Abstract
Objective
To determine if autologous platelet-rich plasma (PRP) injection into a degenerative intervertebral disc, without Modic changes on magnetic resonance imaging (MRI), improve pain and function.
Design
Prospective, randomized controlled study.
Setting
Outpatient spine practice (Stichting Rugpoli, Netherlands).
Participants
Adults with chronic low back pain referred to Stichting Rugpoli, according to the Dutch General Practitioners Guidelines, unresponsive to conservative treatment, without Modic changes on MRI.
Methods
Provocation discography was performed to confirm the suspected disc was the source of pain. Participants were randomized to receive 1.0 cc intradiscal PRP (intervention) or 1.0 cc Saline with 0.2g Kefzol (control). Data on pain (Numeric Rating Scale), physical function (Roland Morris Disabilty Questionnaire, RMDQ), and participants’ general perceived health (SF-12) were collected at 1 week, 4 weeks, 2 months, 6 months, 9 months and 1 year. A repeated-measures analysis (mixed model) was used for comparing the outcomes of the groups.
Results
Of the initial 98 (49 intervention, 49 control) patients randomized, 89 (91%) (44 intervention, 45 control) with complete outcome data were analyzed. Groups were balanced at baseline. After twelve months no differences between groups were found in the average pain (improved 21/44 in intervention vs 16/45 in control, p = 0.244), the disability scores (RMDQ minimal 3 points improvement 22/44 in intervention vs 24/45 in control, p = 0.753) and the SF-12 (mean difference physical health −1.19, 95% CI -5.39 to 2.99, p = 0.721, and mental health −0.34, 95% CI -3.99 to 3.29, p = 0.834). One serious adverse event occurred (spondylodiscitis) after intervention.
Conclusion
Participants who received intradiscal PRP showed no significant improvement in pain or functionality compared to the control group at 1 year follow up.
1. Introduction
Chronic low back pain, a common condition that frequently lacks an exact etiology and a specific treatment, is defined as pain between the lowest rib and the upper buttock fold persistent for at least three months [1]. It is the leading cause of disability worldwide [[2], [3], [4]]. Provocative discography is used to confirm that intervertebral disc is the likely source of pain ([5]). Degenerative changes in the intervertebral disc [[6], [7], [8]], a process in which the cartilage dehydrates and the disc height decreases, is often associated with this diagnosis. Several proinflammatory mediators produced by degenerative discs ([9]) are believed to cause persistent inflammation of the discs’ nucleus and within the annular tears. Through these annular tears granulation tissue and free nerve endings can grow associated with low back pain [[10], [11], [12], [13]].
Degenerative changes may occur in the endplates (10, [14,15]), which can be seen on an magnetic resonance imaging (MRI) scan as Modic Changes ([6,[16], [17], [18]]). There is a positive association between discography and Modic Changes type 1 and 2 ([19]). There is also increasing evidence that Modic Changes are related to a low grade infection ([20]).
There is no gold standard treatment for discogenic low back pain (3), and the options include minimal invasive interventions as well as fusion surgery or total disc replacement as a last resort [[21], [22], [23], [24], [25], [26]]. In the last decades, biological treatment modalities like Platelet Rich Plasma (PRP) have evolved ([27]). Growth factors released by these platelets are believed to stimulate healing. Examples of growth factors released by platelets are transforming growth factor beta (TGF-β), insulin-like growth factor (ILGF), platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF). Many of these growth factors play an important role in stimulating duplication, activation and growth of mesenchymal cells, such as osteoblasts, fibroblasts and endothelial cells. Although several previous studies showed positive effect of PRP for different musculoskeletal disorders, the effect on the intervertebral disc and its clinical outcomes (pain and functioning) are unclear.
The intervertebral disc is an avascular structure and as such has low concentrations of growth factors and impaired healing capabilities. Intradiscal injection of PRP is aimed at reducing the degenerative process and to promote healing. PRP contains growth factors and bioactive proteins that influence the healing of tendon, ligament, muscle, and bone ([28]). It was first used in 1987 in autologous transfusion support of cardiac surgery patients and subsequently used for other treatments [[29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44]]. PRP is blood derived. It is processed in a way (centrifuging) that high concentration platelets remain in the plasma and it releases growth factors in high concentrations when activated ([28,45,46]). A recent RCT showed promising results of PRP injections into a degenerated lumbar disc at eight weeks follow up ([47]). No major complications were reported in this trial but one case report described an infection after intradiscal PRP injection ([48]). Given the risks involved, it is important to demonstrate effectiveness of PRP in a randomized trial. Thus, the objective of our study was to determine if autologous platelet-rich plasma (PRP) injection into a degenerative intervertebral disc, without Modic changes on magnetic resonance imaging (MRI), improve pain and function compared to control.
2. Materials and methods
After obtaining medical ethical review board approval (NL46021.044.13/ABR, 08-05-2014), the trial was conducted in Stichting Rugpoli (Delden, the Netherlands), a specialized multidisciplinary outpatient clinic for musculoskeletal disorders, specialized in low back pain treatment. The study is reported according to CONSORT statement. The study was conducted according to the Declaration of Helsinki.
2.1. Study design
Prospective, single blind, randomized controlled trial of patients with discogenic chronic low back pain without Modic Changes on MRI, treated with a PRP injection into the intervertebral disc. The study participants and research nurse assassing outcomes were blinded, but the performing physician was unable to be blinded because of the difference in viscosity between PRP and saline was clearly sensible.
