Abstract
Objectives
To evaluate the effectiveness of cervical transforaminal epidural steroid injection (CTFESI) for the treatment of unilateral cervical radicular pain.
Design
Single-group prospective cohort study.
Methods
Outcomes included ≥50% reductions in Numeric Rating Scale (NRS) for arm pain, ≥30% Neck Disability Index (NDI-5) improvement, health-related quality of life (EQ-5D), global improvement (PGIC), personal goal achievement (COMBI), Chronic Pain Sleep Index (CPSI), and healthcare utilization at one, three, six, and 12 months. Data analysis included descriptive statistics with the calculations of 95% confidence intervals (CIs), contingency table analysis, and multilevel logistic regression (LR) analysis, including a worst-case (WC) sensitivity analysis in which missing data were treated as treatment failure. Participants who were treated surgically were considered failures in the categorical analyses.
Results
33 consecutively enrolled participants (63.6% females, 51.2 ± 12.2 years of age, BMI 28.3 ± 4.5 kg/m2) were analyzed. Success rates for ≥50% reduction in NRS for arm pain at one, three, six and 12 months were 57.6% (95% CI 40.8–72.8%), 71.9% (95% CI 54.6–84.4%), 64.5% (95% CI 46.9–78.9%), and 64.5% (95% CI 46.9–78.9%). Success rates for ≥30% improvement in NDI-5 were 60.6% (95% CI 43.7–75.3%), 68.8% (95% CI 51.4–82.0%), 61.3% (95% CI 43.8–76.3%), and 71.0% (95% CI 53.4–83.9%). In WC analysis, success rates for ≥50% arm NRS and NDI-5 were 0–4.3% lower between 1 and 12 months. PGIC scores were at least “much improved” or “very much improved,” in 48.4–65.6% of participants between 1 and 12 months. 6.1%, 6.1%, and 3.0% had one, two, or three repeat injections, respectively. 18.2% of participants underwent surgery by 12 months. Participants showed significant improvements in arm NRS and NDI-5 after treatment (p < 0.05), multilevel logistic regression models showed no significant decline in improvements across the follow-up time points (p > 0.05).
Conclusion
Statistically significant and clinically meaningful improvements in pain and disability were observed after CTFESI for up to 12 months in individuals with unilateral cervical radicular pain.
Keywords: Cervical radiculopathy, Transforaminal, Epidural, Pain, Injection
1. Introduction
Cervical radiculopathy is a common source of neck and radiating arm pain. Population-based cohort studies have estimated that the annual prevalence of cervical radiculopathy at 83 per 100,000 individuals, with age-related cervical spondylosis and disc herniation being the most common causes [1]. Although the natural history of cervical radiculopathy is favorable, a significant proportion of patients seek medical care due to severe pain and disability [2]. First-line treatments for cervical radiculopathy include activity modification, physical therapy, and oral analgesics, but where conservative management fails cervical epidural steroid injections (ESI) may be performed to reduce pain and improve function.
Epidural administration of anesthetic and corticosteroid is hypothesized to reduce inflammation, stabilize neural membranes of C-fibers, and reduce nociceptive activity in the dorsal root ganglion [[3], [4], [5]]. Multiple approaches exist for cervical ESI which appear similarly effective [[6], [7], [8], [9]]. Previous systematic reviews have concluded that cervical transforaminal epidural steroid injection (CTFESI) reduces pain and disability associated with cervical radicular pain with single arm meta-analysis demonstrating reduction in pain scores by ≥50% in 48% (95% CI: 34–61%) and 62% (95% CI: 49–75%) of patients at one and three months after CTFESI, respectively [10]. Further, a systematic review published in the Journal of Bone and Joint Surgery concluded that CTFESI reduces pain above the minimally clinical important difference (MCID) for cervical radiculopathy [11]. Few studies have reported outcomes beyond three months, and little is known about the impact of CTFESI on surgical rates.
Given these knowledge gaps, further research is needed to better describe the effectiveness of CTFESI in those with refractory radicular pain. The present study was conceived to measure long-term pain reduction and functional improvement after CTFESI but also quantify effects on quality of life, sleep quality, personal goal achievement, analgesic consumption, healthcare utilization, and surgical rates. This study was funded by a generous grant from the International Pain and Spine Intervention Society (IPSIS).
2. Methods
2.1. Study design and participants
This was a single-center, prospective, observational study evaluating the effectiveness of CTFESI for treating refractory unilateral cervical radicular pain (NCT# 04544683). After IRB approval (IRB# 00116,040), participants were recruited from outpatient Neurosurgery, Orthopedic Surgery, and Physical Medicine and Rehabilitation clinics at the University of Utah. Consecutively identified individuals were screened for eligibility. Primary inclusion criteria were adults with at least 4/10 cervical radicular pain (with arm pain greater than neck pain) for at least six weeks, but not longer than six months secondary to either one-level cervical disc herniation, disc-osteophyte complex, or degenerative foraminal stenosis on MRI. Primary exclusion criteria included BMI >35, active litigation or remuneration related to pain, multilevel unilateral or bilateral radicular symptoms, history of cervical spine surgery, or prior epidural steroid injection for the current episode of pain. Appendix A lists the complete inclusion/exclusion criteria.
2.2. Recruitment, enrollment, data collection
Between March 2019 and May 2022, clinical research coordinators identified potential participants from procedure and clinic schedules. Upon confirmation of eligibility, baseline measurements were recorded and input into a web-based clinical research database. Study data were collected in-person, via internet link, and/or telephone and managed using REDCap (Research Electronic Data Capture) [12], a secure, web-based software platform designed to support data capture for research studies.
2.3. Study intervention
CTFESI was performed by three Physical Medicine and Rehabilitation physicians with subspecialty training in either Pain Medicine or Sports Medicine. Up to three repeat injections were allowed during the study based on the treating physician's discretion.
Participants were positioned supine or lateral recumbent on a fluoroscopy table, and the cervical spine was prepared and draped in a sterile fashion. After injecting 1–2 mL of 1% lidocaine into the superficial tissues, a 25-gauge spinal needle was guided under fluoroscopy to the target position in the neuro-foramen. Once the target position was confirmed with multiplanar fluoroscopy, 0.5–3 mL of contrast was injected under live fluoroscopy with and without digital subtraction imaging. After demonstration of epidural contrast spread without vascular uptake, the injectate was delivered, a mixture consisting of 1 mL of dexamethasone sodium phosphate (10 mg/mL) and 0.5 mL of 1 or 2% preservative-free lidocaine.
Participants who achieved significant pain relief after the initial injection and subsequently experienced a recurrence of their index pain were offered a repeat procedure, at the discretion of the treating physician. “Usual pain” was defined as cervical radicular pain (upper extremity or shoulder girdle/periscapular pain) greater than axial neck pain.
