Abstract
Background
Primaquine (PQ) has activity against mature P. falciparum gametocytes and proven transmission blocking efficacy (TBE) between humans and mosquitoes. WHO formerly recommended a single transmission blocking dose of 0.75 mg/kg but this was little used. Then in 2012, faced with the emergence of artemisinin-resistant P. falciparum (ARPf) in SE Asia, the WHO recommended a lower dose of 0.25 mg/kg to be added to artemisinin-based combination therapy in falciparum-infected patients in low transmission areas. This dose was considered safe in glucose-6-phosphate dehydrogenase deficiency (G6PDd) and not requiring G6PD testing. Subsequent single low-dose primaquine (SLDPQ) studies have demonstrated safety in different G6PD variants. Dosing remains challenging in children under the age of 5 because of the paucity of PQ pharmacokinetic (PK) data. We plan to assess the anti-infectivity efficacy of SLDPQ using an allometrically scaled, weight-based regimen, with a target dose of 0.25 mg/kg, in children with acute uncomplicated falciparum malaria.
Methods
This study is an open label, randomised 1:1, phase IIb study to assess TBE, tolerability, pharmacokinetics and acceptability of artesunate pyronaridine (ASPYR) administered alone or combined with SLDPQ in 56 Burkinabe children aged ≥ 6 months– < 5 years, with uncomplicated P. falciparum and a haemoglobin (Hb) concentration of ≥ 5 g/dL. We will assess TBE, using direct membrane feeding assays (DMFA), and further investigate PQ pharmacokinetics, adverse events, Hb dynamics, G6PD, sickle cells, thalassaemia and cytochrome 2D6 (CYP2D6) status, acceptability of flavoured PQ [CAST—ClinSearch Acceptability Score Test®], and the population’s knowledge, attitude and practices on malaria.
Expected results and discussion
We expect children to accept tablets, confirm the TBE and gametocytocidal effects of SLDPQ and then construct a PK infectivity model (including age, sex, baseline Hb, G6PD and CYP2D6 status) to define the dose response TBE relationship that may lead to fine tuning our SLDPQ regimen. Our study will complement others that have examined factors associated with Hb dynamics and PQ PK. It will provide much needed, high-quality evidence of SLDPQ in sick African children and provide reassurance that SLDPQ should be used as a strategy against emerging ARPf in Africa.
Trial registration
ISRCTN16297951. Registered on September 26, 2021
Supplementary Information
The online version contains supplementary material available at 10.1186/s13063-024-08428-8.
Keywords: Malaria, Children, Infectivity, Gametocytes, Artesunate pyronaridine, Single low-dose primaquine
Introduction
Background and rationale {6a}
In humans, malaria parasites (sporozoites) are injected by feeding female Anopheles mosquitoes and pass via the liver to then invade red blood cells, grow and reproduce. This asexual cycle causes symptomatic disease and may also result in asymptomatic parasitaemia and anaemia. As part of the life cycle in humans, some parasites also develop into male and female forms, called gametocytes. When mosquitoes bite humans, they also take up gametocytes which sexually recombine to form oocysts in the mosquito gut. Inside oocysts sporozoites develop and mature before migrating to the salivary glands ready to be injected back into humans at the next mosquito feed to continue the parasite’s life cycle.
Plasmodium falciparum is the main malaria species in the world causing 249 million cases and 608,000 deaths in 2022 in 85 malaria endemic countries. The World Health Organization (WHO) African Region still bears the largest burden of malaria morbidity with 233 million cases in 2022, accounting for 94% of cases and 580,000 deaths; 76% occurring in children under the age of 5 years [1, 2].
Faced with the high burden of malaria, control measures recommended by WHO include rapid diagnosis and treatment of cases, malaria chemoprevention (seasonal malaria chemoprevention and intermittent preventive treatment in pregnancy and in infants < 1 year), the distribution of long-lasting insecticide impregnated nets (LLINs) and indoor residual spraying (IRS) [3].
Although these tools and interventions have led to substantial gains in the fight against malaria over the past 20 years, there are several reasons to believe that they are insufficient to maintain current progress and to achieve the goal of malaria elimination set in the WHO ‘Global Technical Strategy for malaria 2016–2030’ [2]. Inadequate use and insufficient coverage of the current malaria control interventions, the high prevalence of low-level parasitaemia in asymptomatic individuals, the persistence and spread of malaria drug resistance, including artemisinin resistance, the false negative results by rapid diagnostic tests (RDTs) because of dual pfhrp2 and pfhrp3 deleted parasites, and vectors resistant to insecticides are some of the factors which could compromise the goal of malaria elimination in the next 10 years [2, 4, 5].
Of these, the greatest threat to sub-Saharan Africa is the recent emergence of artemisinin-resistant P. falciparum (ARPf). Artemisinin resistance is marked by single nucleotide polymorphisms in the propeller domain of pfkelch13. Independent of the ARPf in South East Asia, it is now well documented across East Africa with evidence of declining efficacy of artemether-lumefantrine, a widely used artemisinin-based combination therapy (ACT) in Africa [6–12]. History has documented the devastating impact of drug resistance on malaria-related morbidity and mortality [13] and sustained efforts are needed to avert a future disaster in sub-Saharan Africa.
ARPf was first documented in SE Asia in the early 2000s and has spread beyond the initial focus of western Cambodia [14–16] with a catastrophic decline in the efficacy of dihydroartemisinin-piperaquine, a key ACTs [17]. Increased gametocyte carriage has been shown in patients infected with ARPf [16] and demonstrated higher male to female gametocyte ratios in PfKelch13-mutant isolates compared to PfKelch13 wild-type infections [16]; both could contribute significantly to increase transmissibility and spread of ARPf.
In 2012, the WHO recommended adding single low-dose primaquine (SLDPQ) to ACTs when treating patients with acute uncomplicated P. falciparum without testing for glucose-6-phosphate dehydrogenase deficiency (G6PDd). The primary aim was to stop the spread of ARPf in the low transmission areas across SE Asia [18]. Subsequent studies have shown high transmission blocking efficacy (TBE) in reducing mosquito infectivity and substantial reductions in gametocyte carriage [19–22]. If deployed on a large scale, SLDPQ could reduce the pool of gametocytes and the spread of P. falciparum malaria, making SLDPQ an important tool to eliminate ARPf in Africa that control programmes should consider deploying.
The WHO recommended dose of 0.25 mg/kg dose failed to consider allometric dosing for young children and the studies of Dicko et al. and Goncalves et al. gave exact mg/kg doses [19, 23, 24]. SLDPQ PK data suggest that PQ exposure is influenced by age, weight and CYP2D6 genotype [24, 25]. This suggests a nonlinear relationship between exposure and dose, i.e. children had lower exposure than older children for the same mg/kg dose and should be considered in studies of PQ involving young children.
An age-based SLDPQ regimen suitable for sub-Saharan Africa has been developed [26] and tested in a large placebo-controlled trial [27] of uncomplicated falciparum malaria in G6PDd African children aged 0.5–11 years. SLDPQ had a similar tolerability as placebo and the same transfusion rate as placebo (0.9%), supporting data from earlier safety studies [28]. PK data from this study demonstrated higher median PQ and carboxyPQ exposures [25] compared to asymptomatic parasitaemic children dosed by Goncalves et al. [24] and is partly explained by transmission blocking properties of PQ.
Despite the growing evidence base of the safety and efficacy of SLDPQ, the uptake of SLDPQ remains low due residual anxiety of precipitating acute haemolysis in patients with G6PDd as well as the unavailability of quality-assured paediatric formulations that are approved by the WHO.
To meet these challenges, a consortium called ‘Developing Paediatric Primaquine’ has been established between Europe and Africa (www.Welcome - DPP PROJECT (dpp-project.org)). They aim to develop child-friendly formulations masking the bitter taste of primaquine to enhance adherence [29], assess the acceptability of flavoured primaquine tablets by the paediatric population, and prequalify them across a range of dosage strengths (2.5, 5, 7.5 and 15 mg), to make them widely available through global supply of antimalarials. Part of this effort is to conduct a randomised trial of artesunate pyronaridine (ASPYR) administered alone or combined with SLDPQ in falciparum-infected children to assess PK, safety and TBE. We will use a weight-based, allometrically scaled SLDPQ regimen designed by the research team; the generated PK data will complement our PK data from the age dosed regimen [25] and should lead to a robust SLDPQ regimen.