2.2. Population
Adults with chronic low back pain referred to Stichting Rugpoli, according to the Dutch General Practitioners Guidelines, without Modic changes on the MRI, unresponsive to conservative treatment. Internal investigation at Stichting Rugpoli shows that 274 out of 500 patients with chronic low back pain present with lumbar degenerative disc changes. Conservative treatment including McKenzie therapy, load change and pain treatment (medication) results in improvement for approximately 34% of these patients after one year. However, approximately 66% do not respond enough to this conservative treatment. Using inclusion and exclusion criteria listed in Table 1, approximately 50% of this group is eligible for participation in the study. The rationale behind PRP in the degenerative disc is that it repairs the collagen injury and annular tear. Patients with Modic changes on the MRI were excluded from this RCT. There is increasing evidence that Modic changes are related to a low grade infection (20). For this specific condition there is a stepped care treatment protocol at Rugpoli including NSAID's, intradiscal dexamethasone injection and as a last step 100 days of antibiotic treatment.
Table 1.
In- and exclusion criteria.
| Inclusion | Exclusion |
|---|---|
|
|
Discography is used to confirm that a “suspected” degenerative disc is the cause for low back pain. Discography was performed according to the SIS guidelines. Baseline group differences between PRP + and PRP- were assessed using covariance analysis.
2.3. Procedure
Patients included are asked to stop medication Aspirin and NSAID 24 h prior to treatment and to discontinue NSAID's for at least two months after the procedure. The minimum time between the provocative discography and the procedure is two weeks. All included patients signed the Informed Consent Form. After inclusion randomization takes place by computer using “Windows Version 6.0 of Randomization Program Rand.exe”. 30 Minutes prior to treatment a 32 cc blood sample is drawn from the patient with a 20 gauge needle. 3 cc Citrate is then added to this blood to bind the ionized calcium, in order to prevent clotting. If the patient is randomized for PRP + further preparation takes place by the research doctor and a second Rugpoli employee for double check of the procedure. This employee is not the research nurse, for the purpose of blinding. For the PRP preparation the Smart PReP 2 procedure was used. The blood was processed using a centrifuge (Harvest Technologies Corporation, Plymouth, MA). The blood is centrifuged at 1000 RPM for 15 min in a specially designed capsule. This divides the plasma and platelets from the red and white blood cells. The resulting plasma still contains 5% red blood cells and 1% white blood cells. This plasma is again centrifuged, resulting in two layers. The upper layer consists of Platelet Poor Plasma, the lower layer is the Platelet Rich Plasma. This procedure results in plasma with 1.500.000/μl platelet concentration [49].
If the patient is randomized for PRP-, the research doctor prepares a syringe with 1 cc NaCl with 0.2g Kefzol. The prepared syringes are labelled with patient name, age and residence. The patient is blinded for the procedure by prone positioning in a way the preparation and administration of the injection are not visible. The treating physician cannot be blinded for the procedure due to obvious difference in viscosity between PRP+ and PRP-. The injection technique used at the Rugpoli is according to SIS guidelines. The single needle technique was used, and the procedure was performed without administration of prophylactic intravenous antibiotics. The procedure is performed without contrast to confirm the placement of the injectate. This rationale is that the contrast medium may interact with the PRP and thereby reduce its effectiveness. After the injection the patient is transported to a recovery room and observed for 1 h. During this time the patient remains in prone position. After 1 h the patient is mobilized and eventually discharged when the patient feels well enough. Patients are informed not to take NSAID's for at least two months. Follow up at one week consists of completing NRS and RMDQ questionnaires, either at the clinic or by telephone by the research nurse whom is not informed on which preparation has been injected. Also, the injection location is checked at this moment for signs of infection or inflammation. Further follow up questionnaires were completed and submitted online at 1, 2, 4, 6, 9 and 12 months.
2.4. Outcome measures
The primary outcome measures were changes in pain as measured with the Numeric Rating Scale (NRS) [50], and changes in disability as measured with the Roland Morris Disability Questionnaire (RMDQ) [51]. Secondary outcome measures were changes in self-reported mental and physical health as measured with SF-12 [52]. These measurements were applied before the intervention (index) and at one year follow up. Measurement were repeated at 1 week, 4 weeks, 2, 4, 6 and 9 months as secondary time points. To blind the outcome measurements, the research nurse and the patient were not informed about the preparations injected.
2.5. Statistical analysis
A minimally clinical important change for pain was defined 2 points on the NRS scale, and for functioning 3 points on the RMDQ ([50,51]). Based on a previous RCT ([47]) decrease in pain to NRS 5 from 7 and decrease in disability to RMDQ 9 from 12 points was expected after 9 and 12 months. To detect this difference with the two-sample t-test at 90% power and p < 0.05, approximately 80 participants (40 per group) were required ([53,54]). Allowing for 20% drop out we intended to recruit 100 participants. NRS pain score for average and worst pain, and functionality score as measured with RMDQ of both groups are continuous variables. Quantitative analysis was performed on these primary outcome measures. Secondary outcome measures were self-reported physical and mental health measured with SF-12. The Chi2-test was used for categorical data. Continuous data with normal distribution such as age were compared using the t-test and for the non-normal distribution the Mann-Whitley U test. Differences in outcome were measured at 1 week, 1,2,4,6,9 and 12 months.
