Abstract
Background
Reports of pulmonary aspergillosis and mucormycosis co-infections are rare; thus, limited guidance is available on early diagnosis and treatment. We present a case of mixed pulmonary Aspergillus and Mucor infection and review the literature regarding this co-infection. The diagnosis and treatment methods are summarized to improve clinicians’ understanding of the disease and to facilitate early diagnosis and treatment.
Case presentation
A 60-year-old male farmer with poorly controlled diabetes mellitus was admitted to hospital with a fever of unknown origin that had been present for 15 days and pulmonary aspergillosis complicated by Mucor spp. infection. Because multiple lobes were involved, the infection worsened despite surgical resection and antifungal therapy. Finally, we treated this patient with a bronchoscopic infusion of amphotericin B. After four courses of bronchoscopic amphotericin B infusion, we observed rapid clinical improvement and subsequent resolution of pulmonary infiltrates.
Conclusion
Our case highlights the use of bronchoscopy in the successful clinical treatment of invasive fungal diseases of the lung.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12890-024-03234-z.
Keywords: Pulmonary aspergillosis, Pulmonary mucormycosis, Invasive fungal infections, Amphotericin B, Bronchoscopic treatment
Background
The incidence of invasive fungal diseases (IFD) is increasing owing to the aging of the population, an increasing incidence of diabetes mellitus (DM) and malignant tumors, and widespread use of immunosuppressants during the coronavirus disease 2019 (COVID-19) pandemic [1–3]. Both Aspergillus and Mucor spp. are fungi that are opportunistic pathogens and can invade the bronchus and lungs of people with severely impaired immune function, resulting in acute lung inflammation. These pathogens are highly invasive and can enter the blood via the pulmonary blood vessels, resulting in blood vessel embolization, lung tissue ischemia, and necrotic inflammation [4]. Invasive pulmonary aspergillosis (IPA) has a mortality rate of 35–80% [5]. Invasive pulmonary mucormycosis (IPM) has a rapid onset and progression, with a mortality rate of 76–87% [6]. However, mixed infections of pulmonary Aspergillus and Mucor spp. are rarely reported. Pulmonary mycosis is characterized by rapid spread, tissue necrosis, and structural damage of the lungs. In addition, fibrotic and granulation tissues may form in response to immune activity or treatment by antifungal drugs. Consequently, these features may hinder the action of intravenous drugs because of poor local blood circulation. Herein, we describe a case of mixed pulmonary Aspergillus and Mucor spp. infection that was successfully treated with combined bronchoscopic treatment and provide a literature review of 22 published case reports.
Case presentation
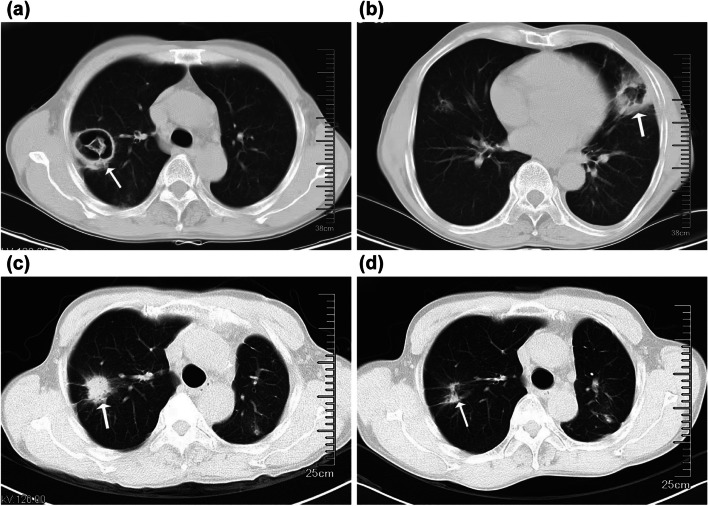
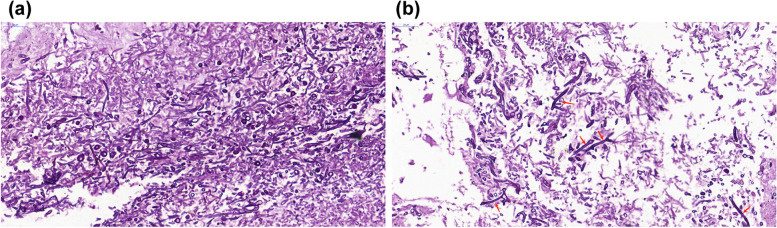