Objectives {7}
The primary study objective is to assess the TBE of SLDPQ using an allometrically scaled, weight-based regimen in paediatric patients (< 5 years) with acute uncomplicated falciparum malaria.
The secondary objectives are to assess/document: (i) oocyst intensity and sporozoite index, (ii) oocyst and sporozoite prevalence, (iii) gametocyte dynamics, (iv) tolerability, (v) PK profiles of PQ and carboxyPQ, using a population PK approach, (vi) acceptability of the flavoured paediatric primaquine tablets and ASPYR granules, and (vii) knowledge, attitudes and practices survey on malaria and health seeking behaviour.
Trial design {8}
This is an open, randomised, parallel, phase IIb trial assessing the TBE, tolerability and PK of SLDPQ in paediatric patients from Burkina Faso with acute uncomplicated Plasmodium falciparum malaria. Patients will be randomised to receive either ASPYR alone (control) or ASPYR + SLDPQ (intervention).
Methods: participants, interventions and outcomes
Study setting {9}
The study will be conducted by the Groupe Action de Recherche en Santé (GRAS) in Sabou town in the Sabou health district (SHD) in Boulkiemdé region, located about 100 km directly to the west of Ouagadougou, the capital of Burkina Faso. The SHD covers an area of 449 km2 and has 112,485 inhabitants who live mostly in small rural villages in houses made of mud or cement walls and thatched or metal roofs. The population is stable and the Mossi are the predominant ethnic group; farming is their main occupation.
SHD is in a Sahelian eco-climatic zone, characterised by a single rainy season from May to October followed by a long dry season. This explains the highly seasonal nature of malaria transmission with most cases seen during or immediately following the rainy season. There are 20 peripheral heath facilities in SHD, which are the primary points of contact with the health system for the population. The study will be conducted at the St. Maximilian de Kolbe Hospital, in Sabou town. It is the main national health service referral centre for the district and provides out- and inpatient care.
The trial unit in the hospital is organised to ensure that all the key steps in the path of a clinical trial participant journey run smoothly by the use of dedicated rooms for consenting potential participants, clinical examination, biological sampling, administration, and patient surveillance and resuscitation. Biological samples are processed in an onsite laboratory that is well equipped and is able to read malaria slides. Biochemistry and haematology samples that require storage for later processing are kept temporarily in refrigerators (2–8 °C) and freezers (− 20 °C, − 30 °C), then transferred to the GRAS headquarters where they will be stored at − 80 °C until shipment or processed. Investigational medicinal products are stored in a dedicated storeroom that is maintained at the appropriate storage temperature, in accordance with the manufacturer’s instructions, using remote temperature monitoring devices.
Eligibility criteria {10}
The inclusion and exclusion criteria are shown below.
Inclusion criteria
These are as follows: (i) written informed consent given by a parent/legal guardian, (ii) aged ≥ 6 months and < 5 years, (iii) weight ≥ 5 kg, (iv) fever (axillary ≥ 37.5 °C, tympanic ≥ 38 °C) or fever history ≤ 72 h and clinically uncomplicated disease, (iv) P. falciparum parasitaemia 1000–250,000/μL (mono-/mixed Plasmodium species) detected by light microscopy, and (v) able and willing to comply with the protocol for the duration of the study.
Exclusion criteria
Any one of the following is an exclusion criterion: (i) general danger signs or signs of severe falciparum malaria according to the definitions of WHO [30], e.g. prostration, respiratory distress, reduced consciousness and persistent vomiting, (ii) P. falciparum parasitaemia > 250,000/μL (> 5% parasitaemia), (iii) haemoglobin (Hb) < 5 g/dL, (iv) treatment for a significant illness, e.g. HIV/AIDS, tuberculosis and leprosy, or currently taking a drug known to cause haemolysis in G6PDd, (v) known to be allergic to PQ or ASPYR, (vi) on regular medication that might interfere with antimalarial drug pharmacokinetics, (vii) antimalarials taken within the last 2 weeks, (viii) taken a herbal medicine within the last 4 weeks, (ix) previous participation in a malaria vaccine trial, (x) previous enrolment in the current trial or current enrolment in another trial, (xi) severe malnutrition—defined as a mid-upper arm circumference (MUAC) < 115 mm, and (xii) febrile due to a disease other than malaria (e.g. measles, acute lower respiratory tract infection, severe diarrhoea with dehydration) or other known underlying chronic or severe diseases (e.g. cardiac, renal or hepatic diseases, HIV/AIDS).
Who will take informed consent? {26a}
The inform consent will be obtained by the PI and the study physicians. Parents of eligible children will provide written informed consent in a private setting prior to screening and possible enrolment. The consent form will be signed or thumb-printed by the child’s parent/guardian prior to any study procedure. The parent/guardian will also tick the box to allow the child’s blood to be stored for future analysis related to this study. Providing a thumbprint is equivalent to a written signature for adults who cannot read or write.
A copy will be given to the parent/guardian and another copy will be kept on site. If a parent/guardian is unable to read or sign, an impartial witness will document that all information has been provided and consent has been freely given. Once informed consent has been obtained, children will be screened for eligibility and included or excluded according to the inclusion/exclusion criteria.
Additional consent provisions for collection and use of participant data and biological specimens {26b}
We will request consent from participants for their inclusion in the study, which will include the collection of blood samples for the purpose of assessing the safety of the drug in question and the incidence of infection in mosquitoes.
Interventions
Choice of comparator {6b}
ASPYR has been selected as a comparator because it is one of the ACT drugs commonly used to treat malaria in the study area.
Intervention {11a}
The intervention drugs are ASPYR and PQ.
ASPYR
We will use Pyramax®, manufactured by Shing Poon. ASPYR granules will be used for patients weighing between 5 and 20 kg, and tablets for higher weights (Table 1). ASPYR will be dissolved in ~ 10 mL, i.e. 2 teaspoons, of water and administered once a day for 3 days.
Table 1.
Dose of SLDPQ
| Weight kg | Dose in mg | mg/kg dose received |
|---|---|---|
| 5– < 8 | 2.5 | 0.31–0.50 |
| 8– < 12 | 3.75 | 0.34–0.47 |
| 12– < 18 | 5.0 | 0.29–0.42 |
| 18– < 31 | 7.5 | 0.25–0.42 |
Primaquine
The flavoured PQ paediatric tablets, in strengths of 2.5, 3.75, 5 and 7.5 mg PQ base, will be supplied by IPCA, the drug manufacturer from India. Administration is once before the first dose of ASPYR to assess acceptability. For young children who cannot swallow, PQ will be dissolved in 5 mL of water and the dose administered (Table 2).
Table 2.
Dosing table for primaquine
| Weight/kg | Dose in mg | Dose in mg/kg |
|---|---|---|
| 5– < 8 | 2.5 | 0.31–0.50 |
| 8– < 12 | 3.75 | 0.34–0.47 |
| 12– < 18 | 5 | 0.29–0.42 |
| 18– < 36 | 7.5 | 0.25–0.42 |
| 36– < 70 | 15 | 0.21–0.42 |
| ≥ 70 | 22.5 | ≤ 0.32 |
All doses of study drugs will be administered by research nurses. A small snack or milk will be given with every dose to reduce the risk of PQ-induced abdominal pain. Patients will be observed for 60 min. Any patient who vomits within the first 30 min will be retreated with the same dose and observed for an additional 30 min. If the vomiting occurs between 31 and 60 min, patients will receive an additional half dose. If the patient vomits again, oral treatment will be stopped and rescue therapy will be given according to national guidelines.
Acceptability study
The acceptability of ASPYR and flavoured PQ will be assessed using the CAST—ClinSearch Acceptability Score Test® by assigning grades to the different aspects of administering PQ, e.g. was the dose taken complete, partly or not at all; patient’s reaction during the administration (positive, neutral or negative); and preparation and administration time, classified as short (< 1′), medium (1′ to 2′30″) or long (> 2′30″). Additional parameters are methods used to ease/achieve PQ administration, e.g. dividing the intake of the dose which cannot be taken as a whole; altering the intended use; using food/drink to allow intake or to mask the drug taste; using a device not provided such as a syringe; promising a reward; and using restraint to achieve administration. All of these data are then integrated into a multivariable model to derive an acceptability score visualised into a 3D reference framework [31, 32].