2.4. Outcome measures
This study examined the effectiveness of CTFESI using multiple validated scales considered important in pain research [13]. Pain was measured using the NRS scale for both arm and neck pain. Arm pain was defined and explained to participants as being discomfort experienced in the periscapular, shoulder, upper arm, lower arm, or hand, while neck pain was considered pain localized to the cervical spine. Other measures recorded at baseline and follow-up time points included Neck Disability Index (NDI-5) [14], Patient Global Impression of Change (PGIC) [15], EuroQol Health-related Quality of life (EQ-5D-5L) [16], key activity restoration from the Clinical Outcome Measurement Brief Instrument (COMBI) [17], analgesic use via Medication Quantification Scale (MQS-III) [18], and Chronic Pain Sleep Inventory (CPSI) [19]. The primary outcome was the proportion of participants reporting ≥50% NRS arm pain reduction at one, three, six, and 12 months after CTFESI. Secondary outcomes also measured at these time points included the proportion of participants with a minimally import clinical change (MCIC) of ≥50% NRS neck pain reduction, ≥30% improvement in NDI-5 [20], ≥0.03 change in EQ-5D score [21], ≥6.8 point change (equivalent to 10 oral morphine equivalents) in MQS-III score [17], ≥30% change in CPSI score [19], substantial or complete restoration of at least three of four key activities from COMBI (described as “a lot” or “completely” restored) [17], and global improvement rated as “improved” or “much improved (PGIC scores 6–7). Rates of repeat injection, surgery, and other cervical spine pain-related healthcare utilization were also captured throughout the study.
Additionally, demographic, clinical, radiographic, and psychological variables were captured at baseline (Table 1). Radiographic variables included categorization of the structural reason for radicular pain (i.e., cervical disc herniation, disc-osteophyte, or foraminal stenosis), and quantification of severity of foraminal stenosis according to the Park classification [22]. The Park classification system is as follows: Grade 0, indicating no significant stenosis or perineural fat obliteration; Grade 1 (mild), with less than 50% nerve root circumference involvement of perineural fat and no morphological change in the nerve root; Grade 2 (moderate), with greater than 50% nerve root circumference involvement of perineural fat yet without morphological change; and Grade 3 (severe), characterized by extensive perineural fat obliteration accompanied by morphological collapse of the nerve root.
Table 1.
Demographics and clinical characteristics of patients.
| Variable | Frequency (%) |
|---|---|
| Gender | |
| Male | 12 (36.4) |
| Female | 21 (63.6) |
| Obesity | |
| Yes | 15 (45.5) |
| No | 18 (54.6) |
| History of tobacco use | |
| Yes | 3 (9.1) |
| No | 30 (90.9) |
| Current tobacco use | |
| Yes | 2 (6.1) |
| No | 31 (93.9) |
| Level of radicular pain - C4 | |
| Yes | 0 (0.0) |
| No | 33 (100.0) |
| Level of radicular pain - C5 | |
| Yes | 0 (0.0) |
| No | 33 (100.0) |
| Level of radicular pain - C6 | |
| Yes | 18 (54.6) |
| No | 15 (45.4) |
| Level of radicular pain - C7 | |
| Yes | 14 (42.4) |
| No | 19 (57.6) |
| Level of radicular pain - C8 | |
| Yes | 1 (3.0) |
| No | 32 (97.0) |
| Cervical disc herniation | |
| Yes | 11 (33.3) |
| No | 22 (66.7) |
| Disc-osteophyte complex | |
| Yes | 15 (45.5) |
| No | 18 (54.6) |
| Foraminal stenosis related to boney elements | |
| Yes | 9 (27.3) |
| No | 24 (72.7) |
| Severity of stenosis (Park Classification) | |
| Grade of 0 & 1 | 9 (27.3) |
| Grade of 2 | 7 (21.2) |
| Grade of 3 | 17 (51.5) |
| Spurlings test | |
| Negative | 10 (30.3) |
| Positive | 23 (69.7) |
| Myotomal strength asymmetry | |
| Yes | 4 (12.1) |
| 29 (87.9) | |
| DTR asymmetry on exam | |
| Yes | 2 (6.1) |
| No | 31 (93.9) |
| Duration of pain | |
| 6 weeks to 3 months | 13 (39.4) |
| 3–6 months | 20 (60.6) |
| Description of pain: burning/electric | |
| Yes | 14 (42.4) |
| No | 19 (57.6) |
| Description of pain: aching | |
| Yes | 20 (60.6) |
| No | 13 (39.4) |
| Description of pain: sharp | |
| Yes | 23 (69.7) |
| No | 10 (30.3) |
| Description of pain: other (vs. no) | |
| Yes | 9 (27.3) |
| No | 24 (72.7) |
| Depression | |
| Yes | 9 (27.3) |
| No | 24 (72.7) |
| Anxiety | |
| Yes | 7 (21.2) |
| No | 26 (78.8) |
| PHQ total score | |
| None-minimal | 18 (54.6) |
| Mild-moderate | 13 (39.4) |
| Moderately-severe to severe | 2 (6.1) |
| Age [mean (SD)] | 51.2 (12.2) |
| Height (cm) [mean (SD)] | 170.8 (11.6) |
| Weight (kg) [mean (SD)] | 83.0 (16.8) |
| Body mass index (kg/m2) [mean (SD)] | 28.3 (4.5) |
| PHQ total [mean (SD)] | 5.8 (6.3) |
2.5. Statistical analysis
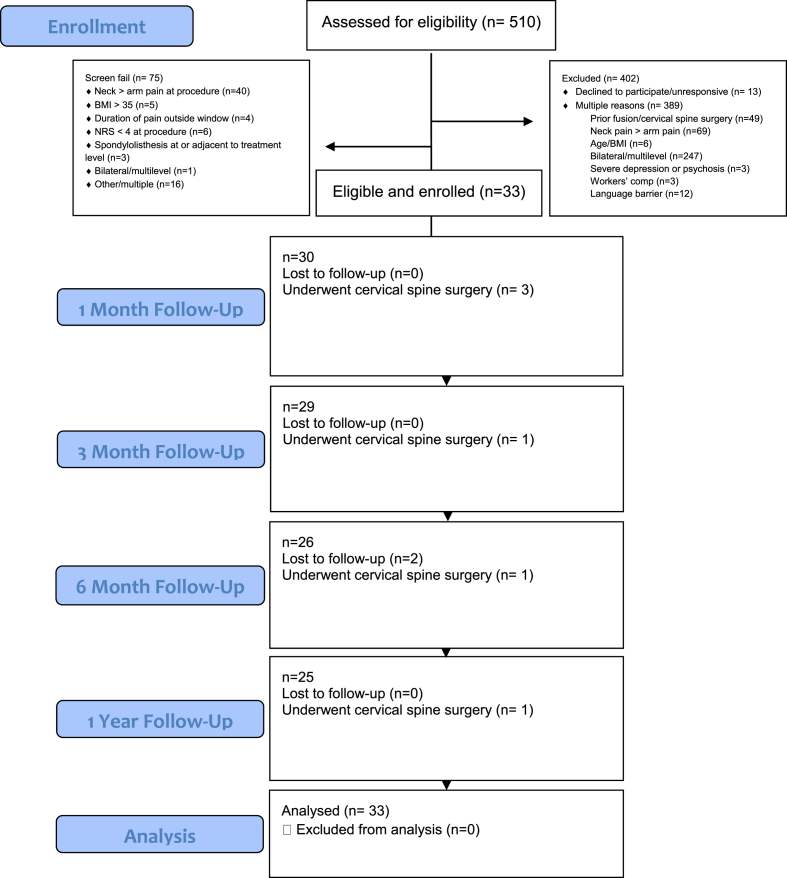
The sample size for this study was determined based upon the findings of Dreyfuss et al. [23] wherein 60% of subjects who received a cervical TFESI with dexamethasone reported at least 50% improvement in NRS score (95% CI 35–85%) at 4 weeks. To distinguish the lower bound of a 95% confidence interval from a theoretical placebo/sham response rate of 30%, but also from less than a 50% responder rate, a sample size of 105 participants was considered necessary (95% CI 51–69%, assuming a 60% responder rate). To account for a conservative 10% attrition rate by the 4-week primary endpoint, we initially sought to enroll 117 participants. During the study, the authors encountered unanticipated challenges in participant enrollment, primarily due to stringent inclusion and exclusion criteria (see Appendix A). The most significant hurdles stemmed from prospective participants' previous cervical spine surgeries, predominant neck pain compared to arm pain, or the presence of multi-level or bilateral symptoms, as illustrated in Fig. 1. These factors contributed to an enrollment rate of 6%. After three years, only 28% of the target sample size was enrolled, and given the low rate of enrollment, the authors determined that continuing the study was not feasible.