3. Results
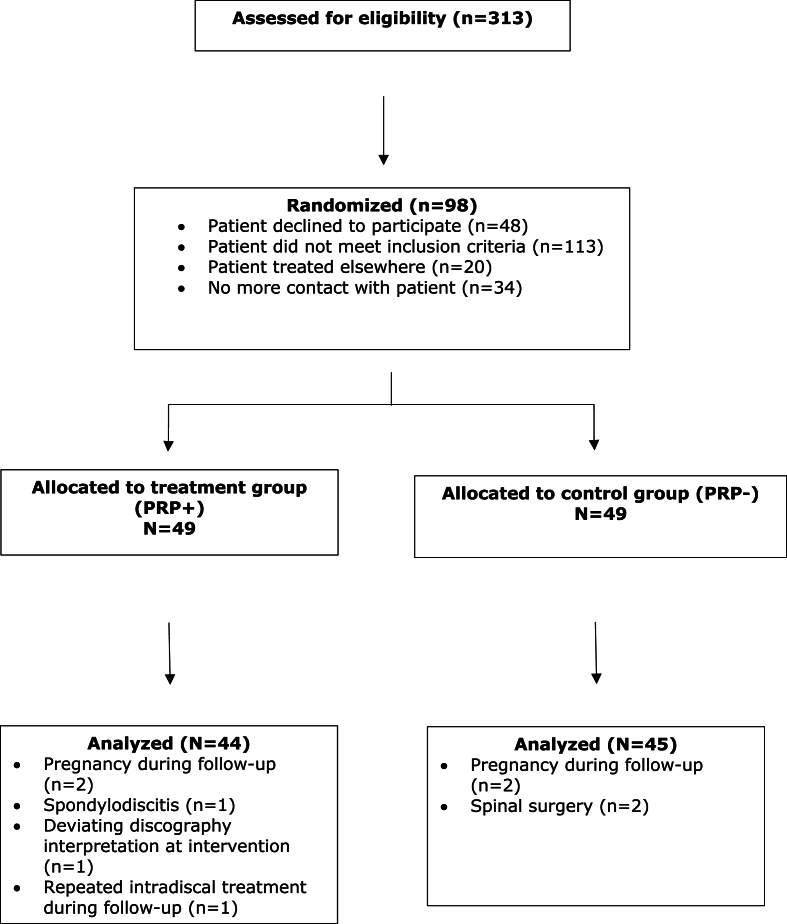
Between July 2014 and May 2018, 313 patients were assessed for inclusion by three interventional spine physiatrists at Stichting Rugpoli. Inclusion was based on the in- and exclusion criteria as listed in Table 1. Two hundred fifteen patients either did not meet the inclusion criteria, went for treatment at a different clinic, did not return for further treatment or were not willing to participate. A total of 98 patients met the inclusion criteria and were randomized in two equal groups (49/49). Nine patients were excluded (five in PRP + group and four in PRP- group). Reasons for exclusion were pregnancy [4], spondylodiscitis (1), deviating discography interpretation (1), repeated intradiscal procedure during follow up period (1) and surgical intervention during follow up period (2). Eighty-nine of the initial 98 included patients were analyzed; PRP+ [44] and PRP– [45]. A flow chart is shown in Fig. 1.
Fig. 1.
Study inclusion randomization and follow-up flow chart.
For randomization “Windows Version 6.0 of Randomization Program Rand.exe” was used. Demographics are listed in Table 2.
Table 2.
Baseline characteristics of included participants.
| Intervention PRP+ (n = 44) | Control PRP– (n = 45) | |
|---|---|---|
| Age (mean/SD) | 40.3/10.4 | 39.1/11.5 |
| Gender (female|male n/%) | 28/63.6% | 16/36.4% | 26/57.8% | 19/42.2% |
| Multiple level intradiscal procedure (n = ) | 8 (18.2%) | 9 (20%) |
| RMDQ Mean/sd | 12.636/5.3574 | 13.422/4.3978 |
| Average pain Mean/sd |
6.295/1.2310 | 6.022/1.4846 |
| Worts pain mean/sd | 8.205/1.3221 | 8.200/1.2541 |
No differences were found in baseline characteristics between both groups (Table 2). Mean age was 40.3(PRP+) versus 39.1(PRP-). There were more female patients in both groups; 28 out of 44 (64%/PRP+) and 26 out of 45 (58%/PRP-). Similar numbers for a multilevel procedure were seen in the PRP+ (n = 8/18%) and PRP- (n = 9/20%) group. Improvement in pain of at least two points (average/worst) was not significantly different between PRP+ and PRP- after twelve months. For average pain 21/44 (48%) in the PRP + group (95% CI 34–62) achieved a minimal two points change in NRS compared to 16/45 (35%) in the PRP- group (95% CI 23–50). For worst pain this was 16/44 (36%) in the PRP + group (95% CI 23–51) compared to 18/45 (40%) in the PRP- group (95% CI 27–55). No significant differences were found in the primary and secondary outcome measures (disability p = 0.753, average pain p = 0.244, worst pain p = 0.724, using the Pearson Chi2; physical health p = 0.721 and mental health p = 0.834) (Table 3).
Table 3.
Primary and secondary outcomes; the number of patients achieving a minimum two points change in NRS for pain and the number of patients achieving a minimum 3 points change in RMDQ for disability.
| Intervention PRP+ (n = 44) | Control PRP– (n = 45) | p-value | |
|---|---|---|---|
| Primary outcome | |||
| Average Paina | 21 | 16 | 0.244 |
| Worst paina | 16 | 18 | 0.724 |
| RMDQb | 22 | 24 | 0.753 |
| Secondary outcomes | |||
| SF-12 Physical health baseline vs 1 year | −1.19 95% CI -5.39 to 2.99 |
0.721 | |
| SF-12 Mental health Baseline vs 1 year |
−0.34 95% CI -3.99 to 3.29 |
0.834 | |
2 points or more improvement on NRS rating scale.
3 points or more improvement on RMDQ scale.