Qualitative study
A knowledge, attitudes and practice (KAP) survey on malaria and health seeking behaviour will be conducted. This will consist of a list of mostly yes/no questions about malaria, e.g. how commonly affected people are, its dangers, what families do when there is a fever in the family, do they take all the prescribed drugs and are drugs kept for other children? We will also collect basic economic data, including willingness to pay, household income, cost of visiting the clinic and loss of work days.
Focus group discussions on broader issues will include issues around PQ adherence, how to improve it, monitoring side effects in the community and using a drug like PQ that aims to improve health in the community but has no individual benefit. Other issues will include knowledge about malaria, home management of fevers/treatment seeking behaviour and perceptions on the use of tablets vs. granules.
Discussions will be facilitated by the team social scientist. We plan for a sample size of approx. 8 to 12 caregivers/legal guardians as well as the heads of families. The process will be explained to caregivers/legal guardians when informed consent is taken. Sessions will be lasting approx. 60 min but caregivers/legal guardians can leave sessions at any time during the process.
Follow-up
All caregivers/legal guardians will be invited to allow their child to be admitted to hospital for at least 72 h for close monitoring, receive directly observed therapy for all doses of their treatment and allow the study investigations and acceptability (CAST® questionnaire) and qualitative surveys to be conducted. If patients are well on study day 2, they will be discharged. Children of caregivers/legal guardians who decline this invitation will be followed up as outpatients; this will include daily visits to the hospital so as to administer the treatment. All study drugs will be given supervised by the research team.
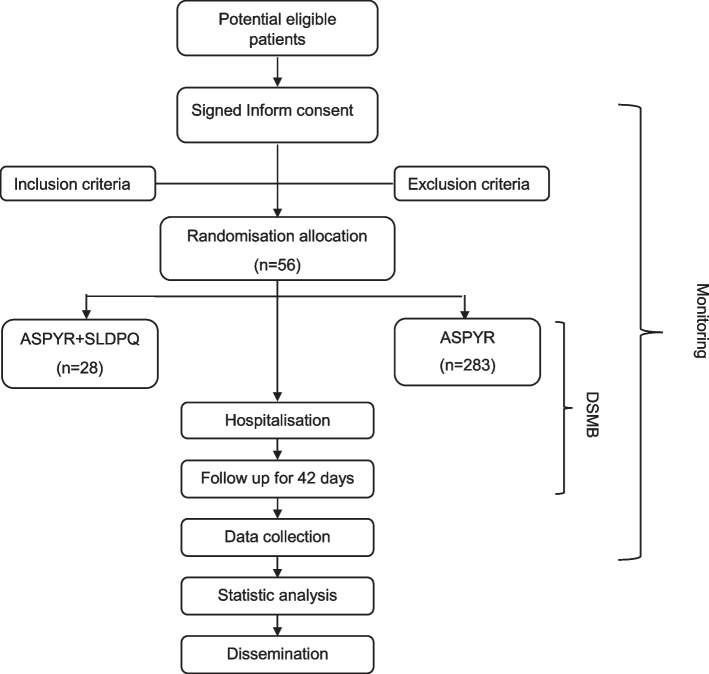
The flow diagram of the study process is shown in Fig. 1. Each participant will be assigned seven visits (one visit every week), after 3 days of hospitalisation and will be followed up until day 42. The schedule for enrolment, intervention and assessment is shown in Table 3. Infectivity will be assessed by direct membrane feeding assays (DMFA) at days 0, 1, 2 and 7. Retention of participant is promoted by close and frequent contact with the caregiver/legal guardian, field worker and study physician via telephone and study visits. In case of relevant issues, visits outside the study schedule are offered.
Fig. 1.
The flow diagram of the study progress
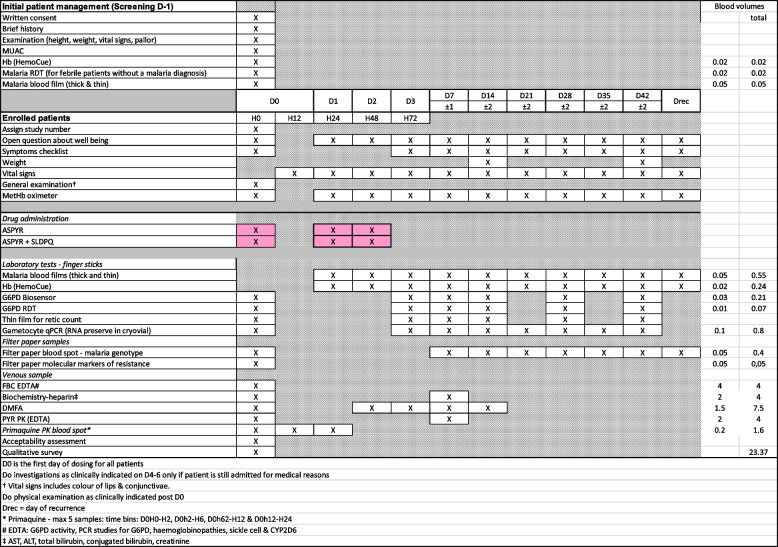
Table 3.
The study schedule
Ethics statement
The study was approved by the Oxford Tropical Research Ethics Committee of the University of Oxford and the Health Research Ethics Committee of Burkina Faso. The trial registration number is ISRCTN16297951. This protocol has been reported according to the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) 2013 statement.
Criteria for discontinuing or modifying allocated interventions {11b}
Patients will be informed that they are free to discontinue the study drug or withdraw from the study at any time and for any reason. The investigators may discontinue the study drug or withdraw a patient from the study, if they believe it is not in the best interest of the patient to continue the study.
Strategies to improve adherence to interventions {11c}
We will engage with local communities before the study starts to inform them of the study, its aims, risks and benefits. Peripheral health facilities will be encouraged to refer subjects who meet the study criteria.
The follow-up is 6 weeks and this may result in loss to follow-up but this is very unlikely in the first 48 h, thus protecting our day 2 primary outcome. All the same, we will take steps to reduce loss to follow-up by emphasising the usefulness of the study, recruiting patients who live close to the research clinic, paying for transport, snacks and refreshments, contacting patients by mobile phone who do not attend for follow-up and going to patient’s home if they do not have a mobile phone.
Study drug accountability and disposal
Drug accountability will be done by the research team member, according to a standard operating procedure (SOP).
Relevant concomitant care permitted or prohibited during the trial {11d}
All ‘outpatient’ acute illnesses occurring during the trial will be treated free of charge. The study insurance will take care of significant drug-related harms.
Caregivers will be asked not to visit local pharmacies, doctors and drug vendors to buy drugs or herbal medicines but febrile patients may use paracetamol. All drugs that are taken by patients will be noted on the concomitant drug form and any details of adverse events (AEs) noted on the AE CRF. Certain drugs and grapefruit juice are not allowed to be taken in the study if they are potentially haemolytic or may interfere with the PK of the study drugs (Table 4).
Table 4.