Fig. 1.
CONSORT diagram.
Descriptive statistics were calculated for demographics and clinical characteristics of participants, as well as for outcome variables. Specifically, mean and standard deviation (SD) were used for continuous variables, while categorical variables were summarized with frequency and percentage. A 95% confidence interval (CI) was calculated for select statistics. A multivariate analysis, a mixed-effects logistic regression model was fit to the data on categorical outcome variables, using different set (=model) of covariates. An odds ratio (OR) and its 95% CI were calculated for each model to aid in interpretations. Participants who were treated surgically were considered failures in the categorical and logistic regression analyses. Missing data in categorical outcome variables were treated by two approaches: 1) completer analysis in which missing data were excluded and 2) worst-case (WC) analysis in which missing data were treated as treatment failure. All the analyses were conducted using Stata/MP 17.0 (StataCorp LLC, College Station, TX), with an α level of 0.05 as statistical significance.
3. Results
3.1. Demographics
Between March 2019 and May 2022, 510 consecutive individuals were screened for potential enrollment with 33 ultimately meeting study inclusion criteria after review by investigators (see Fig. 1). Demographics and clinical characteristics of the participants are summarized in Table 1. The majority were females (n = 21 or 63.6%), with an average age of 51.2 ± 12.2 years. C6 and C7 were the affected spinal nerve root levels in 55% and 42% of participants, respectively. Most participants suffered from pain for more than three, but less than six months (n = 20 or 60.6%), as compared to six weeks to three months (n = 13 or 39.4%). At affected levels, participant MRI's showed cervical disc herniation (n = 11 or 33.0%), disc-osteophyte complex (n = 15 or 45.5%), or stenosis due to boney elements (n = 9 or 27.3%). Severity of neuroforaminal stenosis on MRI by Park Classification was grade 0–1 (n = 9 or 27.3%), grade 2 (n = 7 or 21.2%), or grade 3 stenosis (n = 17 or 51.5%). Very few participants reported prior (n = 3 or 9.1%) or current tobacco use (n = 2 or 6.1%). A history of depression (n = 9 or 27.3%), or anxiety (n = 7 or 21.2%), was noted in a minority of participants, whereas direct screening with the Patient Health Questionnaire 9 (PHQ-9) demonstrated that mild to moderate depression (n = 13 or 39.4%), or moderately severe to severe depression (n = 2 or 6.1%) was present in nearly half of participants.
3.2. Arm pain numerical rating scale
Table 2 summarizes unadjusted continuous arm NRS scores by follow-up time. Mean arm NRS ranged from 6.5 ± 1.4 (baseline; 95% CI = 6.0, 7.0) to 1.8 ± 2.0 (12-month; 95% CI = 1.0, 2.6). The change in arm NRS from baseline to 1-month follow-up was 3.7 ± 2.5 (95% CI = 2.8, 4.7), while that from baseline to the rest of each follow-up time point was over 4.0. The smallest and largest percentage changes in arm NRS from baseline were 57.4 ± 35.7 (1-month; 95% CI = 44.0, 70.7) and 72.0 ± 30.3 (95% CI = 59.5, 84.5), respectively.
Table 2.
Summary measures of continuous arm numerical rating scale.
| Variable | Follow-up time | N | Mean (SD) | Min, Max |
|---|---|---|---|---|
| Arm NRS | Baseline | 33 | 6.5 (1.4) | 4, 9 |
| 1-month | 30 | 2.8 (2.5) | 0, 9 | |
| 3-month | 28 | 2.1 (2.0) | 0, 8 | |
| 6-month | 26 | 2.1 (2.5) | 0, 8 | |
| 12-month | 25 | 1.8 (2.0) | 0, 7 | |
| Change in arm NRSa | 1-month | 30 | 3.7 (2.5) | −1, 8 |
| 3-month | 28 | 4.4 (2.3) | −2, 8 | |
| 6-month | 26 | 4.3 (2.8) | −2, 8 | |
| 12-month | 25 | 4.6 (2.2) | 0, 9 | |
| % change in arm NRSa | 1-month | 30 | 57.4 (35.7) | −12.5, 100.0 |
| 3-month | 28 | 67.0 (30.3) | −33.3, 100.0 | |
| 6-month | 26 | 66.3 (42.2) | −33.3, 100.0 | |
| 12-month | 25 | 72.0 (30.3) | 0.0, 100.0 |
NRS = Numerical Rating Scale; SD = standard deviation; Min = minimum value; Max = maximum value.
From baseline to each follow-up time point (i.e., value at baseline minus value at each follow-up time point).
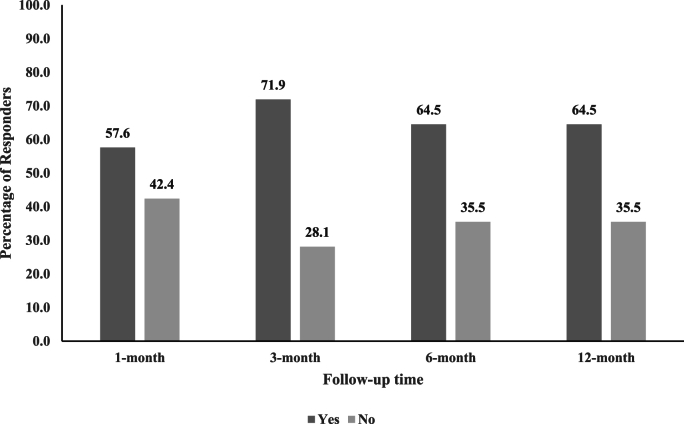
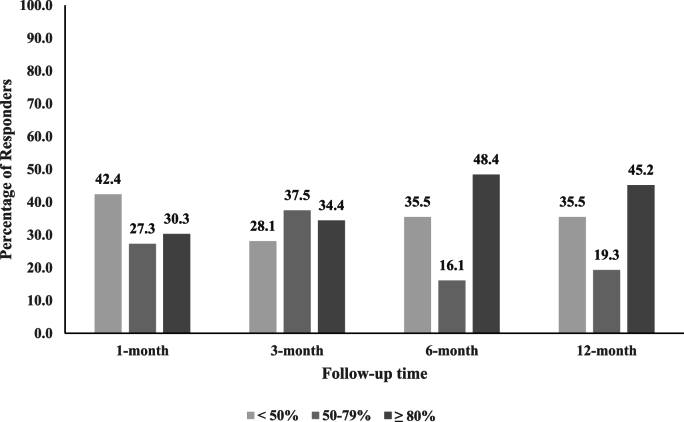
The majority of participants reported ≥50% reduction in arm NRS scores from baseline (Table 3 and Fig. 2). Success rates at 1-month, 3-month, 6-month, and 12-month follow-ups were 57.6% (95% CI = 40.8, 72.8%), 71.9% (95% CI = 54.6, 84.4%), 64.5% (95% CI = 46.9, 78.9%), and 64.5% (95% CI = 46.9, 78.9%), respectively, with similar rates in WC analysis. The smallest number of participants reporting ≥50% reduction in arm NRS was observed at 1-month follow-up (n = 19), whereas 20 or more participants reported ≥50% reduction in arm NRS at the other follow-up time points. Some participants reported ≥80% reduction in arm NRS from baseline (Fig. 3), ranging from 30.3% (95% CI = 15.6, 48.7) at 1-month follow-up to 48.4% (95% CI = 30.2, 66.9%) at 6 -month follow-up, with WC analysis producing comparable results.