A 60-year-old male farmer was admitted to our hospital for treatment due to a fever of unknown origin. He had developed a fever 15 days prior to admission, with a maximum body temperature of 38.5℃, intermittent cough, a small amount of yellow and white phlegm, and chills. The patient was taking glimepiride and metformin sustained-release tablets for diabetes management. His HBA1c was 8% on admission. After admission, insulin was used to control his blood sugar levels. Routine blood tests on admission showed elevated leukocyte count (13.4 × 109/L) and neutrophil percentage (79.8%), an elevated high sensitivity C-reactive protein level (198.00 mg/L), and a procalcitonin level within the normal range (< 0.100 ng/mL). The patient tested negative for viral hepatitis B and C, syphilis, and acquired immunodeficiency syndrome. The serum level of β-D-glucan was 105 pg/ml (0–60 pg/ml). Chest computed tomography (CT) revealed patchy, crescent-shaped, low-density gas shadows in both lungs (Fig. 1a, b). Itraconazole was administered as antifungal therapy along with levofloxacin as antibiotic therapy. The patient underwent diagnostic bronchoscopy, and bronchoalveolar lavage fluid (BALF) was collected for examination. On day 6, the metagenomic second-generation sequencing results of the BALF revealed Rhizobacteria microspora and Aspergillus flavus. The itraconazole was discontinued, and liposomal amphotericin B (L-AmB) was administered intravenously at a dose of 5 mg/kg/day as antifungal therapy. On day 18, chest CT showed that the two lung lesions were larger, the wall was thicker, and the central solid component had increased. The patient underwent left upper lobe and left lower lung cuneiform resection on day 25. Postoperative pathological examination of the lung tissue revealed hyphae of Aspergillus and Mucor (Fig. 2). After surgery, the patient was treated with intravenous voriconazole, cefoperazone, and sulbactam sodium to prevent further infection. On day 66, voriconazole was discontinued, amphotericin B (AmB) was administered for 7 days, and the patient was discharged from the hospital for personal reasons. In October 2022, the patient returned to the hospital for chest CT examination, which showed that the lesions in the upper lobe of the right lung had increased in size (Fig. 1c). During hospitalization, L-AmB combined with atomized AmB was administered intravenously (5 mg/day). AmB 5 mg (total 5 mg) dissolved in 5 ml glucose (5%) was injected into the posterior segment of the right upper lobe several times under bronchoscopy (the lung segment was pre-anesthetized with 1 ml lidocaine injection), and remain in the supine position for 10 min. The patient returned to the hospital 2 weeks later to continue the intravenous administration of L-AmB and atomized AmB, with bronchoscopic infusion of AmB. After treatment, chest CT examination showed obvious absorption of right lung lesions, and no special discomfort was reported during follow-up after discharge (Fig. 1d).
Fig. 1.
Chest computed tomography (CT) (a, b) before starting treatment showing high-density shadows in both lungs and patchy, crescent-shaped low-density gas shadows in the lesions. (c) Chest CT taken 3.5 months later before starting bronchoscopic intramucosal liposomal amphotericin B treatment showing a slightly larger lesion in the right upper lobe of the lung. (d) Chest CT reexamination after 4 treatment courses showing obvious absorption of the lesion in the patient’s right lung
Fig. 2.
