphosphate receptor (type I) increases Ca2+ flux in reconstituted lipid vesicles. J Biol Chem. 1994 Mar 4;269(9):6735–6742. [PubMed] [Google Scholar]
- Nakagawa T., Okano H., Furuichi T., Aruga J., Mikoshiba K. The subtypes of the mouse inositol 1,4,5-trisphosphate receptor are expressed in a tissue-specific and developmentally specific manner. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6244–6248. doi: 10.1073/pnas.88.14.6244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi S., Maeda N., Mikoshiba K. Immunohistochemical localization of an inositol 1,4,5-trisphosphate receptor, P400, in neural tissue: studies in developing and adult mouse brain. J Neurosci. 1991 Jul;11(7):2075–2086. doi: 10.1523/JNEUROSCI.11-07-02075.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauseef W. M., Clark R. A. Separation and analysis of subcellular organelles in a human promyelocytic leukemia cell line, HL-60: application to the study of myeloid lysosomal enzyme synthesis and processing. Blood. 1986 Aug;68(2):442–449. [PubMed] [Google Scholar]
- Nirenberg M., Wilson S., Higashida H., Rotter A., Krueger K., Busis N., Ray R., Kenimer J. G., Adler M. Modulation of synapse formation by cyclic adenosine monophosphate. Science. 1983 Nov 18;222(4625):794–799. doi: 10.1126/science.6314503. [DOI] [PubMed] [Google Scholar]
- Otsu H., Yamamoto A., Maeda N., Mikoshiba K., Tashiro Y. Immunogold localization of inositol 1, 4, 5-trisphosphate (InsP3) receptor in mouse cerebellar Purkinje cells using three monoclonal antibodies. Cell Struct Funct. 1990 Jun;15(3):163–173. doi: 10.1247/csf.15.163. [DOI] [PubMed] [Google Scholar]
- Ross C. A., Danoff S. K., Schell M. J., Snyder S. H., Ullrich A. Three additional inositol 1,4,5-trisphosphate receptors: molecular cloning and differential localization in brain and peripheral tissues. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4265–4269. doi: 10.1073/pnas.89.10.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayers L. G., Brown G. R., Michell R. H., Michelangeli F. The effects of thimerosal on calcium uptake and inositol 1,4,5-trisphosphate-induced calcium release in cerebellar microsomes. Biochem J. 1993 Feb 1;289(Pt 3):883–887. doi: 10.1042/bj2890883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seizinger B. R., Rouleau G. A., Ozelius L. J., Lane A. H., Farmer G. E., Lamiell J. M., Haines J., Yuen J. W., Collins D., Majoor-Krakauer D. Von Hippel-Lindau disease maps to the region of chromosome 3 associated with renal cell carcinoma. Nature. 1988 Mar 17;332(6161):268–269. doi: 10.1038/332268a0. [DOI] [PubMed] [Google Scholar]
- Siess W. Molecular mechanisms of platelet activation. Physiol Rev. 1989 Jan;69(1):58–178. doi: 10.1152/physrev.1989.69.1.58. [DOI] [PubMed] [Google Scholar]
- Somlyo A. V., Bond M., Somlyo A. P., Scarpa A. Inositol trisphosphate-induced calcium release and contraction in vascular smooth muscle. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5231–5235. doi: 10.1073/pnas.82.15.5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suburo A. M., Rodrigo J., Rossi M. L., Martínez-Murillo R., Terenghi G., Maeda N., Mikoshiba K., Polak J. M. Immunohistochemical localization of the inositol 1,4,5-trisphosphate receptor in the human nervous system. Brain Res. 1993 Jan 22;601(1-2):193–202. doi: 10.1016/0006-8993(93)91710-a. [DOI] [PubMed] [Google Scholar]
- Supattapone S., Worley P. F., Baraban J. M., Snyder S. H. Solubilization, purification, and characterization of an inositol trisphosphate receptor. J Biol Chem. 1988 Jan 25;263(3):1530–1534. [PubMed] [Google Scholar]
- Südhof T. C., Newton C. L., Archer B. T., 3rd, Ushkaryov Y. A., Mignery G. A. Structure of a novel InsP3 receptor. EMBO J. 1991 Nov;10(11):3199–3206. doi: 10.1002/j.1460-2075.1991.tb04882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshima H., Nishimura S., Matsumoto T., Ishida H., Kangawa K., Minamino N., Matsuo H., Ueda M., Hanaoka M., Hirose T. Primary structure and expression from complementary DNA of skeletal muscle ryanodine receptor. Nature. 1989 Jun 8;339(6224):439–445. doi: 10.1038/339439a0. [DOI] [PubMed] [Google Scholar]
- Walaas S. I., Nairn A. C., Greengard P. PCPP-260, a Purkinje cell-specific cyclic AMP-regulated membrane phosphoprotein of Mr 260,000. J Neurosci. 1986 Apr;6(4):954–961. doi: 10.1523/JNEUROSCI.06-04-00954.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto-Hino M., Sugiyama T., Hikichi K., Mattei M. G., Hasegawa K., Sekine S., Sakurada K., Miyawaki A., Furuichi T., Hasegawa M. Cloning and characterization of human type 2 and type 3 inositol 1,4,5-trisphosphate receptors. Receptors Channels. 1994;2(1):9–22. [PubMed] [Google Scholar]
- Yamamoto H., Maeda N., Niinobe M., Miyamoto E., Mikoshiba K. Phosphorylation of P400 protein by cyclic AMP-dependent protein kinase and Ca2+/calmodulin-dependent protein kinase II. J Neurochem. 1989 Sep;53(3):917–923. doi: 10.1111/j.1471-4159.1989.tb11792.x. [DOI] [PubMed] [Google Scholar]
- Yoshikawa S., Tanimura T., Miyawaki A., Nakamura M., Yuzaki M., Furuichi T., Mikoshiba K. Molecular cloning and characterization of the inositol 1,4,5-trisphosphate receptor in Drosophila melanogaster. J Biol Chem. 1992 Aug 15;267(23):16613–16619. [PubMed] [Google Scholar]