Abstract
Two different molecular forms of flavodoxin from the green alga Chlorella fusca have been purified to homogeneity and their properties compared. The molecular masses are 22 kDa (flavodoxin I) and 20 kDa (flavodoxin II). Western blots of axenic crude extract show the two bands. Both are single polypeptide chains and their N-terminal sequences differ but are very similar. Each form contains 1 mol of FMN/mol of apoprotein, exhibits a typical flavodoxin u.v.-visible absorption spectrum and does not contain covalently bound phosphate. The oxidation-reduction properties of the FMN in the flavodoxins differ considerably. Redox potentials of flavodoxin I at pH8 are -240 mV for the oxidized/semiquinone couple and -350 mV for the semiquinone/hydroquinone couple. Flavodoxin II gives more electronegative values: -278 mV and -458 mV respectively. Flavodoxin II fulfils better the redox requirements for photosynthetic electron transport and, as expected, it is more efficient at mediating NADP+ photoreduction in the photosynthetic electron flow. A new h.p.l.c. method for flavodoxin purification is described, which is useful for the isolation of very similar anionic proteins.
Full text
PDF
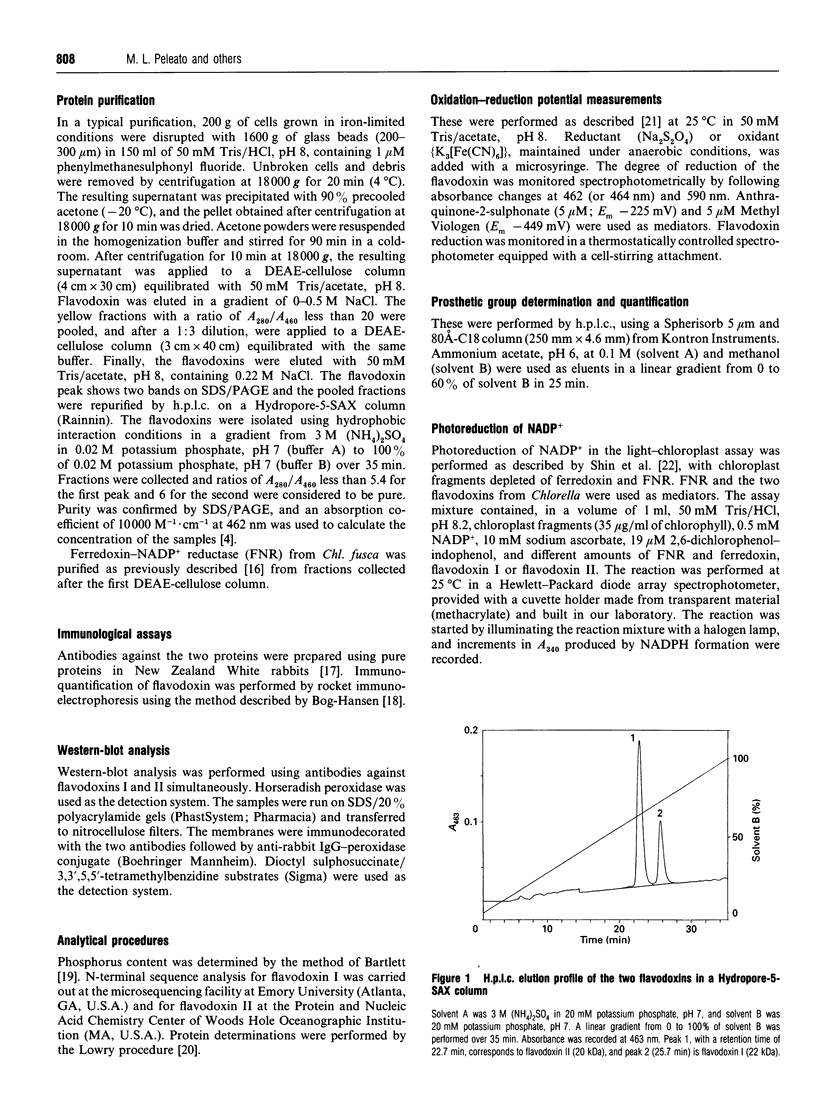
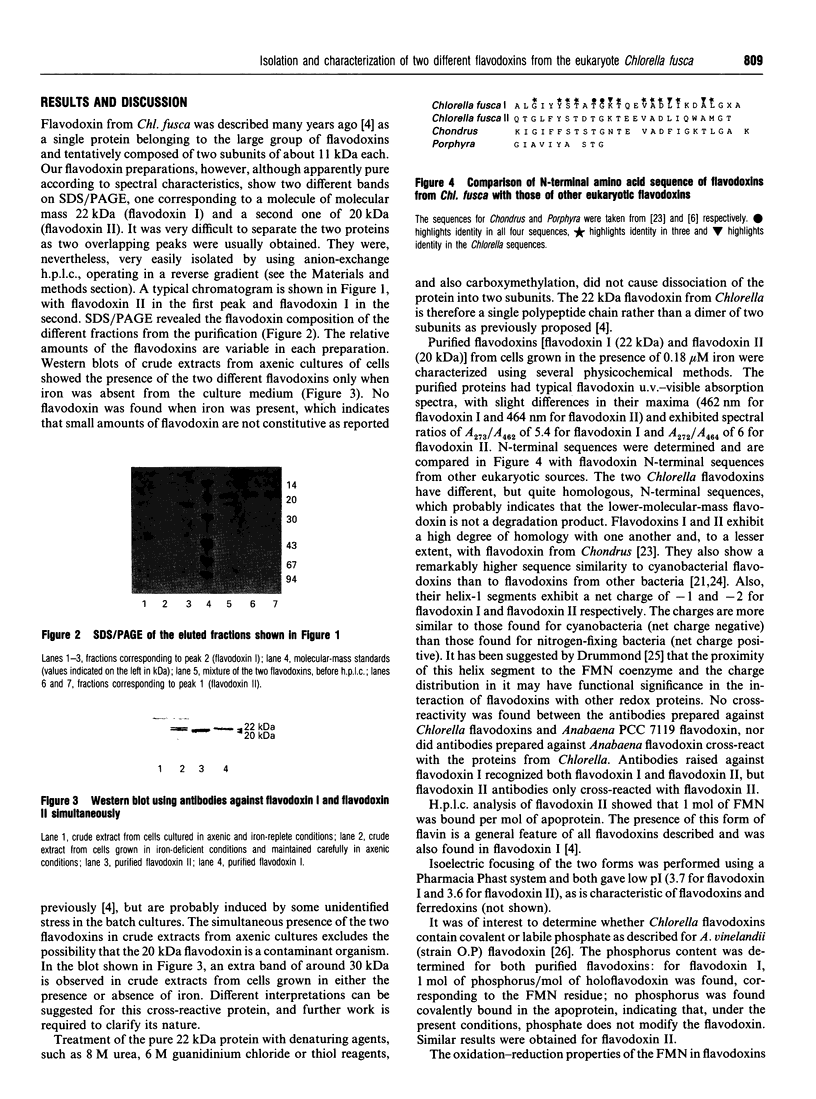
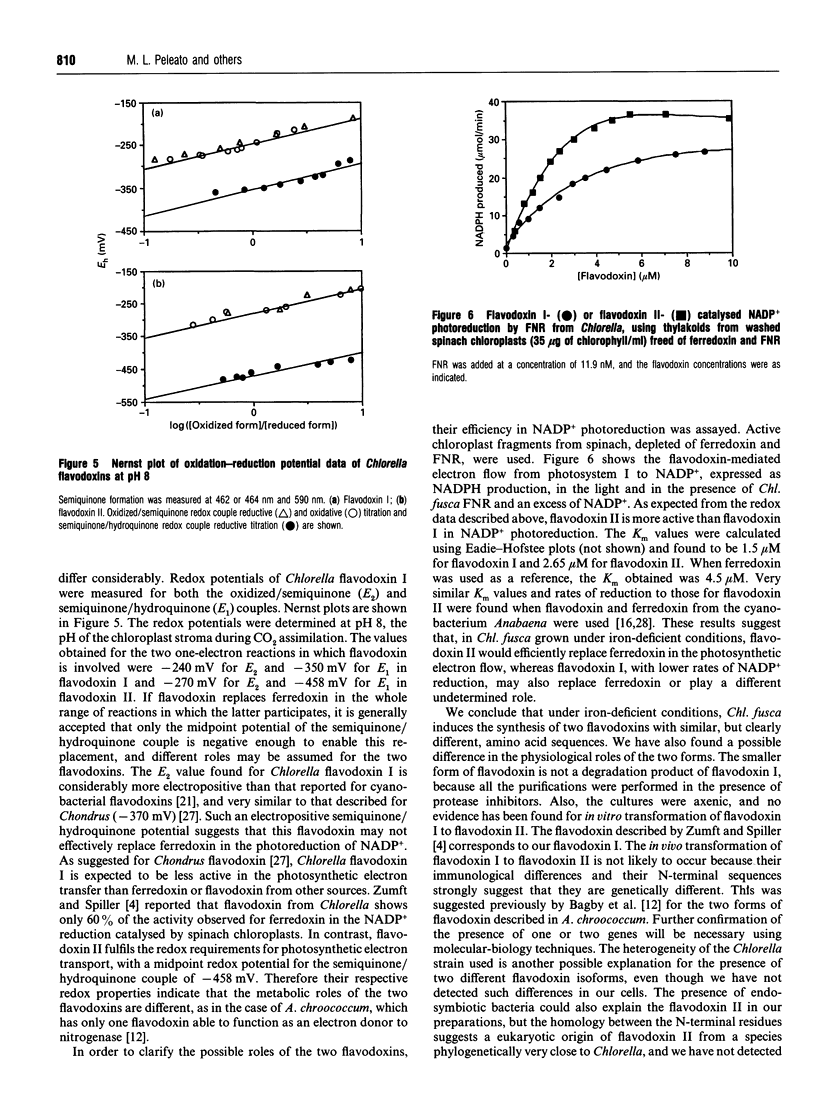

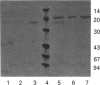
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Bagby S., Barker P. D., Hill H. A., Sanghera G. S., Dunbar B., Ashby G. A., Eady R. R., Thorneley R. N. Direct electrochemistry of two genetically distinct flavodoxins isolated from Azotobacter chroococcum grown under nitrogen-fixing conditions. Biochem J. 1991 Jul 