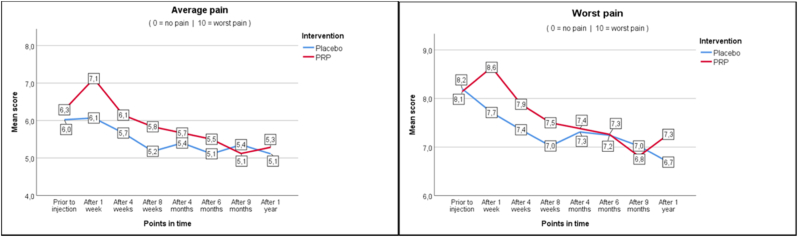
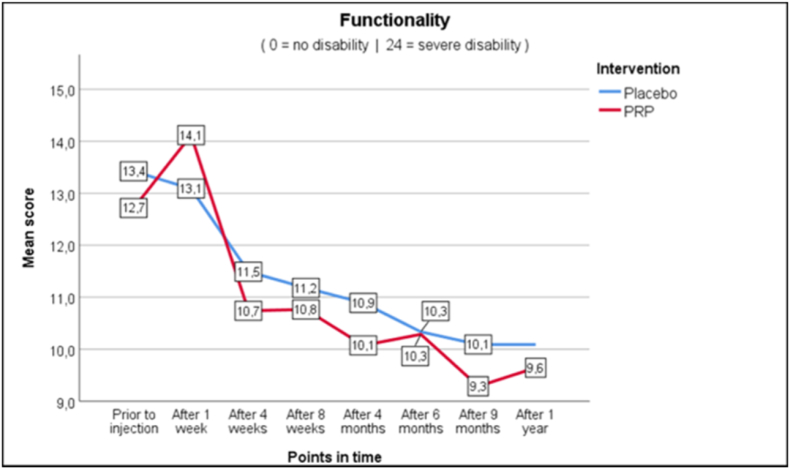
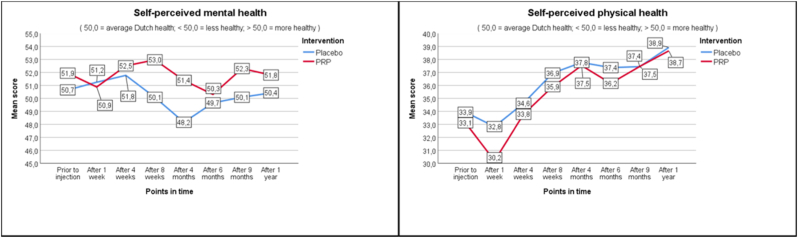
As seen in Fig. 2a–c, average and worst pain scores are very similar at the different follow up moments. In the PRP + group NRS score for average pain changed from 6.3 at baseline to 5.3 (−1.0) at one year, whereas in the PRP- group this changed from 6.0 to 5.1 (−0.9). Pain improvement at primary and secondary timepoints is shown in Table 4 as measured with the Mann-Whitney U test (Table 4). For functionality as measured with RMDQ the scores in the PRP + group changed from 12.7 to 9.6 (−3.1) versus 13.4 to 10.1 (−3.3) in the PRP- group. Also, the secondary outcomes show similar changes in time between the PRP+ and PRP- groups.
Fig. 2.
Outcomes.
Table 4.
Pain Improvement Mann-Whitney U test.
| Mann-Whitney U | test statistics | average pain | Mann-Whitney U | Wilcoxon W | Z | Asymp. Sig. (2-tailed) |
|---|---|---|---|---|
| Categorical improvement average pain after 1 week | 924,000 | 1914,000 | −1358 | 0,175 |
| Categorical improvement average pain after 4 weeks | 775,000 | 1678,000 | −2608 | 0,009 |
| Categorical improvement average pain after 8 weeks | 866,000 | 1856,000 | −1369 | 0,171 |
| Categorical improvement average pain after 16 weeks | 964,000 | 1954,000 | −0,292 | 0770 |
| Categorical improvement average pain after 26 weeks | 956,000 | 1946,000 | −0,363 | 0716 |
| Categorical improvement average pain after 40 weeks | 900,000 | 1935,000 | −0,947 | 0344 |
| Categorical improvement average pain after 1 year | 987,000 | 1977,000 | −0,029 | 0977 |
|
Mann-Whitney U | test statistics | worst pain |
Mann-Whitney U |
Wilcoxon W |
Z |
Asymp. Sig. (2-tailed) |
| Categorical improvement worst pain after 1 week | 836,000 | 1826,000 | −2708 | 0,007 |
| Categorical improvement worst pain after 4 weeks | 797,500 | 1700,500 | −2371 | 0,018 |
| Categorical improvement worst pain after 8 weeks | 867,000 | 1857,000 | −1550 | 0,121 |
| Categorical improvement worst pain after 16 weeks | 930,000 | 1920,000 | −0,719 | 0472 |
| Categorical improvement worst pain after 26 weeks | 945,000 | 1935,000 | −0,506 | 0613 |
| Categorical improvement worst pain after 40 weeks | 927,500 | 1962,500 | −0,690 | 0490 |
| Categorical improvement worst pain after 1 year | 935,000 | 1925,000 | −0,598 | 0550 |
One Serious Adverse Event occurred in this RCT (PRP+); spondylodiscitis after PRP treatment. This patient recovered with antibiotic treatment and surgical debridement.
4. Discussion
To our knowledge this is only the second RCT evaluating the clinical effectiveness of intradiscal PRP injections for patients with chronic discogenic low back pain without Modic changes on MRI.
The strength of this study is that it has higher number of participants compared to previous studies, and one year follow up is present for all participants without cross-over. In contrast with the positive results in the previous RCT [47], we did not observe clinically relevant changes in pain and functioning after intradiscal PRP injection. Given that our study had an a priori sample size estimation for a high level of statistical power to detect a small difference, our negative findings merit consideration as true absence of any clinical effect of PRP.
Inclusion criteria are similar in both studies and identical preparation of PRP was used. In the previous RCT however it is not mentioned if patients Modic changes on the MRI were excluded or not. A possible limitation of this RCT is that patients with Modic changes were excluded. However, the targeted tissue was considered to be the degenerated collagen and annular tear and not the degenerative changes in the endplates which could also be related to a low grade infection.
The PRP+ and PRP- injections were performed without intradiscal contrast because this might influence the PRP effectiveness. This may be considered to be a limitation as there is no fluoroscopic proof of the correct flow of the injectate. However, two weeks prior to the procedure a discography has been performed with contrast to visualize the disc and possible contrast leakage at the annular tear.
The previous study was a double-blind RCT. Our primary study design was also a double-blind RCT. However, the pilot study pointed out that blinding of the treating physician seemed impossible due to clearly sensible differences in viscosity between PRP+ and PRP-. For blinding purpose, a different control injection with similar viscosity to PRP + may be considered for future research on this topic. Although the treating physician could not be blinded, both the patient and the evaluating nurse were blinded for the procedure.