Drugs contraindicated in this study
| Dapsone |
|---|
| Sulphonamides |
| Methylene blue |
| Nalidixic acid |
| Nitrofurantoin |
| Niridazole |
| Aspirin |
| Traditional medicines |
| The following drugs may have a possible risk of haemolysis: |
| Ciprofloxacin, norfloxacin |
| Chloroquine |
| The following drugs are considered to be of doubtful risk of haemolysis: |
| Chloramphenicol |
| Quinine |
| The following drugs may increase the exposure of PQ metabolism by inhibiting the cytochrome (CYP) 450 and should not be prescribed by the research team unless clinically essential: |
| Ketoconazole, itraconazole |
| Cimetidine |
| Grapefruit juice |
| Erythromycin |
| Ritonavir |
| The following drugs may decrease the exposure of PQ, by inducing CYP450. They should not be prescribed by the research team unless clinically essential: |
| Barbiturates |
| Carbamazepine |
| Phenytoin |
| Rifampicin |
| Macrolide antibiotics |
| Glucocorticoids |
| The following drug classes are metabolised by CYP2D6 and may compete with PQ [47]. They should not be prescribed by the research team unless clinically essential: |
| Tricyclic antidepressants, e.g. amitriptyline |
| Major tranquilisers: haloperidol |
| Atypical antipsychotics: risperidone |
| Selective serotonin reuptake inhibitors: paroxetine |
| Beta blockers, e.g. carvedilol, metoprolol |
| Opiates: codeine, tramadol |
| The following drugs are CYP2D6 inhibitors and may reduce the concentrations of PQ’s active oxidative metabolites. They should not be prescribed by the research team unless clinically essential: |
| Amiodarone |
| Cimetidine |
| Diphenhydramine, terbinafine |
| Fluoxetine, paroxetine |
| Quinidine |
| Ritonavir |
Provisions for post-trial care {30}
It should be noted that the provision of post-trial care is not included in the aforementioned programme. All the AEs occurring during the trial will be followed up until the end of the event or its stabilisation. A study insurance policy has been arranged to provide coverage for any harm that may befall a participant as a result of their involvement in the trial.
Outcomes {12}
There are two primary outcomes: (i) the proportion of patients infecting ≥ 1 mosquito in the ASPYR arm vs. ASPYR + SLDPQ arm at D0, 1, 2 and 7 and (ii) the mean within-person percentage change in mosquito infectivity on D1, 2 and 7 vs. D0.
The secondary outcomes measures will be the:
Proportion of mosquitoes with detected oocysts and sporozoites
Oocyst intensity and sporozoite index
Gametocyte carriage over time
Relationships between PQ exposure and anti-infectivity efficacy, haemoglobin dynamics and recovery by day 28
Presence of sickle cell trait/disease, thalassemia and G6PD variants
Cure rate at D42
CAST scores of ASPYR + SLDPQ
Proportions of patients/careers responding to specific questions
Adverse and serious adverse events (AEs, SAEs)
Safety assessment
At each visit, caregiver/legal guardian will be asked about any reported symptoms since the previous visit. On certain study days, a symptom checklist will also be administered. If the research team physician thinks these symptoms require further evaluation, this will be done. The investigator is responsible for the detection and documentation of AEs and SAEs. Any additional relevant laboratory results obtained by the investigator during the course of this study will also be recorded.
All AEs will be treated as clinically indicated and concomitant treatment recorded. If necessary, participants will be referred for specialist care. All patients will be followed up until the AE resolved or stabilised, even if this occurs after study D42. This includes patients who withdraw themselves or have been withdrawn from the study by the PI.
Participant timeline {13}
Blood samples, which will be collected at days 0, 1, 2, 3 and each week until D42, will be collected for the following: safety, direct membrane feeding assay, pharmacokinetics, parasite diagnostic and genotyping. Medical assessment will be done at each contact. Table 3 outlines the study schedule.
Screening visit
Potential participants with a positive rapid diagnostic test (RDT) for malaria who are referred from the peripheral local health facility will be screened. Informed consent will be obtained by one of the study physicians before any study procedure takes place. At this visit, the medical history will be assessed through demography, previous medical and surgical history, prior and current medication and reported symptoms will be recorded. Anthropometric data and vital signs will be measured and a physical examination performed. A finger prick sample will be taken for Giemsa-stained thick and thin blood films and to measure the Hb level. Patients will be enrolled in the study if they meet all of the inclusion criteria and have none of the exclusion criteria. They will be given a subject number and will be randomised to one of the study arms using a computer-generated randomisation.
Hospitalisation
All caregivers/guardians will be invited to allow their child to be admitted to hospital for at least 72 h for close monitoring, receive directly observed therapy for all doses of their treatment and allow the study investigations and acceptability (CAST® questionnaire) and qualitative surveys to be conducted. If patients are well on study D2, they will be discharged. Children of caregivers/guardians who decline this invitation will be followed up as outpatients; this will include daily visits to the hospital so as to administer the treatment. All study drugs will be given supervised by the research team.
Follow-up visit
All patients, whether admitted or who have opted for full outpatient follow-up, will be seen regularly from day 1 to the day 42 according to the schedule in Table 3.
At each visit, clinical and laboratory investigations will be done and they will vary depending on the study day. During the visit, the clinical assessment will include the symptom checklist, temperature, vital signs, the detection of pallor of the nail beds, palms and conjunctivae, and the Masimo pulse-CO oximetry measured methaemoglobin. The venous blood sample will be used for the DMFA, pyronaridine concentration and routine biochemistry. Finger stick samples could be used to assess asexual and sexual parasite counts through thick and thin blood films, gametocyte qPCR, Hb measurement, G6PD biosensor, G6PD RDT and filter paper samples for malaria parasite genotype and PQ PK.
Unscheduled visit
Patients will be asked to return to the clinic at any time of the night or day, if they feel unwell or develop a fever. Patients presenting to the clinic with a fever or other symptoms on unscheduled days will be assessed by the study physician. Their temperature will be recorded, a blood smear will be made and the Hb measured by HemoCue and a filter paper sample taken for parasite genotype. Patients will be treated as clinically indicated and will continue study procedures. Data from unscheduled visits will be recorded in the CRF and will be analysed.
Sample size {14}
We have used the infectivity data from Dicko et al. who conducted a primaquine dose ranging study with dihydroartemisinin-piperaquine (DHAPP) [19]. They found day 2 infectivity rates of 10% in the 0.25 mg/kg PQ arm and 55% in the DHAPP (control) arm. Using these data, a power of 90%, a two-sided alpha of 0.05 and continuity correction, the sample size is 25 patients per arm giving a total of 50 patients. In order to account for some losses to follow-up, we have inflated the sample size by 10%. Thus, we will recruit 56 patients, i.e. 28 patients per arm.
Recruitment {15}
We are working in Sabou town where we have established a network of satellite clinics based around St. Maximilian’s Hospital. This include 20 peripheral heath facilities. It is in a Sudan Sahelian eco-climatic zone. The climate consists of a single rainy season from May to October followed by a long dry season. This defines the highly seasonal malaria transmission with most malaria episodes experienced during or immediately following the rainy season. Through community outreach prior to the study, we will encourage parents of children with fever to attend our health centre. We will also inform the satellite clinics about the study and encourage them to refer sick children who meet the inclusion criteria.
Assignment of interventions: allocation
Sequence generation {16a}
Eligible patients will be randomly assigned on a 1:1 basis to ASPYR or ASPYR + SLDPQ. Treatment sequence will be determined by a computer-generated list.
Concealment mechanism {16b}
The treatment allocation will be contained in opaque sealed envelopes and only opened once a participant is enrolled into the study.
Implementation {16c}
The sealed opaque envelopes will be held by the study pharmacist who will be responsible for ensuring the correct allocation. A study (randomisation) number, starting at 001, will be allocated once a participant is enrolled. The pharmacist will find the envelope with the same allocated study number and open the envelope. The treatment allocation describing the treatment arm will be provided to the physician and the nurse in charge of the drug administration.
Assignment of interventions: blinding
Blinding {17a}
Blinding was not used in this trial due to limited resources for producing a placebo of the primaquine paediatric drug.
Procedure for unblinding {17b}
Not applicable as no blinding was used in this trial due to limited resources for producing a placebo of the primaquine paediatric drug.
Data collection and management
Plans for assessment and collection of outcomes {18a}
All caregivers/legal guardians will be invited to allow their child to be admitted to hospital for at least 72 h for drug administration and close monitoring. After the study drug administration period, the follow-up is 6 weeks with weekly visit at the research unit.
Plans to promote participant retention and complete follow-up {18b}
We will take steps to reduce loss to follow-up by emphasising the usefulness of the study, paying for transport and snacks and refreshments, contacting patients by mobile phone who do not attend for follow-up and going to patient’s home if they do not have a mobile phone.
Data management {19}
A data management plan outlining procedure for collection, curation and storage of data will be created and followed. Study data will be derived from source documents or recorded directly onto a paper case record form (CRF). Data will be entered from the paper CRF to MACRO EDC©, a GCP-compliant data management system. The database is password-protected and includes internal quality checks to identify data that appear inconsistent, incomplete or inaccurate. Study participants will be identified by a unique study number in the database. Names or any other identifying details will not be included in the database or datasets used for analysis. The site principal investigator is responsible for ensuring data collection and entry are accurate and complete and for keeping all screening forms, the case record form and the completed subject identification code list in a secure location.
