Abstract
Objective
Lumbar medial branch denervation is commonly used to treat chronic facetogenic low back pain. Controversy exists regarding risk to adjacent neural structures. The objectives of this cadaveric study were to: (1) dissect, digitize, and model in 3D the branches of the first (L1) to fifth (L5) lumbar dorsal rami located near the junction of the transverse process and lateral neck of the superior articular process; and (2) quantify the minimal distance between the lateral/intermediate and medial branches at the anterior quarter and midpoint of the lateral neck of the superior articular process.
Design
Eighteen formalin-embalmed specimens were dissected, digitized and modeled in 3D. The high-fidelity 3D models were used to compare branching patterns and quantify the mean minimal distance between the lateral/intermediate and medial branches of the lumbar dorsal ramus at the anterior quarter and midpoint of the lateral neck of the superior articular process. A Two-way ANOVA was performed to determine if difference of mean distances was significant.
Results
There was variability in the branching pattern of the lumbar dorsal rami. In 46 cases (51.1%) the lumbar dorsal ramus divided into 2 branches, in 41 cases (45.6%) into 3, and in 3 cases (3.3%) 4. The mean minimal distance between the lateral/intermediate and medial branches was significantly greater at the midpoint (3.2 ± 2.5 mm) than the anterior quarter (1.2 ± 1.8 mm) of the lateral neck of superior articular process.
Conclusion
Minimal distance measurements between the branches of the lumbar dorsal rami at the anterior quarter and midpoint of the lateral neck of the superior articular process were computed. When placing the distal end of the needle tip at the anterior quarter of the lateral neck of the superior articular process, the smaller mean minimal distance between the branches suggests there is a greater risk for inadvertent denervation of the lateral/intermediate branches. Further anatomical and clinical investigations are required.
Keywords: Radiofrequency ablation, Lumbar, 3D modelling, Medial branch, Anatomy
1. Introduction
Lumbar medial branch denervation is a common treatment for chronic facetogenic low back pain [1]. Traditionally, when using a conventional radiofrequency ablation (RFA) electrode, a parallel technique is recommended where the long axis of the exposed needle tip is aligned with the length of the medial branch [[2], [3], [4]]. This parallel technique targets the medial branch as it courses along the middle two quarters of the lateral neck of the superior articular process (SAP) and requires placement of the distal end of the electrode tip at the anterior quarter of the SAP as seen on a lateral radiograph [5]. This placement, previously described by Lau et al. as the “deep position”, results in the distal end of the electrode tip contacting the origin of the medial branch [5].
In a more recent anatomical study, the relationship of the lumbar medial branches to bony and soft tissue landmarks was investigated [6]. Consistent with Lau et al. [5], it was suggested that when using the “deep position”, there is a greater risk of denervating the lateral/intermediate branches of the lumbar dorsal ramus or the ramus itself [6]. To mitigate risk to the other branches that do not innervate the facet joints, it was suggested that the needle tip could be placed at the midpoint of the lateral neck of the SAP (described by Lau et al. as the “withdraw position”). However, in a recent letter to editor, Bryant et al. stated “… the risk of inadvertent dorsal ramus or intermediate branch lesioning using the ‘traditional’ approach targeting the mid-SAP remains unlikely. The illustrations do not reflect the full three dimensions of the relevant anatomy: the dorsal ramus is generally found several millimeters superior to the ventral transverse process and in the neural foramen” [7].
No previous studies were found that quantified and analyzed distances between the branches of the lumbar dorsal ramus at the anterior quarter and midpoint of the lateral neck of the SAP in three dimensions. Therefore, the objectives of this cadaveric study were to: 1) dissect, digitize, and model in 3D the branches of the first (L1) to fifth (L5) lumbar dorsal rami located near the junction of the transverse process and lateral neck of the SAP; and 2) quantify the minimal distance between the lateral/intermediate and medial branches at the anterior quarter and midpoint of the lateral neck of the SAP.