Table 3.
Percentage of participants reporting ≥50% reduction in arm NRS from baseline to each follow-up time point.
| Missing data handling | Follow-up time | ≥50% reduction in arm NRS |
|
|---|---|---|---|
| Yes | No | ||
| Completer analysisa | 1-month | 19 (57.6) | 14 (42.4) |
| 3-month | 23 (71.9) | 9 (28.1) | |
| 6-month | 20 (64.5) | 11 (35.5) | |
| 12-month | 20 (64.5) | 11 (35.5) | |
| Worst-case analysisb | 1-month | 19 (57.6) | 14 (42.4) |
| 3-month | 23 (69.7) | 10 (30.3) | |
| 6-month | 20 (60.6) | 13 (39.4) | |
| 12-month | 20 (60.6) | 13 (39.4) | |
Note: Values are frequency (%).
NRS = Numerical Rating Scale.
Missing data excluded.
Missing data treated as treatment failure.
Fig. 2.
Unadjusted responder analysis for ≥50% reduction in arm numerical rating scale score by follow-up time point.
Missing data are excluded.
Fig. 3.
Unadjusted responder analysis for 50–79% and ≥80% reduction in arm numerical rating scale score by follow-up time point
Missing data are excluded.
Mixed-effects logistic regression models on ≥ 50% reduction in arm NRS score by set (=model) of covariates are summarized in Table 4. There was no statistically significant covariate in any model (p > 0.05). A nonsignificant covariate of follow-up time in these models indicated that the success rate of ≥50% reduction in arm NRS score was sustained throughout the follow-up period.
Table 4.
Mixed-effects logistic regression models on ≥ 50% reduction in arm numerical rating scale by set (=model) of covariates.
| Model | Predictor | OR | (95% CI) | p |
|---|---|---|---|---|
| Radiographic model Clinical variables model |
Follow-up time (vs. 1-month) | |||
| 3-month | 3.76 | (0.74, 19.04) | 0.110 | |
| 6-month | 1.66 | (0.35, 7.82) | 0.519 | |
| 12-month | 1.66 | (0.35, 7.82) | 0.519 | |
| Cervical disc herniation (vs. no) | ||||
| Yes | 16.91 | (0.24, 1177.42) | 0.192 | |
| Disc-osteophyte complex (vs. no) | ||||
| Yes | 10.16 | (0.23, 444.30) | 0.229 | |
| Foraminal stenosis (vs. no) | ||||
| Yes | 19.91 | (0.30, 1300.58) | 0.161 | |
| Severity of stenosis (vs. Grade of 0 & 1) | ||||
| Grade of 2 | 0.04 | (0.00, 1.62) | 0.088 | |
| Grade of 3 | 0.06 | (0.00, 1.45) | 0.083 | |
| Follow-up time (vs. 1-month) | ||||
| 3-month | 4.00 | (0.79, 20.17) | 0.093 | |
| 6-month | 1.72 | (0.37, 8.05) | 0.494 | |
| 12-month | 1.72 | (0.37, 8.05) | 0.494 | |
| Spurlings test (vs. negative) | ||||
| Positive | 0.34 | (0.03, 3.94) | 0.386 | |
| Myotomal strength asymetry (vs. no) | ||||
| Yes | Emptya | |||
| DTR asymmetry on exam (vs. no) | ||||
| Yes | 4.40 | (0.02, 810.77) | 0.578 | |
| Duration of pain (vs. 6 weeks to 3 months) | ||||
| 3–6 months | 3.77 | (0.33, 42.72) | 0.284 | |
| Description of pain: burning/electric (vs. no) | ||||
| Yes | 0.23 | (0.02, 3.02) | 0.263 | |
| Description of pain: aching (vs. no) | ||||
| Yes | 0.73 | (0.05, 9.75) | 0.811 | |
| Description of pain: sharp (vs. no) | ||||
| Yes | 0.25 | (0.02, 3.32) | 0.295 | |
| Description of pain: other (vs. no) | ||||
| Yes | 0.33 | (0.02, 5.46) | 0.441 | |
| Demographics model Psychological model |
Follow-up time (vs. 1-month) | |||
| 3-month | 3.72 | (0.74, 18.68) | 0.110 | |
| 6-month | 1.66 | (0.35, 7.72) | 0.521 | |
| 12-month | 1.66 | (0.35, 7.72) | 0.521 | |
| Gender (vs. female) | ||||
| Male | 1.84 | (0.16, 20.68) | 0.621 | |
| Age | 1.05 | (0.95, 1.17) | 0.363 | |
| Obesity (vs. no) | ||||
| Yes | 0.18 | (0.01, 2.32) | 0.190 | |
| Current Tobacco use (vs. no) | ||||
| Yes | 0.17 | (0.00, 26.33) | 0.489 | |
| Follow-up time (vs. 1-month) | ||||
| 3-month | 3.80 | (0.75, 19.27) | 0.108 | |
| 6-month | 1.69 | (0.36, 7.94) | 0.509 | |
| 12-month | 1.69 | (0.36, 7.94) | 0.509 | |
| Depression (vs. no) | ||||
| Yes | 0.20 | (0.01, 4.46) | 0.307 | |
| Anxiety (vs. no) | ||||
| Yes | 1.19 | (0.05, 28.14) | 0.913 | |
| PHQ total (vs. none to minimal) | ||||
| Mild to moderate | 0.28 | (0.02, 3.67) | 0.333 | |
| Moderately-severe to severe | 0.09 | (0.00, 13.78) | 0.352 | |
Note: Outcome = ≥50% reduction in arm numerical rating scale; Missing data are excluded.
OR = odds ratio; CI = confidence interval.
All patients with myotomal strength asymmetry had ≥50% change in arm numerical rating scale.
3.3. Neck pain numerical rating scale
Mean neck NRS score was 4.5 ± 2.9 (95% CI = 3.5, 5.6) at baseline, and ranged from 2.3 ± 2.1 (95% CI = 1.5, 3.1; 3-month) to 3.1 ± 2.5 (95% CI = 2.1, 4.1; 6-month) during the follow-up period. Changes in neck NRS score were rather smaller than those in arm NRS score, as the minimum and maximum changes were 1.0 ± 3.5 (95% CI = −0.4, 2.5) at 6-month and 1.9 ± 3.9 (95% CI = 0.4, 3.4) at 3-month, respectively. As a result, percentage changes in neck NRS score were less than 40% (minimum = 5.0% at 6-month) at any follow-up time point. Between 36.0% (95% CI = 20.2, 55.5; 6-month) and 53.9% (95% CI = 35.5, 71.2; 3-month) of the participants reported ≥50% reduction in neck NRS from baseline, with the improvement as low as 27.3% (95% CI = 15.1, 44.2) in WC analysis, in which missing data were treated as treatment failure. Meanwhile, as much as 30.8% (95% CI = 14.3, 51.8; 3- and 12-month) of the participants reported ≥80% reduction in neck NRS from baseline.