Participants’ records will be stored in binders or scanned and stored electronically.
Once the database is complete and declared clean, it will be locked for data analysis, using Stata v.15 or higher (Stata Corporation, TX, USA).
Confidentiality {27}
The study staff will ensure that the participants’ anonymity is maintained. The participants will be identified only by a subject number on all study documents and all electronic database. All documents will be stored securely and only accessible to study staff and authorised personnel. The study will comply with the Data Protection Act 2018, which requires that personal data must not be kept as identifiable data for longer than necessary for the purposes concerned.
Plans for collection, laboratory evaluation and storage of biological specimens for genetic or molecular analysis in this trial/future use {33}
The blood volumes per test are detailed in the schedule. The total calculated volume of blood is a little under 20 mL. We anticipate that we may have to take extra blood, e.g. clotted samples or clinical reasons. Therefore, we will consent participants for up to 25 mL of blood.
Samples may be sent outside of Burkina Faso for additional analyses, following all applicable local regulations and according to a material transfer agreement between GRAS and MORU (or another laboratory) which will be complete prior to any sample shipment.
Biological specimens will be maintained until the end of the study. This is to allow time for all study-related testing. The specimens will be maintained at the GRAS laboratory in Ouagadougou, Burkina Faso and the MORU. No personal-identifying information associated with the study will be stored on site with the specimens. Following completion of the study, all specimens will be kept indefinitely in a secure location. If, before destruction, future unrelated studies are proposed for the specimens, such studies will be initiated with appropriate ethics committee approvals and, if deemed appropriate by such committees, informed consent from participants.
Statistical methods
Statistical methods for primary and secondary outcomes {20a}
All patients who received at least one dose of study drug will be included in the analysis. All analyses will be detailed later in a prospectively written analysis plan.
The main strategy for analysis of the primary end point will be assessed using the mixed effects linear models. Furthermore, the generalised linear mixed models with a binomial distribution will be fitted to model the PCR-measured gametocyte prevalence rates and continuous data (e.g. Hb, parasite counts). The models will include the follow-up days, treatment (ASPYR vs. ASPYR + SLDPQ), the presence of gametocytes prior to treatment on D0, recrudescence status (recrudescent vs. cured) and the D0 Hb concentration.
The patient identifier will be coded as a random factor to account for repeated measures on the same individual. Initially univariate analyses will be done to assess relationship of the outcome with each of the factors using a logistic regression. Identified significant factors from this model will then be included in a multivariable generalised linear mixed model to model the gametocyte dynamics. We will also analyse the infectivity data to obtain the mean within-person percentage change in mosquito infectivity on D1, 2 and 7 vs. baseline.
Univariate analyses of proportional data will be done using chi-squared or Fisher’s exact test, as appropriate. Similarly, for continuous data (e.g. Hb, parasite counts), univariate analyses will be performed by t-tests or their non-parametric equivalents, as appropriate. Analyses will be two sided and a p value of < 0.05 is considered statistically significant.
Interim analyses {21b}
There are no plans for interim analyses because the study enrols a small number of participants.
Methods for additional analyses (e.g. subgroup analyses) {20b}
No subgroup analysis is planned.
Methods in analysis to handle protocol non-adherence and any statistical methods to handle missing data {20c}
The analysis of protocol deviations will be conducted using per-protocol methods.
Plans to give access to the full protocol, participant-level data and statistical code {31c}
The dataset is not associated with this study protocol paper. However, the protocol will be made available to the research community after publication. At the completion of the trial study, de-identified data will be made available to the research community. More information is available through trial registration: ISRCTN16297951.
Oversight and monitoring
Composition of the coordinating centre and trial steering committee {5d}
Oxford University, as the sponsor, holds overall responsibility for delivering all trial elements, reporting and fostering team spirit. A monitoring plan will be created, and periodic meetings will be scheduled to track study progress and address any issues. A permanent communication system will also be established. Prior to the commencement of the clinical trial, the relevant local stakeholders will be duly informed. In accordance with the prevailing regulatory framework, safety reports will be submitted to the relevant authorities.
Composition of the data monitoring committee, its role and reporting structure {21a}
An independent Data Safety Monitor Board (DSMB) has been established and consists of qualified volunteers with the necessary knowledge of clinical trials. There is one chairwoman, one statistician and three other members. None has a conflict of interest. A DSMB charter has been written and has been approved by the DSMB. It outlines the DSMB responsibilities, frequency of meetings and, notably, details of safety monitoring and which data the DSMB wishes to see in real time, e.g. Hb data in patients, and all SAEs irrespective of the causality to study drugs. At the time of working, the study has not started but the DSMB has given input into the protocol.
Adverse event reporting and harms {22}
Safety management
Safety reporting will be done in accordance with Good Clinical Practices (GCP) guidelines. During the study, at each visit, we will collect and record on the CRF adverse events of all severities with a focus on the AEs of interest, listed below:
Early (≤ 1 h) nausea, vomiting and abdominal pain
Late (> 1 h) nausea, vomiting and abdominal pain
Hb concentration < 5 g/dL
Passing dark urine graded ≥ 5 or more on the Hillmen colour chart, witnessed by a research team member [33]
Jaundice
Itching
Rash
Any drug-related AE that requires inpatient treatment
Any grade 3 or 4 laboratory abnormalities
A methaemoglobin result > 10%
All SAEs and AEs will be promptly documented on the AE/SAE CRF from the moment of inclusion in the study to discontinuation of the patient from study participation. Any events occurring between screening and randomisation will be considered as baseline, pre-existing conditions.
All SAEs, irrespective of their relationship to the study drugs, will be reported to the sponsor safety team within 24 h of the research team becoming aware of the SAE.
Assessment of causality
Any AE that occurs will be assessed by the team physician to determine the relationship between the AE and the study drugs. Because two drugs are being used in this study, the AE may be related to one or both drugs. This drug-AE relationship will be graded as unrelated (clearly not related to the study drug), unlikely related (doubtfully related to the study drug), possibly related (may be related to the study drug), probably related (likely related to the study drug) and definitely related (clearly related to the study drug). Unrelated or unlikely related means that the AE is probably produced by the participant’s clinical state or by other modes of therapy administered to the participant. Possibly, probably and definitely related means that the AE follows within a ‘reasonable time frame’ after the study drug/s has/have been given and is not thought to be due to any other cause.
Follow‑up of AEs and SAEs
All AEs/SAEs will be treated as clinically indicated and concomitant treatment recorded on the concomitant medication CRF. If necessary, participants will be referred for specialist care.
The research team will refer patients for treatment to the local hospital/clinic for treatment following national guidelines. All patients will be followed up until the AE resolved or stabilised even if this occurs past study D42. This includes patients who withdraw themselves or have been withdrawn from the study by the PI.
Frequency and plans for auditing trial conduct {23}
The investigator and the sponsor’s representative will ensure that this study is conducted in full conformity with relevant regulations and with the 2018 ICH guidelines for GCP. The study will be monitored and this will be coordinated by MORU’s clinical trials support group (CTSG). The first monitoring will take place after about 5 patients have been recruited. If this is satisfactory, the 2nd monitoring will be scheduled after 50% of the patients have been enrolled. If the 2nd monitoring is also satisfactory, the 3rd monitoring will be done after the last patient is enrolled.
On site visits are expected to take 2–3 days and will include feedback to the study principal investigator (PI) and the team. A report will be written that will be shared with the site PI, CTSG and the sponsor. The site will be given 2 weeks to respond to the report and to highlight any remedial action points. In the event that the site needs additional visits, the following criteria will be used to make that determination: compliance issues, e.g. significant protocol violations and site staff turnover, requiring additional training.
Plans for communicating important protocol amendments to relevant parties (e.g. trial participants, ethical committees) {25}
The investigator will submit and obtain approval of all amendments to the original approved documents before implementation. These will be approved by the National Ethical Committee (Comité d’Éthique pour la Recherche en Santé: 284 N°2017/000098/MS/MERSI/CERS) and the National Regulatory Board (Ministry of Health: 285 N°5004520186EC0000) in Burkina Faso. Furthermore, the protocol will be approved by the Oxford Tropical Research Ethics Committee (OxTREC).