2. Material and methods
2.1. Cadaveric specimens
Eighteen formalin-embalmed specimens with mean age of 75.4 ± 13.8 years (8 females/10 males) were used in this study. No other demographic data were available. Specimens with visible signs of trauma or previous spine surgery were excluded. A total of 90 lumbar dorsal rami were dissected, digitized, and modeled in 3D. Ethics approval was received from the University of Toronto Health Sciences Research Ethics Board (protocol #27210). The anatomical relationship of the lumbar dorsal rami to surrounding bony and soft tissue landmarks was documented with photographs.
2.2. Dissection, digitization, and 3D modeling protocol
In each specimen, the skin and fascia of the low back were removed to expose the underlying latissimus dorsi. Next, the erector spinae muscle group was exposed and the fiber bundles of the longissimus thoracis pars lumborum were carefully removed to expose the lateral and intermediate branches of the lumbar dorsal rami (L1-L5). The lateral and intermediate branches were meticulously dissected proximally towards the intervertebral foramen to locate the origin of the medial branch of the lumbar dorsal ramus. Next, each articular branch originating from the medial branch was dissected distally to its termination in the lumbar facet joint capsules. Following dissection, the lumbar dorsal rami and their branches, along with bony surfaces, were digitized using a Microscribe G2X Digitizer (Immersion Corporation, San Jose, CA, USA; accuracy ±0.23 mm). The diameter of each branch of the lumbar dorsal rami (located at the junction of the transverse process and lateral neck of SAP) was also digitized for subsequent volumetric reconstruction. Following digitization, the vertebral column and sacrum, in each specimen, were skeletonized leaving the capsule of the facet joints and vertebral ligaments intact. The skeletonized specimens were scanned using a Faro Laser ScanArm (FARO Technologies, Lake Mary, Florida, USA; accuracy ±35 μm) to create a high-resolution surface scan. The digitized nerves and bony surfaces were registered with the scanned surface data using Blender3D (Blender Foundation, Amsterdam, NL) to generate high-fidelity 3D models as in situ. The branches of the lumbar dorsal rami were volumetrically reconstructed as cylinder tubes with diameters of the digitized thickness of each nerve. The course of the lumbar dorsal rami and their branches were documented relative to anatomical landmarks and their spatial relationship compared between specimens.
2.3. Distance measurement protocol
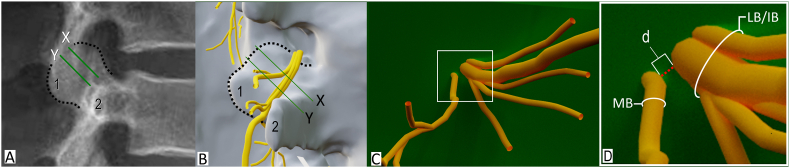
To standardize the distance measurement between branches of the lumbar dorsal ramus, two planes were digitally created and positioned at the anterior quarter and midpoint of the lateral neck of SAP (as seen on direct lateral radiographs) at the L1-L5 spinal levels (Fig. 1A). Using Blender3D's built-in measurement tool, the minimal distance between the edge of the lateral/intermediate branch and the edge of the medial branch was measured at the anterior quarter and midpoint of the lateral neck of SAP on the high-fidelity 3D models (Fig. 1B).
Fig. 1.
Methodology. A. Lateral radiograph with planes positioned at the anterior quarter (X) and midpoint of the lateral neck of the superior articular process (Y). B. Direct lateral view of 3D model with planes positioned at the anterior quarter and midpoint. C. Posterior view of lumbar dorsal ramus. D. Inset of white box in C showing minimal distance (d) between the medial and lateral/intermediate branches of lumbar dorsal ramus. Dotted line indicates outline of superior articular process; MB, medial branch; LB/IB, lateral and intermediate branches; 1, mamillary process; 2, transverse process.
2.4. Data and statistical analysis
The mean minimal distance between the lateral/intermediate and medial branches of the L1 to L5 dorsal rami at the anterior quarter and midpoint of the lateral neck of SAP were computed and compared. Differences between mean distances at each vertebral level were analyzed using a two-way ANOVA with subsequent post-hoc Tukey HSD analysis. Statistical analysis was performed using SPSS v28.0.1.0.