Hyphae of Mucor and Aspergillus observed on periodic acid-Schiff stain of lung biopsy samples (40×)
Literature review
A search was conducted on the PubMed database using “pulmonary mucormycosis” and “pulmonary aspergillosis” as key search terms to identify case reports of mixed Aspergillus and Mucor pulmonary infections published in English. The literature search revealed 22 published cases of mixed infection. The details are summarized in Table 1. Among these patients, the majority were male (n = 17, 74%) with a median age of 54 years (interquartile range [IQR], 22–74 years). Treatment outcomes were reported for 22 cases, with 9 (41%) showing improvement and 13 (59%) resulting in death. The most commonly reported underlying conditions were DM (n = 8, 34.8%), hematologic malignancies (n = 8, 34.8%), and COVID-19 treated with immunosuppressants (n = 7, 30%). Other underlying conditions included trauma (n = 2, 8.7%) and pulmonary tuberculosis, liver cirrhosis, and organ transplantation (one case each, 4.3%). Chest CT findings included the typical anti-halo sign (n = 3, 15%), crescent sign (n = 2, 10%), plaque infiltration and consolidation (n = 6, 30%), and empty expression (n = 7, 35%). BALF analysis and lung biopsy were performed in 10 (43.5%) and 14 cases (60.9%), respectively. Sputum culture was used for diagnostic purposes in five cases (n = 5, 21.7%). Fourteen patients received antifungal treatment, among whom 11 (78.6%) died and 3 (21.4%) recovered. Nine patients received antifungal treatment combined with surgery, of whom two died (22.2%) and six recovered (66.7%). We did not identify any reports of treatment using bronchoscopic AmB infusion, as in our patient.
Table 1.
Published cases of pulmonary aspergillosis and mucormycosis co-infection
| Case | Age (years) |
Sex | Underlying disease | Disease duration | Chest CT | Diagnostic specimen | Therapeutic drug | Bronchoscopic therapy | Surgery | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 [7] | 52 | M | Heart transplant recipient | 1 year | Pulmonary nodular mass | Biopsy | VCZ→PCZ + L-AmB | No | Yes | Died |
| 2 [8] | 58 | M | Liver cirrhosis | 25 days | Patchy infiltration | BALF | VCZ + L-AmB | No | No | Died |
| 3 [9] | 27 | F | DM | 18 days | Patchy infiltration | BALF, Biopsy | VCZ + L-AmB | No | No | Died |
| 4 [10] | 54 | F | DM | 78 days | Nodule | BALF, Biopsy | VCZ + L-AmB + PCZ | No | Yes | Improved |
| 5 [11] | 52 | M | DM | 6 months | Cavity lesion | BALF, Biopsy | AmB | No | Yes | Improved |
| 6 [12] | 65 | M | DM | 21 days | Antihalation sign | Biopsy | L-AmB | No | No | Improved |
| 7a | 60 | M | DM | 9 months | Patchy, crescentic | BALF, Biopsy | VCZ→L-AmB | Yes | Yes | Improved |
| 8 [13] | 48 | M | DM + COVID-19 | NM | Void expression | Biopsy | L-AmB | No | Yes | NM |
| 9 [14] | 69 | M | COVID-19 | 120 days | Void expression | Biopsy | VCZ | No | No | Died |
| 10 [15] | 74 | M | COVID-19 | 26 days | Void expression | BALF | VCZ→L-AmB | No | No | Died |
| 11 [16] | 70 | M | COVID-19 | 43 days | Void expression | Sputum | L-AmB | No | No | Died |
| 12 [17] | 50 | M | COVID-19 | 21 days | Antihalation sign | Sputum | VCZ→L-AmB + PCZ | No | No | Died |
| 13 [18] | 22 | M | HM | 16 days | Consolidation | BALF, Biopsy | L-AmB + PCZ + VCZ | No | No | Died |
| 14 [19] | 44 | M | HM | 4 months | Void expression | Sputum | L-AmB→PCZ | No | No | Improved |
| 15 [20] | 55 | M | HM | 40 days | Double ground glass shadows | BALF | L-AmB | No | No | Died |
| 16 [12] | 70 | M | Phthisis | 21 days | Void, Air crescent | Biopsy | L-AmB | No | Yes | Improved |
| 17 [21] | 40 | F | HM | NM | NM | Biopsy | AmB | No | No | Died |
| 18 [22] | 42 | M | HM | 9 months | Ground glass shadows | BALF, Biopsy | L-AmB + PCZ | No | Yes | Died |
| 19 [17] | 60 | M | HM + DM + COVID-19 | 30 days | NM | Sputum | VCZ→L-AmB | No | No | Died |