Dissemination plans {31a}
At the end of the study, the results will be shared with the scientific community, stakeholders and the local community through the publication of manuscripts, attendance at conferences, congresses and meetings. We will also update our web site.
Discussion
Blocking malaria transmission between humans and mosquitoes is an important part of the strategy to eliminate Plasmodium falciparum malaria and has taken on increasing importance since the emergence of ARPf in eastern Africa. PQ is available widely and cheap and has been used in SE Asia for some years. However, few countries in Africa are currently deploying it despite the growing evidence base of its safety and efficacy [34].
When the WHO recommended adding SLDPQ to ACTs to block the transmission of P. falciparum without testing for G6PD deficiency, it recommended a target dose of 0.25 mg/kg but there was no detailed dosing advice for young children. Since 2012, studies have established a dose-dependent effect of SLDPQ on gametocyte carriage in symptomatic [21] and asymptomatic [23, 35] P. falciparum-infected individuals and mosquito infectivity using DMFA [19, 36]. A recent meta-analysis supports the 0.25 mg/kg dose for gametocytocidal and TBE and also reconfirmed the greater gametocytocidal effect of artemether-lumefantrine compared to dihydroartemisinin-piperaquine [37].
Crucially, however, studies assessing transmission blocking efficacy have excluded acutely ill children < 5 years and only included a limited number of asymptomatic children < 5 years, but these two vulnerable populations both experience lower PQ exposures compared to older children [24, 25]. Goncalves et al.’s study of asymptomatic P. falciparum-infected children aged 2–14 years (median 8 and 10 years) failed to define the TBE of SLDPQ (0.25 and 0.4 mg/kg) because too few children were infectious post day 0 and Okebe et al.’s study (median age 10 years) had the same issue—only 2/170 participants (median age 10 years) were infectious to mosquitoes [35]. Only Dicko et al. were able to define TBE in their study of mostly asymptomatic but gametocyte positive children (≥ 5 years) and adults (median age 12 years) [19]. They also gave PQ on day 0, in accordance with the WHO recommendation in contrast to Eziefula, Goncalves, Okebe and Raman [21, 23, 35, 38].
One weakness of many PQ studies is the exclusion of G6PD-deficient individuals. To fill this critical gap, a large study of 1137 children covering the full range of G6PD status aged 6 months–11 years reconfirmed lower PQ and carboxyPQ exposures, dosed on day 0, that was related to greater clearance/kg of PQ in the 150 children who underwent intense PK sampling [25]. The study also established the safety of the age dose regimen used [26] in the large number (n = 284) of G6PDd children and 108 G6PD heterozygous female children [39].
What will our proposed study add? We are proposing a SLDPQ regimen that has been designed using allometrically scaled body weight as a covariate with the exponents fixed to 0.75 for clearance parameters and 1 for volume parameters [40]. The proposed regimen has a 3.75 mg of PQ band; this tablet strength was on the WHO prequalification list but was later removed for reasons that remain unclear. This regimen underscores the need for us to collect PK data to inform the optimal dose of SLDPQ and inform future studies to determine the optimal SLDPQ dose in malnourished children and in children with ARPf infections. Unfortunately, funding has not been forthcoming despite robust proposals.
To our knowledge, the proposed randomised trial in under the age of 5 years in Burkina Faso will be one of the first in Africa to treat symptomatic uncomplicated malaria to assess TBE and determine the pharmacokinetics of the WHO-recommended SLDPQ regimen with its 0.25 mg/kg target dose. The study will also provide the first information on a range of PQ tablet strengths (2.5, 3.75, 5 and 7.5 mg), where one tablet equals one dose, and determine the acceptability of the tropical flavouring in masking the bitter taste of PQ. The qualitative aspects of the study will provide useful information from the community’s point of SLDPQ, which is an intervention designed to confer a community rather than a direct individual benefit.
The rationale for using ASPYR is that it is an approved first-line antimalarial used to treat uncomplicated malaria in Burkina Faso and efficacious [41, 42] and well tolerated [43, 44] and there remains limited work on PQ combined with ASPYR in healthy Thai adults [45] and falciparum-infected Malians aged at least 5 years [46].
Findings from this research and other related research will allow us to determine an optimal dose of SLDPQ that can be used without G6PDd testing. While waiting for these results, NMCPs should not hesitate to start deploying SLDPQ to counter the spread of ARPF in Africa.
Trial status
Protocol 3.0 of 8 April 2022. The recruitment will start in June 2024 and should be completed by December of the same year.
Supplementary Information
Acknowledgements
The authors thank the EDCTP and the staff of MORU and Groupe de Recherche Action en Santé in Ouagadougou. WRT and MM are supported by the Wellcome Trust of Great Britain through its core grant (220211) to the Mahidol Oxford Tropical Medicine Research Unit research programme. For the purpose of Open Access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission.
Abbreviations
- ACT
Artemisinin-based combination therapy
- AEs
Adverse events
- ARPf
Artemisinin-resistant P. falciparum
- ASPYR
Artesunate pyronaridine
- CAST®
ClinSearch Acceptability Score Test
- COI
Conflicts of interest
- CRF
Case report form
- CTSG
Clinical trials support group
- DHAPP
Dihydroartemisinin-piperaquine
- DMFA
Direct membrane feeding assay
- DSMB
Data Safety Monitor Board
- GCP
Good Clinical Practices
- GRAS
Groupe Action de Recherche en Santé
- Hb
Haemoglobin
- IRS
Indoor residual spraying
- KAP
Knowledge, attitudes and practices
- LLINs
Long-lasting insecticide impregnated nets
- MORU
Mahidol Oxford Tropical Medicine Research Unit
- MUAC
Mid-upper arm circumference
- NMCP
National Malaria Control Programme
- P.f
Plasmodium falciparum
- PI
Principal investigator
- PK
Pharmacokinetics
- PQ
Primaquine
- RDTs
Rapid diagnostic tests
- SAEs
Serious adverse events
- SHD
Sabou health district
- SOP
Standard operating procedure
- SPIRIT
Standard Protocol Items: Recommendations for Interventional Trials
- SLDPQ
Single low-dose primaquine
- TBE
Transmission blocking efficacy
- WHO
World Health Organization
Authors’ contributions {31b}
The criteria for authorship will follow international guidelines and will be reported in the format of the journal concerned. AO, JNP, TK, AR, PM, SBS and WRT conceived this study, developed the protocol, managed the ethical submissions and reviewed the manuscript. MM will develop the statistical analysis plan and reviewed manuscript. TV and FR are responsible for the formal analysis of acceptability evaluations. All authors have read and approved the final manuscript.
Funding {4}
This publication is based on the Developing Paediatric Primaquine research project which is part of the EDCTP2 programme supported by the European Union (RIA2019PD2893-DPP). The funders had no role in the study design, execution, and analysis and decisions regarding publication.
Availability of data and materials {29}
The final database will be shared amongst the site PI and key members of the research team. The database may be shared with researchers not directly involved in this study but only after the main paper has been published and in accordance with GRAS and MORU guidelines on data sharing. The database will only be shared if future publications are not compromised.
Declarations
Ethics approval and consent to participate {24}
The protocol, informed consent form and participant information sheet were submitted to the Oxford Tropical Research Ethics Committee (OxTREC) and local ethics committees (the National Ethical Committee ‘Comité d’Éthique pour la Recherche en Santé: 284 N°2017/000098/MS/MERSI/CERS’) and regulatory authorities (Ministry of Health: 285 N°5004520186EC0000) in Burkina Faso as per local requirements, for written approval.
Consent for publication {32}
All the authors agree for this publication. It should be noted that no identifying images or other personal or clinical details of participants are presented here, nor will they be presented in reports of the trial results. The participant information materials and informed consent form are available from the corresponding author upon request.
Competing interests {28}
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Organization WH. World malaria report 2023. 2023. [Google Scholar]
- 2.Organization GWH. World malaria report 2020: 20 years of global progress and challenges. Licence: CC BY-NC-SA 3.0 IGO. 2020.