Mixed-effects logistic regression models examining ≥50% reduction in neck NRS score by the same sets of covariates as those in ≥50% reduction in arm NRS showed that none of the covariates in any model (radiographic, clinical, demographic, or psychological models) was significant (p > 0.05), except anxiety in the psychological model (p = 0.027). Specifically, the odds of achieving ≥50% reduction in neck NRS for participants with anxiety was 97% (OR = 0.03; 95% CI = 0.01, 0.67) lower than the odds for those without anxiety.
3.4. Neck Disability Index
NDI-5 was, on average, 9.4 ± 3.9 (95% CI = 8.0, 10.8) at baseline, while, during follow-up, it was as low as 2.7 ± 2.5 (95% CI = 1.7, 3.8; 12-month; Table 5). Consequently, the changes in NDI-5 from baseline ranged from 4.9 ± 4.6 (95% CI = 3.2, 6.6 at 1-month to 6.6 ± 4.0 (95% CI = 4.9, 8.3) at 12-month, resulting in percentage changes in NDI-5 between 49.2 ± 36.0% (95% CI = 35.7, 62.6; 1-month) and 69.6 ± 29.4% (95% CI = 57.5, 81.8; 12-month). Most participants achieved ≥30% improvement in NDI-5 scores from baseline to all follow-up time points, which also held true in WC analysis (Table 6). Specifically, as many as 71.0% (95% CI = 53.4, 83.9) of the participants reported ≥30% improvement in NDI-5 scores at 12 months.
Table 5.
Summary measures of continuous Neck Disability Index.
| Variable | Follow-up time | N | Mean (SD) | Min, Max |
|---|---|---|---|---|
| NDI | Baseline | 33 | 9.4 (3.9) | 2, 19 |
| 1-month | 30 | 4.3 (3.3) | 0, 12 | |
| 3-month | 28 | 3.4 (3.0) | 0, 13 | |
| 6-month | 26 | 4.0 (2.6) | 0, 9 | |
| 12-month | 25 | 2.7 (2.5) | 0, 9 | |
| Change in NDIa | 1-month | 30 | 4.9 (4.6) | −2, 19 |
| 3-month | 28 | 5.8 (4.4) | −4, 15 | |
| 6-month | 26 | 5.2 (4.8) | −3, 16 | |
| 12-month | 25 | 6.6 (4.0) | 0, 16 | |
| % change in NDIa | 1-month | 30 | 49.2 (36.0) | −33.3, 100.0 |
| 3-month | 28 | 56.9 (43.1) | −100.0, 100.0 | |
| 6-month | 26 | 43.5 (55.0) | −150.0, 100.0 | |
| 12-month | 25 | 69.6 (29.4) | 0.0, 100.0 |
NDI = Neck Disability Index; SD = standard deviation; Min = minimum value; Max = maximum value.
From baseline to each follow-up time point (i.e., value at baseline minus value at each follow-up time point).
Table 6.
Percentage of participants reporting ≥30% reduction in NDI-5 from baseline to each follow-up time point.
| Missing data handling | Follow-up time | ≥30% reduction in NDI |
|
|---|---|---|---|
| Yes | No | ||
| Completer analysisa | 1-month | 20 (60.6) | 13 (39.4) |
| 3-month | 22 (68.8) | 10 (31.2) | |
| 6-month | 19 (61.3) | 12 (38.7) | |
| 12-month | 22 (71.0) | 9 (29.0) | |
| Worst-case scenario analysisb | 1-month | 20 (60.6) | 13 (39.4) |
| 3-month | 22 (66.7) | 11 (33.3) | |
| 6-month | 19 (57.6) | 14 (42.4) | |
| 12-month | 22 (66.7) | 11 (33.3) | |
Note: Values are frequency (%).
NDI-5 = Neck Disability Index.
Missing data excluded.
Missing data treated as treatment failure.
3.5. Health-related quality of life
The mean EQ-5D score at baseline was 0.61 ± 0.17 (95% CI = 0.55, 0.67), which improved at all follow-up time points, ranging from 0.80 ± 0.16 (95% CI = 0.74, 0.86) at 1-month to 0.86 ± 0.14 (95% CI = 0.80, 0.91) at 12-month (Table 7). Average changes in EQ-5D were between −0.19 at 1- and 6-month and −0.24 at 3- and 12-month. As few as 61.3% (95% CI = 43.8, 76.3; 6-month) and as many as 78.1% (95% CI = 61.2, 89.0; 3-month) of the participants reported ≥0.03-point improvement in EQ-5D scores from baseline, with similar improvements in the WC analysis (Table 8).
Table 7.
Summary measures of continuous Health-related Quality of life as measured by EQ-5D.
| Variable | Follow-up time | N | Mean (SD) | Min, Max |
|---|---|---|---|---|
| EQ-5D | Baseline | 33 | 0.61 (0.17) | 0.17, 0.87 |
| 1-month | 30 | 0.80 (0.16) | 0.37, 1.00 | |
| 3-month | 28 | 0.85 (0.11) | 0.58, 1.00 | |
| 6-month | 26 | 0.81 (0.15) | 0.46, 1.00 | |
| 12-month | 25 | 0.86 (0.14) | 0.38, 1.00 | |
| Change in EQ-5Da | 1-month | 30 | −0.19 (0.22) | −0.77, 0.21 |
| 3-month | 28 | −0.24 (0.19) | −0.58, 0.20 | |
| 6-month | 26 | −0.19 (0.23) | −0.50, 0.35 | |
| 12-month | 25 | −0.24 (0.15) | −0.50, 0.07 |
EQ-5D = EuroQol Health-related Quality of life; SD = standard deviation; Min = minimum value; Max = maximum value.
From baseline to each follow-up time point (i.e., value at baseline minus value at each follow-up time point).
Table 8.
Percentage of participants reporting ≥0.03-point increase in Health-related Quality of life from EQ-5D at each follow-up time point.
| Missing data handling | Follow-up time | ≥0.03-point increase in EQ-5D |
|
|---|---|---|---|
| Yes | No | ||
| Completer analysisa | 1-month | 25 (75.8) | 8 (24.2) |
| 3-month | 25 (78.1) | 7 (21.9) | |
| 6-month | 19 (61.3) | 12 (38.7) | |
| 12-month | 23 (74.2) | 8 (25.8) | |
| Worst-case scenario analysisb | 1-month | 25 (75.8) | 8 (24.2) |
| 3-month | 25 (75.8) | 8 (24.2) | |
| 6-month | 19 (57.6) | 14 (42.4) | |
| 12-month | 23 (69.7) | 10 (30.3) | |
Note: Values are frequency (%).
EQ-5D = EuroQol Health-related Quality of life.
Missing data excluded.
Missing data treated as treatment failure.
3.6. Clinical Outcome Measurement Brief Instrument
Table 9 Appendix B shows the numbers and percentages of participants who reported substantial or complete recovery of at least three of four key activities between one and 12 months. The lowest and highest percentages were 33.3% (95% CI = 19.8, 50.4) at 1-month and 56.3% (95% CI = 39.3, 71.8) at 6-month, respectively, which was similar in WC analysis.
3.7. Chronic Pain Sleep Inventory
The mean CPSI score at baseline was 51.9 ± 30.7 (95% CI = 41.0, 62.8), whereas, except at 3-month (51.2 ± 35.4), it was higher at any other follow-up time points (mean ranging from 60.0 to 68.3; Table 10 Appendix C). There was great variability of the changes in follow-up CPSI, as shown by high SDs and ranges (minimum to maximum values). Less than 50% of the participants showed ≥30% reduction in CPSI from baseline to any follow-up time point, with the highest percentage reported at 3-month (40.0%; 95% CI = 24.6, 57.7; Table 11 Appendix D).