| 20 [23] | 51 | F | HM | 6 months | Nodule | BALF | L-AmB + PCZ | No | No | Improved |
| 21 [24] | 72 | M | HM + DM + COVID-19 | 64 days | Void | Sputum | VCZ→L-AmB | No | No | Died |
| 22 [25] | 58 | F | HM | 111 days | Consolidation | Biopsy | VCZ→L-AmB | No | Yes | Improved |
| 23 [26] | 24 | F | HM | 3 months | Antihalation sign | Biopsy | VCZ + L-AmB + PCZ | No | Yes | Improved |
AmB amphotericin B, BALF bronchoalveolar lavage fluid, COVID-19 coronavirus disease 2019, CT computed tomography, DM diabetes mellitus, HM hematologic malignancy, L-AmB liposomal amphotericin B, NM not mentioned, PCZ posaconazole, VCZ voriconazole
aCase 7 in Table 1 refers to the present case
Discussion and conclusion
The human respiratory tract is constantly exposed to various harmful microorganisms that can cause disease, particularly in individuals with weakened immune systems. Invasive pulmonary Aspergillus and Mucor are two types of infection that share similar risk factors. Among the 23 cases of mixed pulmonary aspergillosis and mucormycosis, common predisposing factors included hematologic malignancies, DM, and immunosuppressant use after COVID-19. The clinical symptoms of both infections are not distinct and are typically pneumonia-like symptoms [27]. Both IPM and IPA can be accompanied by atypical non-nodular manifestations, including consolidation and ground glass opacities [28]. The pathogenesis of pulmonary Aspergillus and Mucor infections are similar, making it difficult to distinguish between them when making a diagnosis. Although direct histological examination of lung tissue biopsy is the gold standard for the diagnosis of IFDs, it is an invasive and risky procedure, prone to complications such as pneumothorax and pulmonary hemorrhage. Therefore, pulmonary biopsy should not be routinely selected for diagnosis. In clinical practice, BALF and repeated sputum culture are commonly used to detect fungi, aiding early diagnosis. Mucor pulmonary infection progresses more rapidly than Aspergillus pneumonia, with a high fatality rate of up to 65% [29]. Therefore, in patients presenting with hemoptysis, pulmonary consolidation and cavity lesions with a halo sign or anti-halo sign, it is crucial to remain vigilant for the occurrence of Mucor infection. Bronchoscopy should be performed promptly, and BALF and sputum samples should be sent for multiple culture identification.
Based on published case reports, mixed pulmonary Aspergillus and Mucor infection is associated with high mortality, rapid disease progression, and poor prognosis. Early diagnosis and treatment are vital for improving survival rates. Once diagnosed, antifungal treatment is necessary for both infections. However, the treatment methods differ. The most frequently used drugs for treating pulmonary aspergillosis are itraconazole and voriconazole, but voriconazole is not effective for Mucor pneumonia, and its excessive use in treating Aspergillus may increase the risk of Mucor pneumonia [30]. Misdiagnosis or mistreatment can considerably reduce patient survival rates. Currently, AmB (including its liposomal form) and posaconazole are the drugs of choice for treating mucormycosis, and they are also effective for treating aspergillosis. Among the cases reviewed, eight patients received inadequate treatment with voriconazole, which can result in increased mucous virulence and mortality from pulmonary disease. Subsequently, these patients were switched to L-AmB for the treatment of Mucor infection. Therefore, it is important to consider Mucor in invasive fungal infections that do not respond to voriconazole. Current treatment measures for invasive Mucor pulmonary infection include controlling the underlying disease, selecting appropriate surgical interventions, and choosing appropriate antifungal drugs.