- 3.WHO. Guidelines for malaria vector control. 2019. [PubMed] [Google Scholar]
- 4.Bousema T, Okell L, Felger I, Drakeley C. Asymptomatic malaria infections: detectability, transmissibility and public health relevance. Nat Rev Microbiol. 2014;12(12):833–40. 10.1038/nrmicro3364 [DOI] [PubMed] [Google Scholar]
- 5.Heinemann M, Phillips RO, Vinnemeier CD, Rolling CC, Tannich E, Rolling T. High prevalence of asymptomatic malaria infections in adults, Ashanti Region, Ghana, 2018. Malar J. 2020;19(1):366. 10.1186/s12936-020-03441-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uwimana A, Umulisa N, Venkatesan M, Svigel SS, Zhou Z, Munyaneza T, Habimana RM, Rucogoza A, Moriarty LF, Sandford R, et al. Association of Plasmodium falciparum kelch13 R561H genotypes with delayed parasite clearance in Rwanda: an open-label, single-arm, multicentre, therapeutic efficacy study. Lancet Infect Dis. 2021;21(8):1120–8. 10.1016/S1473-3099(21)00142-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balikagala B, Fukuda N, Ikeda M, Katuro OT, Tachibana SI, Yamauchi M, Opio W, Emoto S, Anywar DA, Kimura E, et al. Evidence of artemisinin-resistant malaria in Africa. N Engl J Med. 2021;385(13):1163–71. 10.1056/NEJMoa2101746 [DOI] [PubMed] [Google Scholar]
- 8.Tumwebaze PK, Conrad MD, Okitwi M, Orena S, Byaruhanga O, Katairo T, Legac J, Garg S, Giesbrecht D, Smith SR, et al. Decreased susceptibility of Plasmodium falciparum to both dihydroartemisinin and lumefantrine in northern Uganda. Nat Commun. 2022;13(1):6353. 10.1038/s41467-022-33873-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Straimer J, Gandhi P, Renner KC, Schmitt EK. High prevalence of Plasmodium falciparum K13 mutations in Rwanda is associated with slow parasite clearance after treatment with artemether-lumefantrine. J Infect Dis. 2022;225(8):1411–4. 10.1093/infdis/jiab352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Derbie A, Mekonnen D, Adugna M, Yeshitela B, Woldeamanuel Y, Abebe T. Therapeutic efficacy of artemether-lumefantrine (Coartem(R)) for the treatment of uncomplicated falciparum malaria in Africa: a systematic review. J Parasitol Res. 2020;2020:7371681. 10.1155/2020/7371681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bwire GM, Ngasala B, Mikomangwa WP, Kilonzi M, Kamuhabwa AAR. Detection of mutations associated with artemisinin resistance at k13-propeller gene and a near complete return of chloroquine susceptible falciparum malaria in southeast of Tanzania. Sci Rep. 2020;10(1):3500. 10.1038/s41598-020-60549-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mihreteab S, Platon L, Berhane A, Stokes BH, Warsame M, Campagne P, Criscuolo A, Ma L, Petiot N, Doderer-Lang C, et al. Increasing prevalence of artemisinin-resistant HRP2-negative malaria in Eritrea. N Engl J Med. 2023;389(13):1191–202. 10.1056/NEJMoa2210956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trape JF, Pison G, Preziosi MP, Enel C, Desgrees du Lou A, Delaunay V, Samb B, Lagarde E, Molez JF, Simondon F. Impact of chloroquine resistance on malaria mortality. C R Acad Sci III. 1998;321(8):689–97. 10.1016/S0764-4469(98)80009-7 [DOI] [PubMed] [Google Scholar]
- 14.Noedl H, Se Y, Schaecher K, Smith BL, Socheat D, Fukuda MM. Evidence of artemisinin-resistant malaria in western Cambodia. N Engl J Med. 2008;359(24):2619–20. 10.1056/NEJMc0805011 [DOI] [PubMed] [Google Scholar]
- 15.Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ, et al. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361(5):455–67. 10.1056/NEJMoa0808859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ashley EA, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S, Sreng S, Anderson JM, Mao S, Sam B, et al. Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2014;371(5):411–23. 10.1056/NEJMoa1314981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Pluijm RW, Imwong M, Chau NH, Hoa NT, Thuy-Nhien NT, Thanh NV, Jittamala P, Hanboonkunupakarn B, Chutasmit K, Saelow C, et al. Determinants of dihydroartemisinin-piperaquine treatment failure in Plasmodium falciparum malaria in Cambodia, Thailand, and Vietnam: a prospective clinical, pharmacological, and genetic study. Lancet Infect Dis. 2019;19(9):952–61. 10.1016/S1473-3099(19)30391-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White NJ, Qiao LG, Qi G, Luzzatto L. Rationale for recommending a lower dose of primaquine as a Plasmodium falciparum gametocytocide in populations where G6PD deficiency is common. Malar J. 2012;11:418. 10.1186/1475-2875-11-418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dicko A, Brown JM, Diawara H, Baber I, Mahamar A, Soumare HM, Sanogo K, Koita F, Keita S, Traore SF, et al. Primaquine to reduce transmission of Plasmodium falciparum malaria in Mali: a single-blind, dose-ranging, adaptive randomised phase 2 trial. Lancet Infect Dis. 2016;16(6):674–84. 10.1016/S1473-3099(15)00479-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dicko A, Roh ME, Diawara H, Mahamar A, Soumare HM, Lanke K, Bradley J, Sanogo K, Kone DT, Diarra K, et al. Efficacy and safety of primaquine and methylene blue for prevention of Plasmodium falciparum transmission in Mali: a phase 2, single-blind, randomised controlled trial. Lancet Infect Dis. 2018;18(6):627–39. 10.1016/S1473-3099(18)30044-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eziefula AC, Bousema T, Yeung S, Kamya M, Owaraganise A, Gabagaya G, Bradley J, Grignard L, Lanke KH, Wanzira H, et al. Single dose primaquine for clearance of Plasmodium falciparum gametocytes in children with uncomplicated malaria in Uganda: a randomised, controlled, double-blind, dose-ranging trial. Lancet Infect Dis. 2014;14(2):130–9. 10.1016/S1473-3099(13)70268-8 [DOI] [PubMed] [Google Scholar]
- 22.Vantaux A, Kim S, Piv E, Chy S, Berne L, Khim N, Lek D, Siv S, Mukaka M, Taylor WR, et al. Significant efficacy of a single low dose of primaquine compared to stand-alone artemisinin combination therapy in reducing gametocyte carriage in Cambodian patients with uncomplicated multidrug-resistant Plasmodium falciparum malaria. Antimicrob Agents Chemother. 2020;64(6):e02108-19. 10.1128/AAC.02108-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goncalves BP, Tiono AB, Ouedraogo A, Guelbeogo WM, Bradley J, Nebie I, Siaka D, Lanke K, Eziefula AC, Diarra A, et al. Single low dose primaquine to reduce gametocyte carriage and Plasmodium falciparum transmission after artemether-lumefantrine in children with asymptomatic infection: a randomised, double-blind, placebo-controlled trial. BMC Med. 2016;14:40. 10.1186/s12916-016-0581-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goncalves BP, Pett H, Tiono AB, Murry D, Sirima SB, Niemi M, Bousema T, Drakeley C, Ter Heine R. Age, weight, and CYP2D6 genotype are major determinants of primaquine pharmacokinetics in African children. Antimicrob Agents Chemother. 2017;61(5):e02590-16. 10.1128/AAC.02590-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mukaka M, Onyamboko MA, Olupot-Olupot P, Peerawaranun P, Suwannasin K, Pagornrat W, Kouhathong J, Madmanee W, Were W, Namayanja C, et al. Pharmacokinetics of single low dose primaquine in Ugandan and Congolese children with falciparum malaria. EBioMedicine. 2023;96:104805. 10.1016/j.ebiom.2023.104805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor WR, Naw HK, Maitland K, Williams TN, Kapulu M, D’Alessandro U, Berkley JA, Bejon P, Okebe J, Achan J, et al. Single low-dose primaquine for blocking transmission of Plasmodium falciparum malaria - a proposed model-derived age-based regimen for sub-Saharan Africa. BMC Med. 2018;16(1):11. 10.1186/s12916-017-0990-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor WR, Olupot-Olupot P, Onyamboko MA, Peerawaranun P, Weere W, Namayanja C, Onyas P, Titin H, Baseke J, Muhindo R, et al. Safety of age-dosed, single low-dose primaquine in children with glucose-6-phosphate dehydrogenase deficiency who are infected with Plasmodium falciparum in Uganda and the Democratic Republic of the Congo: a randomised, double-blind, placebo-controlled, non-inferiority trial. Lancet Infect Dis. 2023;23(4):471–83. 10.1016/S1473-3099(22)00658-2 [DOI] [PubMed] [Google Scholar]