15;277(Pt 2):313–319. doi: 10.1042/bj2770313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Liu M. Y., LeGall J. Isolation and characterization of flavoredoxin, a new flavoprotein that permits in vitro reconstitution of an electron transfer chain from molecular hydrogen to sulfite reduction in the bacterium Desulfovibrio gigas. Arch Biochem Biophys. 1993 May 15;303(1):44–50. doi: 10.1006/abbi.1993.1253. [DOI] [PubMed] [Google Scholar]
- Drummond M. H. Structure predictions and surface charge of nitrogenase flavodoxins from Klebsiella pneumoniae and Azotobacter vinelandii. Eur J Biochem. 1986 Sep 15;159(3):549–553. doi: 10.1111/j.1432-1033.1986.tb09921.x. [DOI] [PubMed] [Google Scholar]
- Dubourdieu M., Fox J. L. Amino acid sequence of Desulfovibrio vulgaris flavodoxin. J Biol Chem. 1977 Feb 25;252(4):1453–1463. [PubMed] [Google Scholar]
- Fillat M. F., Edmondson D. E., Gomez-Moreno C. Structural and chemical properties of a flavodoxin from Anabaena PCC 7119. Biochim Biophys Acta. 1990 Sep 3;1040(2):301–307. doi: 10.1016/0167-4838(90)90091-s. [DOI] [PubMed] [Google Scholar]
- Fukuyama K., Matsubara H., Rogers L. J. Crystal structure of oxidized flavodoxin from a red alga Chondrus crispus refined at 1.8 A resolution. Description of the flavin mononucleotide binding site. J Mol Biol. 1992 Jun 5;225(3):775–789. doi: 10.1016/0022-2836(92)90400-e. [DOI] [PubMed] [Google Scholar]
- Hase T., Wada K., Matsubara H. A minor component of ferredoxin from Aphanothece sacrum cells. J Biochem. 1975 Sep;78(3):605–610. doi: 10.1093/oxfordjournals.jbchem.a130946. [DOI] [PubMed] [Google Scholar]