3. Results
In total 90 lumbar dorsal rami were dissected, digitized, and modeled in 3D (Fig. 2, Fig. 3). The innervation patterns and distance measurements between the lateral/intermediate and medial branches are described below.
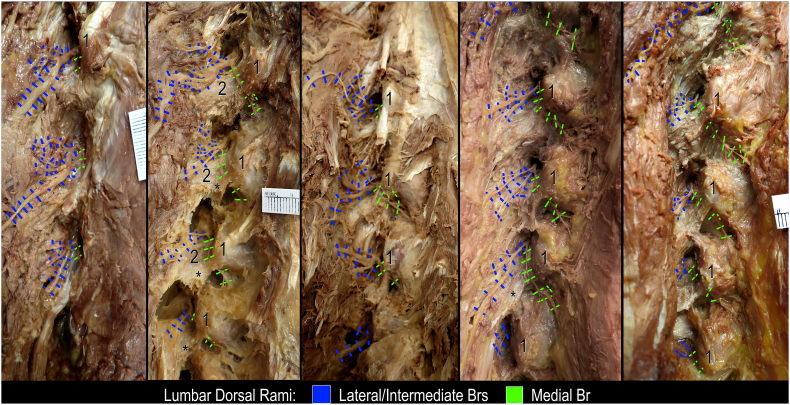
Fig. 2.
Dissection of the lumbar dorsal rami in five different specimens, left posterolateral views. Asterisks indicate accessory process; 1, mamillary process; 2, transverse process (base).
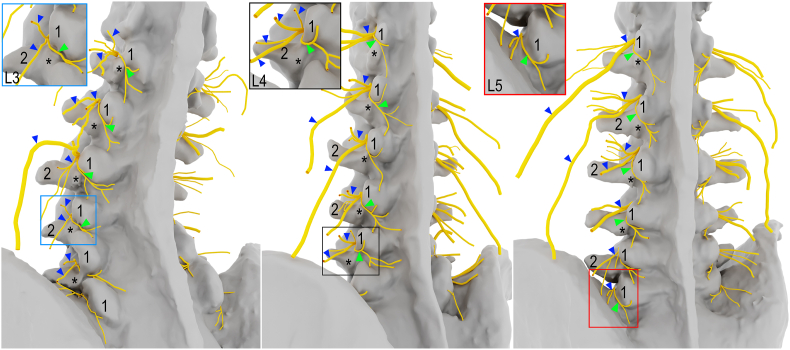
Fig. 3.
High-fidelity 3D modeling of the lumbar dorsal rami reconstructed from cadaveric dissections, left posterolateral views. Asterisks indicate accessory process; Arrowheads (blue), lateral/intermediate branches; Arrowheads (green), medial branches; 1, mamillary process; 2, transverse process.
3.1. Innervation pattern
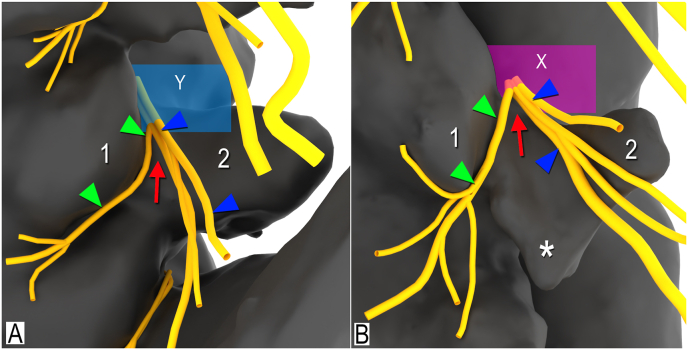
There was variability in the branching pattern of the lumbar dorsal rami (Fig. 4). In 46 cases (51.1%) the lumbar dorsal ramus divided into 2 branches, 41 cases (45.6%) into 3, and in 3 cases (3.3%) 4 (Fig. 4). At the anterior quarter of the lateral neck of SAP the lumbar dorsal rami were found to remain unbranched in 41 cases (45.6%) whereas at the midpoint of the lateral neck of the SAP only 3 cases (3.3%) had not yet ramified (Fig. 5). In all specimens, the medial branch of the lumbar dorsal ramus coursed along the lateral neck of the SAP. At the mamilloaccessory notch, the nerve coursed posteromedial and continued through the substance of multifidus while supplying articular branches to the adjacent and inferior facet joints. The lateral/intermediate branches were found to course in a posterolateral direction and nerves further divided into several branches that coursed superior and inferior through the substance of the longissimus thoracis pars lumborum. The lateral branches further extended distally to the iliocostalis.