Although only one adverse event has occurred (spondylodiscitis after PRP injection) this might raise concerns as the number of patients treated with PRP+ was 44. Other intradiscal therapies have shown very low adverse events, with less than 1 in 1000. In this RCT no intravenous antibiotics were used. The PRP + preparation does not include prophylactic antibiotics whereas the PRP- includes 0.2 g Kefzol. This might explain the spondylodiscitis in the PRP + group.
In a recent review paper and guideline issue for effective use of biologics in the management of low back pain, qualitative evidence for positive effect of PRP has been assessed as Level III (on a scale of Level I to V), based on multiple moderate-quality observational studies and the previously mentioned RCT ([55]). Our study clearly has contradictive results. This is in line with several previous attempts to treat discogenic low back pain using minimal invasive procedures. Examples of such procedures are IDET, Nucleoplasty and Methylene Blue. At first these treatments seem very promising, however, the positive results mentioned in the first studies on such treatments are often difficult or impossible to reproduce [[56], [57], [58]].
Different types of PRP preparation have been described resulting in different concentrations of platelets, white blood cells, fibrin network and exogenous activators. In previous publications on PRP several classifications are proposed. These classifications are often based on the presence and concentration of platelets, white blood cells, fibrin network, and exogenous activators ([[59], [60], [61], [62]]). The American Academy of Orthopaedic Surgeons made recommendations on the classification and use of PRP. This also includes a list of variables that may influence the growth factor profile of PRP ([63]). The three types of variables are defined as “Donor”, “Processing” and “Delivery”. Donor variables are gender, comorbidities, nutritional status and concurrent medications, including NSAID's. Processing variables are blood collection and storage conditions, spin protocol (speed, time), activation protocol (agent, concentration, timing) and storage. Delivery variables are form of delivery (gel, solution), timing of delivery in relation to isolation, timing of delivery in relation to activation and host factors (similar to donor factors), including injury chronicity.
In this study the blood used for PRP was drawn 30 min prior to intradiscal treatment and could be directly prepared. The platelet concentration derived from the centrifuge using the SmartPReP 2 procedure [49](Harvest Technologies Corporation, Plymouth, MA) is known to be 1.500.000/μl and the plasma contains 1% White blood cells. There was no further biochemical analysis of the PRP used in this RCT. In several musculoskeletal tissues PRP treatment has shown promising results but evidence for clinical improvement after intradiscal PRP administration is very limited. A possible explanation might be the fact that the intervertebral disc is an avascular structure and minimal blood supply might be necessary to achieve positive PRP results. Options for future research might include a combination of Mesenchymal Stem Cell therapy and PRP treatment at the same time. However, with lack of clear clinical evidence for both these treatment options a combination of both will likely be ineffective as well. At this time, many clinics provide intradiscal PRP injections as a treatment for discogenic low back pain. There are concerns that other biological treatments, such as Mesenchymal Stem Cell therapy, are being used in several clinics without clear proven effect on the outcome of such therapy. This study highlights the need for further investigation on the effect of intradiscal PRP injections. At this moment there is no clear evidence to support PRP intradiscal treatment as standard care.
5. Conclusion
In this single-blind, statistically powered RCT, participants who received intradiscal PRP showed no significant improvement compared to the control group at 1 year follow up.
Disclosures
There was no funding for this study. There is no conflict of interest for any of the participants in the study.
Acknowledgement
The authors would like to thank Professor K.S.Khan for reviewing the manuscript.
References
- 1.Wessels P. CBO-richtlijn Aspecifieke lage-rugklachten. Huisarts Wet. 2004 May 1;47(5):706. 706. [Google Scholar]
- 2.Hartvigsen J., Hancock M.J., Kongsted A., Louw Q., Ferreira M.L., Genevay S., et al. What low back pain is and why we need to pay attention. Lancet. 2018 Jun 9;391(10137):2356–2367. doi: 10.1016/S0140-6736(18)30480-X. [DOI] [PubMed] [Google Scholar]
- 3.Foster N.E., Anema J.R., Cherkin D., Chou R., Cohen S.P., Gross D.P., et al. Prevention and treatment of low back pain: evidence, challenges, and promising directions. Lancet. 2018 Jun 9;391(10137):2368–2383. doi: 10.1016/S0140-6736(18)30489-6. [DOI] [PubMed] [Google Scholar]
- 4.Geurts J.W., Willems P.C., Kallewaard J.-W., van Kleef M., Dirksen C. Pain Res Manag; 2018. p. 4696180. (The impact of chronic discogenic low back pain: costs and patients' burden). 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walker J., El Abd O., Isaac Z., Muzin S. Discography in practice: a clinical and historical review. Curr Rev Musculoskelet Med. 2008 Jun;1(2):69–83. doi: 10.1007/s12178-007-9009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheung K.M.C., Karppinen J., Chan D., Ho D.W.H., Song Y.-Q., Sham P., et al. Prevalence and pattern of lumbar magnetic resonance imaging changes in a population study of one thousand forty-three individuals. Spine. 2009 Apr 20;34(9):934–940. doi: 10.1097/BRS.0b013e3181a01b3f. [DOI] [PubMed] [Google Scholar]