- Kessler E., Czygan F. C. Physiologische und biochemische Beiträge zur Taxonomie der Gattung cChlorella. IV. Verwertng organischer Stickstoffverindungen. Arch Mikrobiol. 1970;70(3):211–216. [PubMed] [Google Scholar]
- Klugkist J., Haaker H., Wassink H., Veeger C. The catalytic activity of nitrogenase in intact Azotobacter vinelandii cells. Eur J Biochem. 1985 Feb 1;146(3):509–515. doi: 10.1111/j.1432-1033.1985.tb08681.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lampreave F., Piñeiro A. Characterization of a new alpha-glycoprotein as the major serum component in later fetal and newborn pigs. Comp Biochem Physiol B. 1982;72(2):215–219. doi: 10.1016/0305-0491(82)90037-2. [DOI] [PubMed] [Google Scholar]
- SHIN M., TAGAWA K., ARNON D. I. CRYSTALLIZATION OF FERREDOXIN-TPN REDUCTASE AND ITS ROLE IN THE PHOTOSYNTHETIC APPARATUS OF CHLOROPLASTS. Biochem Z. 1963;338:84–96. [PubMed] [Google Scholar]
- Sykes G. A., Rogers L. J. Redox potentials of algal and cyanobacterial flavodoxins. Biochem J. 1984 Feb 1;217(3):845–850. doi: 10.1042/bj2170845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi S., Kimura T., Fukuyama K., Matsubara H., Rogers L. J. The amino acid sequence of a flavodoxin from the eukaryotic red alga Chondrus crispus. Biochem J. 1989 Nov 1;263(3):981–984. doi: 10.1042/bj2630981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoch D. C., Valentine R. C. Ferredoxins and flavodoxins of bacteria. Annu Rev Microbiol. 1972;26:139–162. doi: 10.1146/annurev.mi.26.100172.001035. [DOI] [PubMed] [Google Scholar]
- Zumft W. G., Spiller H. Characterization of a flavodoxin from the green alga Chlorella. Biochem Biophys Res Commun. 1971 Oct 1;45(1):112–118. doi: 10.1016/0006-291x(71)90057-x. [DOI] [PubMed] [Google Scholar]