In our case, initial intravenous treatment with L-AmB was ineffective. Therefore, we performed surgery and debridement. However, due to involvement of multiple lobes, complete resection of the lesions was not possible. Mucor can easily invade blood vessels, leading to vessel obstruction and ischemic necrosis of lung tissue. The disrupted blood flow in the lungs affects the therapeutic concentration of AmB in the lesion [4]. Studies have shown that atominzed inhalation [31], intrathecal injection [32] and transcutaneous retrobulbar injection [33]of Amb have an adjunctive role in antifungal therapy; AmB is a key and effective drug for the treatment of severe invasive fungal infections, given its broad antifungal spectrum and potency. When applied intravenously, the drug concentration in pleural fluid, ascites, and synovial fluid remains below half of its concentration in blood and is even lower in bronchial secretions. However, the drug has strong water solubility, has slow absorption through the airway mucosa, and causes no obvious irritation to the airway mucosa. Given these pharmacological and metabolic characteristics, AmB has irreplaceable advantage for bronchoscopic pulmonary preservation perfusion. Previous clinical reports [34, 35] also indicate that antifungal drugs injected into the pulmonary cavity lesions can alleviate aspergillus lesions, with good clinical effects, in case systemic use of antifungal drugs is ineffective or adverse events cannot be tolerated. To our knowledge, this is the first reported case of mixed pulmonary Aspergillus and Mucor infection successfully treated using intrabronchial AmB infusion under bronchoscopy. Existing literature lacks consensus on antifungal dose regimens for treating pulmonary mixed Aspergillus and Mucor infection, and there are no data available on the exact dosage and duration of intrabronchial AmB therapy. Our patient did not experience any immediate or delayed adverse reactions to bronchoscopic reserved infusion of AmB. The lack of response to intravenous AmB initially observed in our patient was due to the failure to achieve an adequate local concentration of the drug at the affected site. Mucor easily invades blood vessels, leading to vascular obstruction and ischemic necrosis of lung tissue, causing local congestion of the blood flow inside the lungs, which affects the concentration of AmB in the lesion. Bronchoscopic administration enables targeted delivery of high local drug concentrations, resulting in positive clinical outcomes. Therefore, we believe that endobronchial AmB therapy has the potential to become a promising primary treatment modality, reducing the total cumulative antifungal dose and treatment duration. This is particularly important for patients who experience side effects, do not respond to intravenous antifungal drugs, or are not suitable candidates for surgery.
In conclusion, patients with suspected mixed pulmonary Aspergillus and Mucor infection, combined with risk factors for infection, should undergo sputum culture or BALF assessment and pathological examination as early as possible. In addition, antifungal drug infusion under bronchoscopy should be considered as a means of antifungal therapy.
Supplementary Information
Acknowledgements
We thank our colleagues at Nanxishan Hospital for their useful feedback, which helped us to improve this paper.
Abbreviations
- AmB
Amphotericin
- B; BALF
Bronchoalveolar lavage fluid
- COVID-19
Coronavirus disease 2019
- CT
Computed tomography
- DM
Diabetes mellitus
- IFD
Invasive fungal disease
- IPA
Invasive pulmonary aspergillosis
- IPM
Invasive pulmonary mucormycosis
- L-AmB
Liposomal amphotericin B
Authors’ contributions
F-XL drafted the manuscript. D-MQ is the corresponding author. F-XL designed the research study. Y-QL and RL collected the clinical data. D-MQ and L-R revised the manuscript. All authors read and approved the final version of the manuscript.
Funding
This work was supported by the Guangxi Medical and Health Key Discipline Construction Project.