- 7.Hancock M.J., Maher C.G., Latimer J., Spindler M.F., McAuley J.H., Laslett M., et al. Systematic review of tests to identify the disc, SIJ or facet joint as the source of low back pain. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. 2007 Oct;16(10):1539–1550. doi: 10.1007/s00586-007-0391-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verrills P., Nowesenitz G., Barnard A. Prevalence and characteristics of discogenic pain in tertiary practice: 223 consecutive cases utilizing lumbar discography. Pain Med Malden Mass. 2015 Aug;16(8):1490–1499. doi: 10.1111/pme.12809. [DOI] [PubMed] [Google Scholar]
- 9.Burke J.G., Watson R.W.G., McCormack D., Dowling F.E., Walsh M.G., Fitzpatrick J.M. Intervertebral discs which cause low back pain secrete high levels of proinflammatory mediators. J Bone Joint Surg Br. 2002 Mar;84(2):196–201. doi: 10.1302/0301-620x.84b2.12511. [DOI] [PubMed] [Google Scholar]
- 10.Peng B., Hou S., Wu W., Zhang C., Yang Y. The pathogenesis and clinical significance of a high-intensity zone (HIZ) of lumbar intervertebral disc on MR imaging in the patient with discogenic low back pain. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. 2006 May;15(5):583–587. doi: 10.1007/s00586-005-0892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jensen T.S., Karppinen J., Sorensen J.S., Niinimäki J., Leboeuf-Yde C. Vertebral endplate signal changes (Modic change): a systematic literature review of prevalence and association with non-specific low back pain. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. 2008 Nov;17(11):1407–1422. doi: 10.1007/s00586-008-0770-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwarzer A.C., Aprill C.N., Derby R., Fortin J., Kine G., Bogduk N. The prevalence and clinical features of internal disc disruption in patients with chronic low back pain. Spine. 1995 Sep 1;20(17):1878–1883. doi: 10.1097/00007632-199509000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Yang G., Liao W., Shen M., Mei H. Insight into neural mechanisms underlying discogenic back pain. J Int Med Res. 2018 Nov;46(11):4427–4436. doi: 10.1177/0300060518799902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peng B., Hao J., Hou S., Wu W., Jiang D., Fu X., et al. Possible pathogenesis of painful intervertebral disc degeneration. Spine. 2006 Mar 1;31(5):560–566. doi: 10.1097/01.brs.0000201324.45537.46. [DOI] [PubMed] [Google Scholar]
- 15.Peng B.-G. Pathophysiology, diagnosis, and treatment of discogenic low back pain. World J Orthoped. 2013 Apr 18;4(2):42–52. doi: 10.5312/wjo.v4.i2.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cao P., Jiang L., Zhuang C., Yang Y., Zhang Z., Chen W., et al. Intradiscal injection therapy for degenerative chronic discogenic low back pain with end plate Modic changes. Spine J Off J North Am Spine Soc. 2011 Feb;11(2):100–106. doi: 10.1016/j.spinee.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 17.Modic M.T., Ross J.S. Lumbar degenerative disk disease. Radiology. 2007 Oct;245(1):43–61. doi: 10.1148/radiol.2451051706. [DOI] [PubMed] [Google Scholar]
- 18.Çevik S., Yılmaz H. Evaluation of the relationship between clinical symptoms and modic changes. Cureus. 2020 Feb 12;12(2) doi: 10.7759/cureus.6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herlin C., Kjaer P., Espeland A., Skouen J.S., Leboeuf-Yde C., Karppinen J., et al. PLoS ONE; 2018. Modic changes—their associations with low back pain and activity limitation: a systematic literature review and meta-analysis.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6070210/ [Internet] Aug 1 [cited 2020 Mar 30];13(8). Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Albert H.B., Sorensen J.S., Christensen B.S., Manniche C. Antibiotic treatment in patients with chronic low back pain and vertebral bone edema (Modic type 1 changes): a double-blind randomized clinical controlled trial of efficacy. Eur Spine J. 2013 Apr 1;22(4):697–707. doi: 10.1007/s00586-013-2675-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Willems P. Decision making in surgical treatment of chronic low back pain: the performance of prognostic tests to select patients for lumbar spinal fusion. Acta Orthop Suppl. 2013 Feb;84(349):1–35. doi: 10.3109/17453674.2012.753565. [DOI] [PubMed] [Google Scholar]
- 22.Manchikanti L., Staats P.S., Nampiaparampil D.E., Hirsch J.A. What is the role of epidural injections in the treatment of lumbar discogenic pain: a systematic review of comparative analysis with fusion. Korean J Pain. 2015 Apr;28(2):75–87. doi: 10.3344/kjp.2015.28.2.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manchikanti L., Hirsch J.A. An update on the management of chronic lumbar discogenic pain. Pain Manag. 2015 Sep;5(5):373–386. doi: 10.2217/PMT.15.33. [DOI] [PubMed] [Google Scholar]
- 24.Phillips F.M., Slosar P.J., Youssef J.A., Andersson G., Papatheofanis F. Lumbar spine fusion for chronic low back pain due to degenerative disc disease: a systematic review. Spine. 2013 Apr 1;38(7):E409–E422. doi: 10.1097/BRS.0b013e3182877f11. [DOI] [PubMed] [Google Scholar]