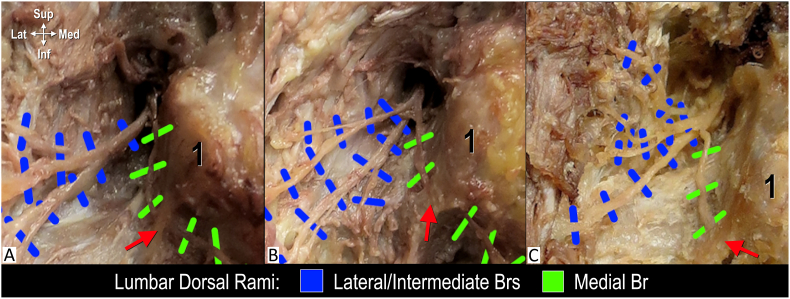
Fig. 4.
Different branching patterns of the lumbar dorsal ramus, posterolateral views. A. Two branches. B. Three branches. C. Four Branches. Red arrow indicates mamilloaccessory ligament (note its position at the posterior margin of the lateral neck of superior articular process); 1, mamillary process.
Fig. 5.
3D modelling of the branching patterns of the lumbar dorsal ramus, right posterolateral views. A. Branching of lumbar dorsal ramus at midpoint of lateral neck of superior articular process (Y, magenta plane). B. Branching at anterior quarter of lateral neck of superior articular process (X, blue plane). Asterisks indicate accessory process; Arrow (red), branching of medial branch from lateral/intermediate branches; Arrowheads (blue), lateral/intermediate branches; Arrowheads (green), medial branches; 1, mamillary process; 2, transverse process.
3.2. Mean distance measurement
There was variation in the minimal distance between the lateral/intermediate and medial branches of the lumbar dorsal rami. The minimal distances are summarized in Table 1. The mean minimal distance between the lateral/intermediate and medial branches was significantly greater at the midpoint (3.2 ± 2.5 mm) than the anterior quarter (1.2 ± 1.8 mm) of the lateral neck of SAP. A two-way ANOVA was performed to analyze the effect of location on the lateral neck of the SAP (anterior quarter or midpoint) and vertebral level (L1-L5) on the distance between the lateral/intermediate and medial branches of the lumbar dorsal rami. The analysis revealed that there was not a statistically significant interaction between the effects of location on lateral neck of the SAP and vertebral level (F(4, 170) = 0.413, p = 0.799). Simple main effects analysis showed that location on the lateral neck of the SAP did have a statistically significant effect on distance between the lateral/intermediate and medial branches of the lumbar dorsal rami (p < 0.001). Simple main effects analysis showed that vertebral level did not have a statistically significant effect on distance between the lateral/intermediate and medial branches of the lumbar dorsal rami (p = 0.528).
Table 1.