3.8. Global impression of change
PGIC was rather consistent during follow-up, as the mean ranged between 5.6 and 5.9, while the median was 6 at all follow-up time points (Table 12 Appendix E). The majority of participants reported being “improved or much improved” (PGIC 6–7) at all follow-up time points, except at 6-month (48.4%), which was also consistent in WC analysis (Table 13 Appendix F).
3.9. Analgesic medication and cervical spine-related healthcare utilization
Details of interventional and surgical healthcare utilization are shown in Table 14 Appendix G. Surgery was performed for a minority of participants (n = 6 or 18.2%), with 50% occurring before the one-month data collection timepoint. 90.9% of participants received only the index injection, whereas one participant received two injections, one participant received three injections, and one participant received four injections.
The mean MQS-3 score at baseline was 6.0 ± 4.8 (95% CI = 4.3, 7.7). Lower MQS-3 scores were reported at follow-up, ranging from 3.6 ± 4.8 (95% CI = 1.6, 5.6) at 12-month to 5.5 ± 5.1 (95% CI = 3.6, 7.5) at 1-month (Table 15 Appendix H). Changes in MQS-3 were between 0.4 ± 5.1 (95% CI = −1.6, 2.3) at 1-month and 2.8 ± 4.9 (95% CI = 0.8, 4.8) at 12-month. As many participants had MQS-3 scores below 6.8 at baseline, less than 20% of the participants ultimately showed ≥6.8-point change during the study period (Table 16 Appendix I).
3.10. Logistic regression models on secondary outcome variables
Mixed-effects logistic regression models examining secondary outcome variables by the same sets of covariates as those in ≥50% reduction in arm NRS analysis showed that none of the covariates in any model (radiographic, clinical variables, demographics, or psychological models) was significant (p > 0.05), except the presence of a positive Spurling's test for NDI-5 scores in the clinical variables model (p = 0.039) and description of pain as “burning/electric” for CPSI scores in the clinical variables model (p = 0.027). Specifically, the odds of achieving ≥30% improvement in NDI-5 for participants with a positive Spurling's test was 90% (OR = 0.10; 95% CI = 0.01–0.89) lower than the odds for those without a positive test. Similarly, the odds of achieving ≥30% improvement in CPSI scores for participants who described their pain as “burning/electric” was about 12 times (OR = 12.30; 95% CI = 1.33–113.48) greater than the odds for those who described their pain differently, though it should be noted that the 95% CI of the OR was wide.
3.11. Adverse effects
There were no serious adverse effects or complications related to CTFESI during this study.
4. Discussion
Following CTFESI, participants reported statistically significant and clinically meaningful improvements in pain and disability for up to one year. Additionally, CTFESI appears to have positively impacted health-related quality of life, personal goal achievement, and reduced pain-related sleep disturbance. Specifically, arm NRS improved by ≥ 50% in 58–65%, NDI-5 improved by ≥ 30% in 61–71%, health related quality of life (EQ-5D) improved by ≥ 0.03 in 61–78%. Between 48 and 66% of participants described their global state as “improved” or “much improved” during the study period. At one month, only 33% reported substantial or complete restoration of most key activities, but this increased to 45–56% at subsequent time points. Success rates for neck pain and pain related sleep disturbance were lower than other outcomes, with 36–54% of the participants reporting ≥50% reduction in neck NRS and 17–40% reporting ≥30% improvement in CPSI scores. During the study, 91% of participants required only a single injection, potentially indicating the effectiveness of the initial injection. Furthermore, surgical avoidance was relatively high, as only 9% of participants underwent surgery after the first month.
Our findings are similar to previously reported outcomes of CTFESI when participants were strictly selected and when the procedure was performed according to recognized guidelines. Dreyfuss et al. reported a 60% responder rate for ≥50% pain relief at short-term follow-up [23] Notably, that study enrolled 30 participants after screening 420, or approximately 7%. We used similarly strict criteria for eligibility, screening 510 with 33, or about 6%, ultimately being eligible. This suggests that CTFESI effectively reduces pain and disability in carefully selected individuals, but these outcomes may not be generalizable to all populations with cervical radicular pain. On the other hand, meta-analysis of multiple studies (some with less strict enrollment criterion and lower screening rates), has demonstrated responder rates between 48 and 62% at short term follow-up [10].
Very few studies have reported long-term outcomes after CTFESI. Data from an RCT comparing interlaminar ESI to CTFESI demonstrated 52% and 61% responder rates for ≥50% pain relief and ≥30% NDI improvement, respectively, at one year for those treated with CTFESI [24]. A cross-sectional study polling individuals treated with CTFESI found that 81% experienced ≥50% pain relief for an average duration of 13.3 ± 9.44 months [25]. Other prospective studies have demonstrated a pattern of continued pain relief at long-term follow-up and surgical avoidance in 80% of patients who underwent ESI [26]. We observed similar proportions of surgical treatment in our study, with 18% undergoing anterior cervical discectomy and fusion (ACDF) or disc arthroplasty during the one-year study period with 50% of these participants undergoing surgery prior to one-month post-CTFESI.
In addition to pain and function outcomes, this study observed significant improvements in health-related quality of life, personal goal achievement, and to a lesser degree, pain-related sleep interference. Despite guidelines emphasizing the importance of assessing sleep quality in clinical trials involving patients with chronic pain, a recent scoping review found that less than 5% of studies have adhered to these recommendations [27]. Patients with pain often report frequent disruptions in sleep patterns including increased arousal, prolonged awakenings, and periodic body movements. Substantial evidence suggests a mutually reinforcing association where pain contributes to sleep disturbance and vice versa [28,29]. Further, pain alleviation has been linked to a reduction in self-reported sleep problems, while improved sleep has been associated with decreased pain symptoms [30]. We observed significant improvement in sleep quality following CTFESI, with 17–40% of participants showing improvement at different time points. However, the data displayed significant variability, evidenced by high standard deviations and broad ranges (minimum to maximum values). This could either indicate that the measurement tool used was not sensitive enough or, more plausibly, that factors other than cervical radicular pain may have influenced sleep quality. The fact that most participants continued to report significant pain-related sleep disturbance raises concern and highlights the need for more comprehensive assessments including this often-overlooked metric in future studies.
Despite the favorable outcomes in this study and others like it, CTFESI may not be an effective treatment in all individuals. Certain factors are known to decrease the probability of treatment success with interventional pain treatments, such as severe depression. Prior studies involving patients with cervical and lumbar radicular pain have demonstrated worse outcomes in those with significant depression, with responder rates as low as 19% amongst depressed patients [31,32]. Regarding depression, we did not find a notable impact on treatment success rates. This is likely because only 6% of participants exhibited more than moderate depression, while 55% had either no or minimal depression in our cohort. Interestingly, we found that a significant number of participants had previously undiagnosed depression, which highlights the importance of point-of-care mental health screening in those seeking treatment for chronic pain conditions.
Certain radiographic factors have also been associated with different outcomes after CTFESI. Studies exist which have demonstrated that more severe central or neuroforaminal stenosis is negatively associated with treatment success of interlaminar and transforaminal ESI [33,34], while other studies have not demonstrated this same relationship [35,36]. Our results were consistent with these latter studies; while approximately 50% of the participants in our study had severe neuroforaminal stenosis (Park grade 3), success rates across all outcomes did not appear significantly different depending on the severity of neuroforaminal stenosis.