- 28.Stepniewska K, Allen EN, Humphreys GS, Poirot E, Craig E, Kennon K, Yilma D, Bousema T, Guerin PJ, White NJ, et al. Safety of single-dose primaquine as a Plasmodium falciparum gametocytocide: a systematic review and meta-analysis of individual patient data. BMC Med. 2022;20(1):350. 10.1186/s12916-022-02504-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ranmal SR, Lavarde M, Wallon E, Issa S, Taylor WR, Nguyen Ngoc Pouplin JLA, Tuleu C, Pense-Lheritier AM. Responsive sensory evaluation to develop flexible taste-masked paediatric primaquine tablets against malaria for low-resource settings. Pharmaceutics. 2023;15(7):1879. 10.3390/pharmaceutics15071879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Severe falciparum malaria. World Health Organization, Communicable Diseases Cluster. Trans R Soc Trop Med Hyg. 2000;94 Suppl 1:S1–90. [PubMed]
- 31.Ruiz F, Vallet T, Pense-Lheritier AM, Aoussat A. Standardized method to assess medicines’ acceptability: focus on paediatric population. J Pharm Pharmacol. 2017;69(4):406–16. 10.1111/jphp.12547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vallet T, Ruiz F, Lavarde M, Pense-Lheritier AM, Aoussat A. Standardised evaluation of medicine acceptability in paediatric population: reliability of a model. J Pharm Pharmacol. 2018;70(1):42–50. 10.1111/jphp.12829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hillmen P, Hall C, Marsh JC, Elebute M, Bombara MP, Petro BE, Cullen MJ, Richards SJ, Rollins SA, Mojcik CF, et al. Effect of eculizumab on hemolysis and transfusion requirements in patients with paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2004;350(6):552–9. 10.1056/NEJMoa031688 [DOI] [PubMed] [Google Scholar]
- 34.Recht J, Ashley EA, White NJ. Use of primaquine and glucose-6-phosphate dehydrogenase deficiency testing: divergent policies and practices in malaria endemic countries. PLoS Negl Trop Dis. 2018;12(4):e0006230. 10.1371/journal.pntd.0006230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okebe J, Bousema T, Affara M, Di Tanna GL, Dabira E, Gaye A, Sanya-Isijola F, Badji H, Correa S, Nwakanma D, et al. The gametocytocidal efficacy of different single doses of primaquine with dihydroartemisinin-piperaquine in asymptomatic parasite carriers in The Gambia: a randomized controlled trial. EBioMedicine. 2016;13:348–55. 10.1016/j.ebiom.2016.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chotsiri P, Mahamar A, Hoglund RM, Koita F, Sanogo K, Diawara H, Dicko A, Simpson JA, Bousema T, White NJ, et al. Mechanistic modeling of primaquine pharmacokinetics, gametocytocidal activity, and mosquito infectivity. Clin Pharmacol Ther. 2022;111(3):676–85. 10.1002/cpt.2512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stepniewska K, Humphreys GS, Goncalves BP, Craig E, Gosling R, Guerin PJ, Price RN, Barnes KI, Raman J, Smit MR, et al. Efficacy of single-dose primaquine with artemisinin combination therapy on Plasmodium falciparum gametocytes and transmission: an individual patient meta-analysis. J Infect Dis. 2022;225(7):1215–26. 10.1093/infdis/jiaa498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raman J, Allen E, Workman L, Mabuza A, Swanepoel H, Malatje G, Frean J, Wiesner L, Barnes KI. Safety and tolerability of single low-dose primaquine in a low-intensity transmission area in South Africa: an open-label, randomized controlled trial. Malar J. 2019;18(1):209. 10.1186/s12936-019-2841-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Onyamboko MA, Olupot-Olupot P, Were W, Namayanja C, Onyas P, Titin H, Baseke J, Muhindo R, Kayembe DK, Ndjowo PO, et al. Factors affecting haemoglobin dynamics in African children with acute uncomplicated Plasmodium falciparum malaria treated with single low-dose primaquine or placebo. BMC Med. 2023;21(1):397. 10.1186/s12916-023-03105-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anderson BJ, Holford NH. Understanding dosing: children are small adults, neonates are immature children. Arch Dis Child. 2013;98(9):737–44. 10.1136/archdischild-2013-303720 [DOI] [PubMed] [Google Scholar]
- 41.Falade CO, Orimadegun AE, Olusola FI, Michael OS, Anjorin OE, Funwei RI, Adedapo AD, Olusanya AL, Orimadegun BE, Mokuolu OA. Efficacy and safety of pyronaridine-artesunate versus artemether-lumefantrine in the treatment of acute uncomplicated malaria in children in South-West Nigeria: an open-labelled randomized controlled trial. Malar J. 2023;22(1):154. 10.1186/s12936-023-04574-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quang Bui P, Hong Huynh Q, Thanh Tran D, Le Thanh D, Quang Nguyen T, Van Truong H, Khim N, Witkowski B, Cong Tran D, Bustos MD, et al. Pyronaridine-artesunate efficacy and safety in uncomplicated Plasmodium falciparum malaria in areas of artemisinin-resistant falciparum in Viet Nam (2017–2018). Clin Infect Dis. 2020;70(10):2187–95. 10.1093/cid/ciz580 [DOI] [PubMed] [Google Scholar]
- 43.Tona Lutete G, Mombo-Ngoma G, Assi SB, Bigoga JD, Koukouikila-Koussounda F, Ntamabyaliro NY, Ntoumi F, Agnandji ST, Groger M, Shin J, et al. Pyronaridine-artesunate real-world safety, tolerability, and effectiveness in malaria patients in 5 African countries: a single-arm, open-label, cohort event monitoring study. PLoS Med. 2021;18(6):e1003669. 10.1371/journal.pmed.1003669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Compaore YD, Zongo I, Some AF, Barry N, Nikiema F, Kabore TN, Ouattara A, Kabre Z, Wermi K, Zongo M, et al. Hepatic safety of repeated treatment with pyronaridine-artesunate versus artemether-lumefantrine in patients with uncomplicated malaria: a secondary analysis of the WANECAM 1 data from Bobo-Dioulasso, Burkina Faso. Malar J. 2021;20(1):64. 10.1186/s12936-021-03593-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jittamala P, Pukrittayakamee S, Ashley EA, Nosten F, Hanboonkunupakarn B, Lee SJ, Thana P, Chairat K, Blessborn D, Panapipat S, et al. Pharmacokinetic interactions between primaquine and pyronaridine-artesunate in healthy adult Thai subjects. Antimicrob Agents Chemother. 2015;59(1):505–13. 10.1128/AAC.03829-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stone W, Mahamar A, Sanogo K, Sinaba Y, Niambele SM, Sacko A, Keita S, Youssouf A, Diallo M, Soumare HM, et al. Pyronaridine-artesunate or dihydroartemisinin-piperaquine combined with single low-dose primaquine to prevent Plasmodium falciparum malaria transmission in Ouelessebougou, Mali: a four-arm, single-blind, phase 2/3, randomised trial. Lancet Microbe. 2022;3(1):e41–51. 10.1016/S2666-5247(21)00192-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lynch T, Price A. The effect of cytochrome P450 metabolism on drug response, interactions, and adverse effects. Am Fam Physician. 2007;76(3):391–6. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The final database will be shared amongst the site PI and key members of the research team. The database may be shared with researchers not directly involved in this study but only after the main paper has been published and in accordance with GRAS and MORU guidelines on data sharing. The database will only be shared if future publications are not compromised.