Minimal distance (mm) between lateral/intermediate and medial branches of lumbar dorsal ramus.
| SP 1 | SP 2 | SP 3 | SP 4 | SP 5 | SP 6 | SP 7 | SP 8 | SP 9 | SP 10 | SP 11 | SP 12 | SP 13 | SP 14 | SP 15 | SP 16 | SP 17 | SP 18 | Mean ± SD | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anterior quarter of lateral neck of SAP | 1.2 ± 1.8 | ||||||||||||||||||
| L1 | X | X | X | 2.6 | X | X | X | X | 0.8 | 3.3 | X | X | 0.1 | X | 0.7 | 0.5 | X | 2.8 | 0.6 ± 1.1 |
| L2 | X | 1.7 | 1.7 | 3.7 | X | X | X | X | X | 1.0 | X | X | X | 2.5 | 1.5 | X | 0.2 | 3.8 | 0.9 ± 1.3 |
| L3 | X | 1.7 | 1.8 | 0.9 | 0.4 | X | X | X | X | 2.1 | X | 1.9 | 1.2 | 1.2 | 2.7 | X | 1.8 | 1.4 | 1.4 ± 0.9 |
| L4 | X | 0.8 | X | 1.9 | 11.6 | X | 2.3 | X | X | X | 1.8 | 1.5 | 1.6 | X | X | X | X | 1.5 | 1.3 ± 2.7 |
| L5 | 2.3 | X | X | 0.8 | 4.5 | 0.9 | x | 6.5 | X | X | 0.6 | 7.2 | 1.4 | 3.0 | 3.1 | X | 0.9 | 3.8 | 2.0 ± 2.3 |
| Midpoint of lateral neck of SAP | 3.2 ± 2.5 | ||||||||||||||||||
| L1 | 1.3 | 2.4 | 2.3 | 5.4 | 2.6 | 2.2 | 0.6 | 0.9 | 3.2 | 4.9 | 2.2 | 2.2 | 2.8 | 1.0 | 3.8 | 3.9 | 2.7 | 7.9 | 2.9 ± 1.8 |
| L2 | 2.7 | 4.0 | 5.1 | 5.8 | 1.4 | 1.0 | 0.5 | 0.5 | 2.3 | 3.4 | 0.8 | 1.8 | 2.5 | 3.1 | 4.5 | 1.8 | 4.4 | 5.7 | 2.9 ± 1.7 |
| L3 | 4.4 | 3.3 | 5.1 | 3.9 | 2.3 | 0.6 | 1.5 | 0.7 | 1.6 | 6.1 | 2.9 | 3.2 | 2.3 | 3.3 | 7.4 | 2.6 | 6.6 | 4.9 | 3.5 ± 2.0 |
| L4 | X | 2.4 | 2.8 | 2.6 | 15.9 | 1.3 | 3.6 | 1.3 | X | 0.8 | 2.2 | 6.9 | 7.0 | 1.4 | 3.1 | 1.0 | 1.6 | 3.6 | 3.2 ± 3.7 |
| L5 | 2.0 | 2.3 | 0.6 | 0.9 | 6.3 | 0.7 | 2.5 | 10.6 | X | 1.0 | 1.4 | 8.7 | 5.0 | 4.9 | 4.1 | 1.4 | 1.4 | 4.3 | 3.2 ± 3.0 |
X, lumbar dorsal ramus has not divided; SAP, superior articular process.
4. Discussion
In the current anatomical study, the course of the branches of the lumbar dorsal rami was documented in 3D relative to the lateral neck of the SAP. The 3D analysis of the anatomical relationship between the branches of the lumbar dorsal rami (i.e. mean minimal distance) enables assessment of which nerves are potentially captured during lumbar medial branch denervation.
Previous anatomical studies have described the anatomy of the lumbar dorsal rami dividing into 2 or 3 branches [5,[8], [9], [10]]. Early literature published by Bogduk et al. described the lumbar dorsal rami dividing into lateral and medial branches “5 mm from the origin of the dorsal ramus” [8]. More recent literature documented the division of the lumbar dorsal ramus into 3 branches [5,10]. Lau et al. found that the “intermediate and lateral branches arise from the dorsal ramus at the same point as the medial branch” [5]. Similarly, Saito et al. reported trifurcation of the first to fifth lumbar dorsal rami stating “triplication also at L5 after our sequential dissection” [10]. In the current study the anatomical findings are consistent with the previous literature. Although the branching patterns were variable, the lumbar dorsal rami were found to divide into either 2–3 main branches, as previously described [5,[8], [9], [10]], and on rare occasions into 4 branches (3.3%). The main branches further arborize into numerous branches that course through the longissimus thoracis pars lumborum.