Limitations of this study must be acknowledged. Due to the absence of a control group, the specific effect of CTFESI cannot be separated from the natural course of cervical radicular pain. Although the natural history of cervical radiculopathy is generally considered favorable, conflicting evidence exists about the expected duration of pain at short and intermediate time points. For example, some studies indicate that up to 66% of patients may continue to experience severe pain and functional limitations despite non-operative treatments [37,38] and only 29% completely recover [39]. These populations may be more representative of patients in our study—those referred to a tertiary care spine clinic. In contrast, population-based cohort studies have shown that after an average follow-up of 5.9 years 90% report being asymptomatic or only mildly affected [1]. It is important to note that a substantial proportion (61%) of participants in our study presented with chronic symptoms persisting between three to six months prior to their enrollment. This duration of symptoms diminishes the probability that the improvements observed can be attributed solely to natural recovery.
This study was also limited by lower-than-anticipated enrollment, largely a result of the stringent inclusion and exclusion criteria, resulting in a smaller study cohort. This restricted our ability to conduct in-depth analyses on demographic and radiographic variables related to treatment success or failure. To address the impact of low enrollment, the authors constructed several sets of regression analyses centered on clusters of variables, including radiographic, clinical, demographic, and psychological factors (Table 4). Despite the small sample size, the study demonstrated statistically significant and clinically meaningful improvements in both the primary and various secondary outcomes. Strengths of the study include high rate of follow-up and robust long term outcome measurement across domains including pain, function, quality of life, pain-related sleep interference, and global improvement.
5. Conclusion
In individuals with unilateral cervical radicular pain, clinically meaningful improvements in pain, disability, health related quality of life, personal goal achievement, and pain-related sleep disturbance were observed for up to 12 months after CTFESI. These findings were also accompanied by reduced analgesic use and relatively low cervical spine pain-related healthcare utilization. This study provides further evidence that CTFESI benefits carefully selected patients with cervical radicular pain. Future, larger-scale studies that include a control group will offer a more accurate assessment of the treatment's relative effectiveness.
Conflicts of interest disclosure statement
Zachary L. McCormick, MD serves on the Board of Directors of the International Pain and Spine Intervention Society (IPSIS). There are no other potential conflicts of interest related to this study to disclose on the part of any of the other authors.
Funding
This study was funded by a research grant from IPSIS.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors wish to IPSIS for their generous support of this work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.inpm.2023.100379.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Murray C.J.L. The state of US health, 1990–2010. JAMA. 2013;310(6):591. doi: 10.1001/jama.2013.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong J.J., Côté P., Quesnele J.J., Stern P.J., Mior S.A. The course and prognostic factors of symptomatic cervical disc herniation with radiculopathy: a systematic review of the literature. Spine J. 2014;14(8):1781–1789. doi: 10.1016/j.spinee.2014.02.032. [DOI] [PubMed] [Google Scholar]
- 3.Johansson A., Hao J., Sjölund B. Local corticosteroid application blocks transmission in normal nociceptive C‐fibres. Acta Anaesthesiol Scand. 1990 doi: 10.1111/j.1399-6576.1990.tb03097.x. [DOI] [PubMed] [Google Scholar]
- 4.Ramesh G., Meisner O.C., Philipp M.T. Anti-inflammatory effects of dexamethasone and meloxicam on Borrelia burgdorferi-induced inflammation in neuronal cultures of dorsal root ganglia and myelinating cells of the peripheral nervous system. J Neuroinflammation. 2015 doi: 10.1186/s12974-015-0461-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li J.Y., Xie W., Strong J.A., Guo Q.L., Zhang J.M. Mechanical hypersensitivity, sympathetic sprouting, and glial activation are attenuated by local injection of corticosteroid near the lumbar ganglion in a rat model of neuropathic pain. Reg Anesth Pain Med. 2011 doi: 10.1097/AAP.0b013e318203087f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCormick Z.L., Conger A., Sperry B.P., Teramoto M., Petersen R., Salazar F., et al. A randomized comparative trial of targeted steroid injection via epidural catheter vs standard transforaminal epidural injection for the treatment of unilateral cervical radicular pain: six-month results. Pain Med. 2020;21(10):2077–2089. doi: 10.1093/pm/pnaa242. https://academic.oup.com/painmedicine/advance-article/doi/10.1093/pm/pnaa242/5892882 [DOI] [PubMed] [Google Scholar]
- 7.Conger A., Kendall R.W., Sperry B.P., Petersen R., Salazar F., Cunningham S., et al. One-year results from a randomized comparative trial of targeted steroid injection via epidural catheter versus standard transforaminal epidural injection for the treatment of unilateral cervical radicular pain. Reg Anesth Pain Med. 2021;46(9):813–819. doi: 10.1136/rapm-2021-102514. [DOI] [PubMed] [Google Scholar]
- 8.McCormick Z.L., Nelson A., Bhave M., Zhukalin M., Kendall M., McCarthy R.J., et al. A Prospective randomized comparative trial of targeted steroid injection via epidural catheter versus standard C7-T1 interlaminar approach for the treatment of unilateral cervical radicular pain. Reg Anesth Pain Med. 2017;42(1):82–89. doi: 10.1097/AAP.0000000000000521. [DOI] [PubMed] [Google Scholar]
- 9.Conger A., Sperry B.P., Cheney C.W., Kuo K., Petersen R., Randall D., et al. Does the contrast dispersion pattern during fluoroscopically guided cervical transforaminal epidural steroid injection predict short-term pain and functional outcomes? An exploratory analysis of prospective cohort data. Pain Med. 2020 doi: 10.1093/pm/pnaa305. https://academic.oup.com/painmedicine/advance-article/doi/10.1093/pm/pnaa305/5912686 Sep 29;00(0). Available from: [DOI] [PubMed] [Google Scholar]
- 10.Conger A., Cushman D.M., Speckman R.A., Burnham T., Teramoto M., McCormick Z.L. The effectiveness of fluoroscopically guided cervical transforaminal epidural steroid injection for the treatment of radicular pain; a systematic review and meta-analysis. Pain Med. 2020;21(1):41–54. doi: 10.1093/pm/pnz127. https://academic.oup.com/painmedicine/advance-article-abstract/doi/10.1093/pm/pnz127/5513392 Available from: [DOI] [PubMed] [Google Scholar]
- 11.Borton Z.M., Oakley B.J., Clamp J.A., Birch N.C., Bateman A.H. Cervical transforaminal epidural steroid injections for radicular pain. Bone Joint J. 2022 doi: 10.1302/0301-620X.104B5.BJJ-2021-1816.R1. https://online.boneandjoint.org.uk/doi/10.1302/0301-620X.104B5.BJJ-2021-1816.R1 104-B(5):567–74. Available from: [DOI] [PubMed] [Google Scholar]