- 25.Thavaneswaran P., Vandepeer M. Lumbar artificial intervertebral disc replacement: a systematic review. ANZ J Surg. 2014 Mar;84(3):121–127. doi: 10.1111/ans.12315. [DOI] [PubMed] [Google Scholar]
- 26.Bydon M., De la Garza-Ramos R., Macki M., Baker A., Gokaslan A.K., Bydon A. Lumbar fusion versus nonoperative management for treatment of discogenic low back pain: a systematic review and meta-analysis of randomized controlled trials. J Spinal Disord Tech. 2014 Jul;27(5):297–304. doi: 10.1097/BSD.0000000000000072. [DOI] [PubMed] [Google Scholar]
- 27.Akeda K., Yamada J., Linn E.T., Sudo A., Masuda K. Platelet-rich plasma in the management of chronic low back pain: a critical review. J Pain Res. 2019;12:753–767. doi: 10.2147/JPR.S153085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foster T.E., Puskas B.L., Mandelbaum B.R., Gerhardt M.B., Rodeo S.A. Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med. 2009 Nov;37(11):2259–2272. doi: 10.1177/0363546509349921. [DOI] [PubMed] [Google Scholar]
- 29.Rossi L.A., Piuzzi N.S., Shapiro S.A. Glenohumeral osteoarthritis: the role for orthobiologic therapies: platelet-rich plasma and cell therapies. JBJS Rev. 2020 Feb;8(2) doi: 10.2106/JBJS.RVW.19.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Molloy T., Wang Y., Murrell G. The roles of growth factors in tendon and ligament healing. Sports Med Auckl NZ. 2003;33(5):381–394. doi: 10.2165/00007256-200333050-00004. [DOI] [PubMed] [Google Scholar]
- 31.Schnabel L.V., Mohammed H.O., Miller B.J., McDermott W.G., Jacobson M.S., Santangelo K.S., et al. Platelet rich plasma (PRP) enhances anabolic gene expression patterns in flexor digitorum superficialis tendons. J Orthop Res Off Publ Orthop Res Soc. 2007 Feb;25(2):230–240. doi: 10.1002/jor.20278. [DOI] [PubMed] [Google Scholar]
- 32.Moraes V.Y., Lenza M., Tamaoki M.J., Faloppa F., Belloti J.C. Platelet-rich therapies for musculoskeletal soft tissue injuries. Cochrane Database Syst Rev. 2013 Dec 23;(12):CD010071. doi: 10.1002/14651858.CD010071.pub2. [DOI] [PubMed] [Google Scholar]
- 33.Lyras D.N., Kazakos K., Agrogiannis G., Verettas D., Kokka A., Kiziridis G., et al. Experimental study of tendon healing early phase: is IGF-1 expression influenced by platelet rich plasma gel? Orthop Traumatol Surg Res OTSR. 2010 Jun;96(4):381–387. doi: 10.1016/j.otsr.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 34.Le Thua T.H., Thua T.H.L., Nguyen D.-T., Pham D.N., Le K.-L., Le Q.N.B., et al. Mini-invasive treatment for delayed or non-union: the use of percutaneous autologous bone marrow injection. Biomed Res Ther. 2015 Nov 24;2(11):389–395. [Google Scholar]
- 35.Taylor D.W., Petrera M., Hendry M., Theodoropoulos J.S. A systematic review of the use of platelet-rich plasma in sports medicine as a new treatment for tendon and ligament injuries. Clin J Sport Med Off J Can Acad Sport Med. 2011 Jul;21(4):344–352. doi: 10.1097/JSM.0b013e31821d0f65. [DOI] [PubMed] [Google Scholar]
- 36.Everts P.A.M., Devilee R.J.J., Oosterbos C.J.M., Mahoney C.B., Schattenkerk M.E., Knape J.T.A., et al. Autologous platelet gel and fibrin sealant enhance the efficacy of total knee arthroplasty: improved range of motion, decreased length of stay and a reduced incidence of arthrofibrosis. Knee Surg Sports Traumatol Arthrosc. 2007 Jul;15(7):888–894. doi: 10.1007/s00167-007-0296-x. [DOI] [PubMed] [Google Scholar]
- 37.Everts P.A.M., Knape J.T.A., Weibrich G., Schönberger J.P.A.M., Hoffmann J., Overdevest E.P., et al. Platelet-rich plasma and platelet gel: a review. J Extra Corpor Technol. 2006 Jun;38(2):174–187. [PMC free article] [PubMed] [Google Scholar]
- 38.Hechtman K.S., Uribe J.W., Botto-vanDemden A., Kiebzak G.M. Platelet-rich plasma injection reduces pain in patients with recalcitrant epicondylitis. Orthopedics. 2011 Jan 1;34(2):92. doi: 10.3928/01477447-20101221-05. [DOI] [PubMed] [Google Scholar]
- 39.Ali M., Mohamed A., Ahmed H.E., Malviya A., Atchia I. The use of ultrasound-guided platelet-rich plasma injections in the treatment of hip osteoarthritis: a systematic review of the literature. J Ultrason. 2018;18(75):332–337. doi: 10.15557/JoU.2018.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sánchez M., Anitua E., Delgado D., Sánchez P., Orive G., Padilla S. Muscle repair: platelet-rich plasma derivates as a bridge from spontaneity to intervention. Injury. 2014 Oct;45(Suppl 4):S7–S14. doi: 10.1016/S0020-1383(14)70004-X. [DOI] [PubMed] [Google Scholar]
- 41.Bielecki T., Gazdzik T.S., Szczepanski T. Benefit of percutaneous injection of autologous platelet-leukocyte-rich gel in patients with delayed union and nonunion. Eur Surg Res Eur Chir Forsch Rech Chir Eur. 2008;40(3):289–296. doi: 10.1159/000114967. [DOI] [PubMed] [Google Scholar]
- 42.Vogrin M., Rupreht M., Dinevski D., Hašpl M., Kuhta M., Jevsek M., et al. Effects of a platelet gel on early graft revascularization after anterior cruciate ligament reconstruction: a prospective, randomized, double-blind, clinical trial. Eur Surg Res Eur Chir Forsch Rech Chir Eur. 2010;45(2):77–85. doi: 10.1159/000318597. [DOI] [PubMed] [Google Scholar]
- 43.Edwards S.G., Calandruccio J.H. Autologous blood injections for refractory lateral epicondylitis. J Hand Surg. 2003 Mar;28(2):272–278. doi: 10.1053/jhsu.2003.50041. [DOI] [PubMed] [Google Scholar]