The findings of the current study provide quantitative evidence that the branches of the lumbar dorsal ramus are closer together at the anterior quarter of the lateral neck of the SAP as compared to the midpoint. Furthermore, in almost half of the specimens examined, the dorsal ramus had not yet divided into medial and lateral/intermediate branches at the anterior quarter. Therefore, when placing a conventional RF cannula along (i.e. parallel) to the medial branch, advancement of the distal end of the conventional needle tip to the anterior quarter of the lateral neck of the SAP will likely result in greater risk of an RF lesion encroaching upon and capturing the lateral/intermediate branches (or the dorsal ramus proper if it has not yet divided) as compared to placement at the midpoint of the SAP. In a recent case study, it was reported that the use of the traditional RFA technique to denervate the L3-L5 medial branches resulted in adverse effects where “the patient complained of pain and numbness on the left buttock and posterolateral thigh, with associated swelling” [11]. Previous anatomical literature have described that the superior cluneal nerves are formed from the lateral branches of the lumbar dorsal rami [12]. The area supplied by the superior cluneal nerves overlaps with the region of numbness described in the case report [11]. Therefore, a potential anatomical explanation for the adverse effects is the inadvertent denervation of the lateral branches of the lumbar dorsal rami.
Although the incidence is rare, adverse effects related to the traditional RFA technique are anatomically possible and have been clinically reported [11]. There are two potential explanations for this low incidence rate. The first postulation is related to the anatomy of the lumbar dorsal rami. In the current study, the branching pattern of the lumbar dorsal rami was found to be variable. The number of branches varied from 2 to 4 when examined at the lateral neck of the SAP. Potentially, partial denervation of the lateral/intermediate branches does occur but may be insufficient to manifest as adverse effects in the majority of patients. Additionally, anatomical variation in the spinal contribution to the superior cluneal nerve may factor in the low incidence rate. Previous anatomical studies have described variable spinal contributions to the superior cluneal nerve originating from L1-L5 [12] and only L1-L3 [13]. Therefore, denervation at lower lumbar levels may not present clinically as adverse effects. The second postulation is related to needle placement angulation. In a recent letter to the editor, Bryant et al. stated “(the) dorsal ramus is generally found several millimeters superior to the ventral transverse process”. This anatomy is consistent with the findings in the current study. Bryant et al. further suggested the risk of denervation of the dorsal ramus is “unlikely” when using the traditional RFA technique [7]. This statement is supported by recent anatomical literature that suggested the caudal angulation used clinically is not sufficient to achieve true parallel placement along the medial branch [6,14,15]. As a consequence, the distal end of the conventional needle tip would be placed inferior to the lumbar dorsal ramus and/or its lateral and intermediate branches such that denervation of these structures would not occur. However, insufficient caudal angulation of the traditional RFA technique may not optimize clinical outcomes, as it is commonly assumed that the length of the medial branch being denervated is associated with the duration of pain relief [[2], [3], [4]]. If greater caudal angulation is used in order to potentially maximize the length of the medial branch captured, interventionalists should be aware that this may result in positioning the distal end of the RF needle tip closer to the lateral/intermediate branches. This may lead to inadvertent denervation and potentially increase the incidence of adverse effects. Pending future anatomical and clinical validation research, spine pain interventionalists should be cognizant that placement at the anterior quarter of the lateral neck of the SAP has a higher chance of inadvertent denervation of the lateral/intermediate branches.
More recently, non-parallel multi-tined cannula approaches have been clinically used and have shown comparable improvements in pain, disability, and quality of life to parallel approaches [16]. Similar to the parallel technique, targeting the posterior half of the lateral neck of the SAP may be optimal to mitigate risk of denervating the lateral/intermediate branches of the lumbar dorsal ramus and/or the dorsal ramus proper when performing a non-parallel approach. This is based on the results of the current study, which found the distance between the intermediate/lateral and medial branches is less at the anterior quarter of the lateral neck of the SAP than the midpoint. Early clinical evidence supports the feasibility of using the conventional parallel technique to target the posterior half of the lateral neck of the SAP [17]. Further anatomical and clinical investigations are required to assess the effectiveness of expanded lesions at the posterior half of the lateral neck of the SAP.