- 12.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inf. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith S.M., Dworkin R.H., Turk D.C., McDermott M.P., Eccleston C., Farrar J.T., et al. Interpretation of chronic pain clinical trial outcomes: IMMPACT recommended considerations. Pain. 2020;161(11):2446–2461. doi: 10.1097/j.pain.0000000000001952. https://journals.lww.com/10.1097/j.pain.0000000000001952 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walton D.M., MacDermid J.C. A brief 5-item version of the Neck Disability Index shows good psychometric properties. Health Qual Life Outcomes. 2013;11(1):1. doi: 10.1186/1477-7525-11-108. Available from: Health and Quality of Life Outcomes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hurst H., Bolton J. Patients' global impression of change (PGIC) scale. J Manipul Physiol Therapy. 2004;27:26–35. [Google Scholar]
- 16.Soer R., Reneman M.F., Speijer B.L.G.N., Coppes M.H., Vroomen P.C.A.J. Clinimetric properties of the EuroQol-5D in patients with chronic low back pain. Spine J. 2012 doi: 10.1016/j.spinee.2012.10.030. [DOI] [PubMed] [Google Scholar]
- 17.Stojanovic M.P., Higgins D.M., Popescu A., Bogduk N. COMBI: a convenient tool for clinical outcome assessment in conventional practice. Pain Med. 2015 doi: 10.1111/pme.12581. https://academic.oup.com/painmedicine/article-lookup/doi/10.1111/pme.12581 Mar 1 [cited 2018 Sep 10];16(3):513–9. Available from: [DOI] [PubMed] [Google Scholar]
- 18.Gallizzi M., Gagnon C., Harden R.N., Stanos S., Khan A. Medication quantification scale version III: internal validation of detriment weights using a chronic pain population. Pain Practice [Internet] 2008 Jan;8(1):1–4. doi: 10.1111/j.1533-2500.2007.00163.x. 10.1111/j.1533-2500.2007.00163.x. [DOI] [PubMed] [Google Scholar]
- 19.Ayearst L., Harsanyi Z., Michalko K.J. The Pain and Sleep Questionnaire three-item index (PSQ-3): a reliable and valid measure of the impact of pain on sleep in chronic nonmalignant pain of various etiologies. Pain Res Manag [Internet. 2012;17(4):281–290. doi: 10.1155/2012/635967. http://www.ncbi.nlm.nih.gov/pubmed/22891194 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hung M., Saltzman C.L., Kendall R., Bounsanga J., Voss M.W., Lawrence B., et al. What are the MCIDs for PROMIS, NDI, and ODI instruments among patients with spinal conditions? Clin Orthop Relat Res. 2018;476(10):2027–2036. doi: 10.1097/CORR.0000000000000419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salaffi F., Stancati A., Silvestri C.A., Ciapetti A., Grassi W. Minimal clinically important changes in chronic musculoskeletal pain intensity measured on a numerical rating scale. Eur J Pain. 2004 doi: 10.1016/j.ejpain.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Park H.J., Kim S.S., Lee S.Y., Park N.H., Chung E.C., Rho M.H., et al. A practical MRI grading system for cervical foraminal stenosis based on oblique sagittal images. Br J Radiol. 2013;86(1025):1–7. doi: 10.1259/bjr.20120515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dreyfuss P., Baker R., Bogduk N. Comparative effectiveness of cervical transforaminal injections with particulate and nonparticulate corticosteroid preparations for cervical radicular pain. Pain Med [Internet. 2006 May 1;7(3):237–242. doi: 10.1111/j.1526-4637.2006.00162.x. https://academic.oup.com/painmedicine/article-lookup/doi/10.1111/j.1526-4637.2006.00162.x Available from: [DOI] [PubMed] [Google Scholar]
- 24.Conger A., Kendall R.W., Sperry B.P., Petersen R., Salazar F., Cunningham S., et al. One-year results from a randomized comparative trial of targeted steroid injection via epidural catheter versus standard transforaminal epidural injection for the treatment of unilateral cervical radicular pain. Reg Anesth Pain Med. 2021;46(9):813–819. doi: 10.1136/rapm-2021-102514. [DOI] [PubMed] [Google Scholar]
- 25.Kesikburun S., Aras B., Kelle B., Yavuz F., Yaşar E., Taşkaynatan M.A. The effectiveness of cervical transforaminal epidural steroid injection for the treatment of neck pain due to cervical disc herniation: long-term results. Pain Manag. 2018 Sep 1;8(5):321–326. doi: 10.2217/pmt-2018-0002. [DOI] [PubMed] [Google Scholar]
- 26.Lee S.H., Kim K.T., Kim D.H., Lee B.J., Son E.S., Kwack Y.H. Clinical outcomes of cervical radiculopathy following epidural steroid injection: a prospective study with follow-up for more than 2 years. Spine (Phila Pa. 1976;37(12):1041–1047. doi: 10.1097/BRS.0b013e31823b4d1f. 2012 May 20. [DOI] [PubMed] [Google Scholar]
- 27.Neilson B.D., Dickerson C., Young J.L., Shepherd M.H., Rhon D.I. Measures of sleep disturbance are not routinely captured in trials for chronic low back pain: a systematic scoping review of 282 trials. J Clin Sleep Med. 2023 Jun 1 doi: 10.5664/jcsm.10672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cole J.C., Dubois D., Kosinski M. Use of patient-reported sleep measures in clinical trials of pain treatment: a literature review and synthesis of current sleep measures and a conceptual model of sleep disturbance in pain. Clin Therapeut. 2007;29(Suppl):2580–2588. doi: 10.1016/j.clinthera.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 29.Smith M.T., Haythornthwaite J.A. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Med Rev. 2004 Apr;8(2):119–132. doi: 10.1016/S1087-0792(03)00044-3. [DOI] [PubMed] [Google Scholar]
- 30.Turk D.C., Cohen M.J.M. Sleep as a marker in the effective management of chronic osteoarthritis pain with opioid analgesics. Semin Arthritis Rheum. 2010 Jun;39(6):477–490. doi: 10.1016/j.semarthrit.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 31.Kim E.J., Chotai S., Schneider B.J., Sivaganesan A., McGirt M.J., Devin C.J. Effect of depression on patient-reported outcomes following cervical epidural steroid injection for degenerative spine disease. Pain Med [Internet] 2018:1–6. doi: 10.1093/pm/pny196. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=medp&NEWS=N&AN=30357417 0(0. [DOI] [PubMed] [Google Scholar]
- 32.Kim E.J., Chotai S., Stonko D.P., Wick J.B., Schneider B.J., McGirt M.J., et al. Patient-reported outcomes after lumbar epidural steroid injection for degenerative spine disease in depressed versus non-depressed patients. Spine J. 2017 Apr;17(4):511–517. doi: 10.1016/j.spinee.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 33.Sencan S., Edipoglu I.S., Yazıcı G., Yucel F.N., Gunduz O.H. Are foraminal stenosis severity and herniation level associated with the treatment success of cervical interlaminar epidural steroid injection? Pain Physician. 2020;23(3):326–332. [PubMed] [Google Scholar]
- 34.Ray W.Z., Akbari S., Shah L.M., Bisson E. Correlation of foraminal area and response to cervical nerve root injections. Cureus. 2015;7(7):4–11. doi: 10.7759/cureus.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim M.S., Lee D.G., Chang M.C. Outcome of transforaminal epidural steroid injection according to severity of cervical foraminal stenosis. World Neurosurg [Internet] 2018 Feb;110 doi: 10.1016/j.wneu.2017.11.014. cited 2018 Jun 24. [DOI] [PubMed] [Google Scholar]
- 36.Kim J., Kim K., Lee M., Kim S. Correlation between intravascular injection rate, pain intensity, and degree of cervical neural foraminal stenosis during a cervical transforaminal epidural block. J Pain Res. 2021;14:3017–3023. doi: 10.2147/JPR.S330858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lees F., Turner Jw. Natural history and prognosis of cervical spondylosis. Br Med J. 1963 Dec 28;2(5373):1607–1610. doi: 10.1136/bmj.2.5373.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dillin W., Booth R., Cuckler J., Balderston R., Simeone F., Rothman R. Cervical radiculopathy. A review. Spine. 1986 Dec;11(10):988–991. doi: 10.1097/00007632-198612000-00003. [DOI] [PubMed] [Google Scholar]
- 39.DePalma A.F., Subin D.K. Study of the cervical syndrome. Clin Orthop Relat Res. 1965;38:135–142. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.