- 44.Kunze K.N., Hannon C.P., Fialkoff J.D., Frank R.M., Cole B.J. Platelet-rich plasma for muscle injuries: a systematic review of the basic science literature. World J Orthoped. 2019 Jul 18;10(7):278–291. doi: 10.5312/wjo.v10.i7.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Amable P.R., Carias R.B.V., Teixeira M.V.T., da Cruz Pacheco I., Corrêa do Amaral R.J.F., Granjeiro J.M., et al. Platelet-rich plasma preparation for regenerative medicine: optimization and quantification of cytokines and growth factors. Stem Cell Res Ther. 2013 Jun 7;4(3):67. doi: 10.1186/scrt218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Passaretti F., Tia M., D'Esposito V., De Pascale M., Del Corso M., Sepulveres R., et al. Growth-promoting action and growth factor release by different platelet derivatives. Platelets. 2014;25(4):252–256. doi: 10.3109/09537104.2013.809060. [DOI] [PubMed] [Google Scholar]
- 47.Tuakli-Wosornu Y.A., Terry A., Boachie-Adjei K., Harrison J.R., Gribbin C.K., LaSalle E.E., et al. Lumbar intradiskal platelet-rich plasma (PRP) injections: a prospective, double-blind, randomized controlled study. Pharm Manag PM R. 2016 Jan;8(1):1–10. doi: 10.1016/j.pmrj.2015.08.010. quiz 10. [DOI] [PubMed] [Google Scholar]
- 48.Beatty N.R., Lutz C., Boachie-Adjei K., Leynes T.A., Lutz C., Lutz G. Spondylodiscitis due to Cutibacterium acnes following lumbosacral intradiscal biologic therapy: a case report. Regen Med. 2019;14(9):823–829. doi: 10.2217/rme-2019-0008. [DOI] [PubMed] [Google Scholar]
- 49.Dohan Ehrenfest D.M., Rasmusson L., Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF) Trends Biotechnol. 2009 Mar 1;27(3):158–167. doi: 10.1016/j.tibtech.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 50.Childs J.D., Piva S.R., Fritz J.M. Responsiveness of the numeric pain rating scale in patients with low back pain. Spine. 2005 Jun 1;30(11):1331–1334. doi: 10.1097/01.brs.0000164099.92112.29. [DOI] [PubMed] [Google Scholar]
- 51.Roland M., Fairbank J. The roland-morris disability questionnaire and the oswestry disability questionnaire. Spine. 2000 Dec 15;25(24):3115–3124. doi: 10.1097/00007632-200012150-00006. [DOI] [PubMed] [Google Scholar]
- 52.Chiarotto A., Boers M., Deyo R.A., Buchbinder R., Corbin T.P., Costa L.O.P., et al. Core outcome measurement instruments for clinical trials in nonspecific low back pain. Pain. 2018;159(3):481–495. doi: 10.1097/j.pain.0000000000001117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Benner A., Machin David, Campbell Michael J., Fayers Peter M., Pinol Alain P.Y. second ed. Blackwell Science Ltd; 1999. Sample size tables for clinical studies; pp. 494–495. Oxford, 1997. No. of pages: x+315. Price: £45. ISBN 0-86542-870-0. Stat Med. [Google Scholar]
- 54.Zar J.H. second ed. Prentice-Hall; Englewood Cliffs, N.J: 1984. Biostatistical analysis; p. 718. [Google Scholar]
- 55.Navani A., Manchikanti L., Albers S.L., Latchaw R.E., Sanapati J., Kaye A.D., et al. Responsible, safe, and effective use of biologics in the management of low back pain: American society of interventional pain physicians (ASIPP) guidelines. Pain Physician. 2019;22(1S):S1–S74. [PubMed] [Google Scholar]
- 56.Kallewaard J.W., Wintraecken V.M., Geurts J.W., Willems P.C., van Santbrink H., Terwiel C.T.M., et al. A multicenter randomized controlled trial on the efficacy of intradiscal methylene blue injection for chronic discogenic low back pain: the IMBI study. Pain. 2019 Apr;160(4):945–953. doi: 10.1097/j.pain.0000000000001475. [DOI] [PubMed] [Google Scholar]
- 57.Helm S., Ii, Simopoulos T.T., Stojanovic M., Abdi S., El Terany M.A. Effectiveness of thermal annular procedures in treating discogenic low back pain. Pain Physician. 2017;20(6):447–470. [PubMed] [Google Scholar]
- 58.Helm S., Hayek S.M., Benyamin R.M., Manchikanti L. Systematic review of the effectiveness of thermal annular procedures in treating discogenic low back pain. Pain Physician. 2009 Feb;12(1):207–232. [PubMed] [Google Scholar]
- 59.Amable P.R., Carias R.B.V., Teixeira M.V.T., da Cruz Pacheco I., Corrêa do Amaral R.J.F., Granjeiro J.M., et al. Platelet-rich plasma preparation for regenerative medicine: optimization and quantification of cytokines and growth factors. Stem Cell Res Ther. 2013 Jun 7;4(3):67. doi: 10.1186/scrt218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.DeLong J.M., Russell R.P., Mazzocca A.D. Platelet-rich plasma: the PAW classification system. Arthrosc J Arthrosc Relat Surg Off Publ Arthrosc Assoc N Am Int Arthrosc Assoc. 2012 Jul;28(7):998–1009. doi: 10.1016/j.arthro.2012.04.148. [DOI] [PubMed] [Google Scholar]
- 61.Lubkowska A., et al. Growth factor content in PRP and their applicability in medicine. https://www.ncbi.nlm.nih.gov/pubmed/23648195 PubMed - NCBI [Internet]. [cited 2020 Apr 24]. Available from: [PubMed]
- 62.Harrison P. The use of platelets in regenerative medicine and proposal for a new classification system: guidance from the SSC of the ISTH. J Thromb Haemostasis. 2018;16(9):1895–1900. doi: 10.1111/jth.14223. [DOI] [PubMed] [Google Scholar]
- 63.LaPrade R.F., Dragoo J.L., Koh J.L., Murray I.R., Geeslin A.G., Chu C.R. AAOS research symposium updates and consensus: biologic treatment of orthopaedic injuries. J Am Acad Orthop Surg. 2016 Jul;24(7):e62–78. doi: 10.5435/JAAOS-D-16-00086. [DOI] [PMC free article] [PubMed] [Google Scholar]