Limitations in the current study include a small sample size and measurement of only the minimal distance between the branches of the lumbar dorsal ramus. Due to the laborious and time consuming process of cadaveric dissection, large scale anatomical research is not feasible. However, our sample size is greater than the recommended 12 per group when conducting a pilot study with no previous data [18]. Additionally, the assessment of risk of inadvertent denervation was based on the minimal distance between the medial branch and the lateral or intermediate branch of the lumbar dorsal ramus. Due to the variation in the branching of the lumbar dorsal ramus, not all distances between the arborizations of the lateral/intermediate branches were measured relative to the medial branch. Rather, only the closest distance was measured and used in the assessment. Consequently, the clinical implications and postulations discussed in the current anatomical study require further in vivo validation research.
5. Conclusions
In this anatomical study the course of the lateral, intermediate and medial branches of the lumbar dorsal rami were documented in 3D. Minimal distance measurements between the branches of the lumbar dorsal rami at the anterior quarter and midpoint of the lateral neck of the SAP were computed. When placing the distal end of the needle tip at the anterior quarter of the lateral neck of the SAP, the smaller mean minimal distance between the branches suggests there is a greater risk for inadvertent denervation of the lateral/intermediate branches. Further, as almost half of the lumbar dorsal rami in our sample had not yet ramified at the anterior quarter, there is also greater risk of denervation of the lumbar dorsal ramus proper. Regardless of the RFA technique utilized, positioning the distal end of the needle tip at the midpoint of the lateral neck should be considered. Further anatomical and clinical investigations are required.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Eldon Loh reports financial support was provided by International Pain and Spine Intervention Society. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors wish to thank the individuals who donated their bodies and tissues for the advancement of education and research. This study was funded by the International Pain and Spine Intervention Society Research Grant awarded to EL & JT.
Contributor Information
John Tran, Email: johnjt.tran@utoronto.ca.
Emma Campisi, Email: emma.campisi@mail.utoronto.ca.
Alexandria Roa Agudelo, Email: alexandria.roaagudelo@sjhc.london.on.ca.
Anne MR. Agur, Email: anne.agur@utoronto.ca.
Eldon Loh, Email: eldon.loh@sjhc.london.on.ca.
References
- 1.Manchikanti L., Sanapati M.R., Pampati V., et al. Update of utilization patterns of facet joint interventions in managing spinal pain from 2000 to 2018 in the US fee-for-service Medicare population. Pain Physician. 2020;23:E133–E149. [PubMed] [Google Scholar]
- 2.Bogduk N. Lumbar radiofrequency neurotomy. Clin J Pain. 2006;22(4):409. doi: 10.1097/01.ajp.0000182845.55330.9f. [DOI] [PubMed] [Google Scholar]
- 3.Loh J.T., Nicol A.L., Elashoff D., Ferrante F.M. Efficacy of needleplacement technique in radiofrequency ablation for treatment of lumbar facet arthropathy. J Pain Res. 2015;8:687–694. doi: 10.2147/JPR.S84913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schneider B.J., Doan L., Maes M.K., et al. Standards Division of the Spine Intervention Society. Systematic review of the effectiveness of lumbar medial branch thermal radiofrequency neurotomy, stratified for diagnostic methods and procedural technique. Pain Med. 2020;21(6):1122–1141. doi: 10.1093/pm/pnz349. [DOI] [PubMed] [Google Scholar]
- 5.Lau P., Mercer S., Govind J., et al. The surgical anatomy of lumbar medial branch neurotomy (facet denervation) Pain Med. 2004;5(3):289–298. doi: 10.1111/j.1526-4637.2004.04042.x. [DOI] [PubMed] [Google Scholar]
- 6.Tran J., Peng P., Loh E. Anatomical study of the medial branches of the lumbar dorsal rami: implications for image-guided intervention. Reg Anesth Pain Med. 2022;47:464–474. doi: 10.1136/rapm-2022-103653. [DOI] [PubMed] [Google Scholar]
- 7.Bryant D.G., Dovgan J.T., Hunt C., Beckworth W.J., Waring P.H., Rivers W.E. Letter to the editor regarding 'Anatomical study of the medial branches of the lumbar dorsal rami: implications for image-guided intervention'. Reg Anesth Pain Med. 2023;48(2):94. doi: 10.1136/rapm-2022-104119. [DOI] [PubMed] [Google Scholar]
- 8.Bogduk N., Long D.M. The anatomy of the so-called “articular nerves” and their relationship to facet denervation in the treatment of low-back pain. J Neurosurg. 1979;51(2):172–177. doi: 10.3171/jns.1979.51.2.0172. [DOI] [PubMed] [Google Scholar]
- 9.Bogduk N., Long D.M. Percutaneous lumbar medial branch neurotomy: a modification of facet denervation. Spine. 1980;5(2):193–200. doi: 10.1097/00007632-198003000-00015. (Phila Pa 1976) [DOI] [PubMed] [Google Scholar]
- 10.Saito T., Steinke H., Miyaki T., et al. Analysis of the posterior ramus of the lumbar spinal nerve: the structure of the posterior ramus of the spinal nerve. Anesthesiology. 2013;118(1):88–94. doi: 10.1097/ALN.0b013e318272f40a. [DOI] [PubMed] [Google Scholar]
- 11.Reddy A.T., Goyal N., Cascio M., Leal J., Singh K. Abnormal paresthesias associated with radiofrequency ablation of lumbar medial branch nerves: a case report. Cureus. 2023;15(2) doi: 10.7759/cureus.35176. 2023 Feb 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Konno T., Aota Y., Kuniya H., et al. Anatomical etiology of "pseudo-sciatica" from superior cluneal nerve entrapment: a laboratory investigation. J Pain Res. 2017;10:2539–2545. doi: 10.2147/JPR.S142115. Published 2017 Nov 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tubbs R.S., Levin M.R., Loukas M., Potts E.A., Cohen-Gadol A.A. Anatomy and landmarks for the superior and middle cluneal nerves: application to posterior iliac crest harvest and entrapment syndromes: laboratory investigation. J Neurosurg: Spine SPI. 2010;13(3):356–359. doi: 10.3171/2010.3.SPINE09747. [DOI] [PubMed] [Google Scholar]
- 14.Tran J., Loh E. Reply to letter to editor regarding 'anatomical study of the medial branches of the lumbar dorsal rami: implications for image guided intervention'. Reg Anesth Pain Med. 2023;48(2):95–96. doi: 10.1136/rapm-2022-104168. [DOI] [PubMed] [Google Scholar]
- 15.Tran J., Campisi E.S., Agur A.M.R., Loh E. Quantification of needle angles for traditional lumbar medial branch radiofrequency ablation: an osteological study. Pain Med. 2023;24(5):488–495. doi: 10.1093/pm/pnac160. [DOI] [PubMed] [Google Scholar]
- 16.Deng G., Smith A., Burnham R. Prospective within subject comparison of fluoroscopically guided lumbosacral facet joint radiofrequency ablation using a multi-tined (Trident) versus conventional monopolar cannula. Pain Physician. 2022;25(5):391–399. [PubMed] [Google Scholar]
- 17.Tran J., Lawson A., Agur A., Loh E. Parasagittal needle placement approach for lumbar medial branch denervation: a brief technical report. Reg Anesth Pain Med. 2024 doi: 10.1136/rapm-2023-105152. Published Onlione First: 04 January. [DOI] [PubMed] [Google Scholar]
- 18.Julious S.A. Sample size of 12 per group rule of thumb for a pilot study. Pharmaceut Statist. 2005;4:287–291. doi: 10.1002/pst.185. [DOI] [Google Scholar]