Availability of data and materials
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained for the publication of this case report and accompanying images.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rashbi KS, Ali T. COVID-19-Associated mucormycosis: Case series from a tertiary care hospital in South India. Access Microbiol. 2022;4(6):acmi360. 10.1099/acmi.0.000360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garcia-Carnero LC, Mora-Montes HM. Mucormycosis and COVID-19-associated mucormycosis: insights of a deadly but neglected mycosis[J]. J Fungi (Basel). 2022;8(5):445–5. [DOI] [PMC free article] [PubMed]
- 3.Bitar D, Van Cauteren D, Lanternier F, et al. Increasing incidence of zygomycosis (mucormycosis), France, 1997–2006. Emerg Infect Dis. 2009;15(9):1395–401. 10.3201/eid1509.090334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tedder M, Spratt JA, Anstadt MP, et al. Pulmonary mucormycosis: results of medical and surgical therapy. Ann Thorac Surg. 1994;57(4):1044–50. 10.1016/0003-4975(94)90243-7 [DOI] [PubMed] [Google Scholar]
- 5.Schmiedel Y, Zimmerli S. Common invasive fungal diseases: an overview of invasive candidiasis, aspergillosis, cryptococcosis, and Pneumocystis pneumonia. Swiss Med Wkly. 2016;146:w14281. [DOI] [PubMed] [Google Scholar]
- 6.Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41(5):634–53. 10.1086/432579 [DOI] [PubMed] [Google Scholar]
- 7.Webb BJ, Blair JE, Kusne S, et al. Concurrent pulmonary aspergillus fumigatus and mucor infection in a cardiac transplant recipient: a case report. Transpl Proc. 2013;45(2):792–7. 10.1016/j.transproceed.2012.03.056 [DOI] [PubMed] [Google Scholar]
- 8.Persichino JG, Can AD, Van TT, et al. Invasive pulmonary mucormycosis and aspergillosis in a patient with decompensated hepatic cirrhosis. Med Mycol Case Rep. 2018;21:12–5. 10.1016/j.mmcr.2018.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahadevaiah A H, Rajagopalan N, Patil M, et al. Coinfection of pulmonary mucormycosis and aspergillosis presenting as bilateral vocal cord palsy[J]. BMJ Case Rep. 2013;2013(aug20 1):bcr2013009615–bcr2013009615. [DOI] [PMC free article] [PubMed]
- 10.Teng P, Han X, Zhang S, et al. Mixed invasive pulmonary Mucor and aspergillus infection: a case report and literature review. Chin Med J (Engl). 2022;135(7):854–6. 10.1097/CM9.0000000000001839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin L, Xue D, Lin TY, et al. Pulmonary aspergillosis, mucormycosis, and actinomycosis co-infection presenting as a cavitary lesion in a patient with diabetes. Chin Med J (Engl). 2019;132(20):2512–3. 10.1097/CM9.0000000000000468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ravindra CM, Rajaram M, Madhusmita M, et al. Pulmonary aspergillus and Mucor Co-infection: a report of two cases. Sultan Qaboos Univ Med J. 2021;21(3):495–8. 10.18295/squmj.8.2021.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rathna R, D’Souza C, D’Silva C, et al. Two uncommon presentations of COVID-19-Associated Mucormycosis. Cureus. 2022;14(1):e21229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim JH, Kim M, Lim S, et al. A fatal case report of invasive pulmonary aspergillosis and mucormycosis coinfection in an immunocompetent patient with coronavirus disease 2019 in Korea[J]. Acute Crit Care. 2023;38(3):382–8. [DOI] [PMC free article] [PubMed]
- 15.Benhadid-Brahmi Y, Hamane S, Soyer B, et al. COVID-19-associated mixed mold infection: a case report of aspergillosis and mucormycosis and a literature review. J Mycol Med. 2022;32(1):101231. 10.1016/j.mycmed.2021.101231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lai CC, Wu CJ, Lee YC, et al. COVID-19 associated with concomitant mucormycosis and aspergillosis. J Microbiol Immunol Infect. 2022;55(2):353–4. 10.1016/j.jmii.2021.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buil J B, van Zanten A, Bentvelsen R G, et al. Case series of four secondary mucormycosis infections in COVID-19 patients, the Netherlands, December 2020 to May 2021[J]. Euro Surveill. 2021;26(23):2100510. [DOI] [PMC free article] [PubMed]
- 18.Radowsky JS, Strawn AA, Sherwood J, et al. Invasive mucormycosis and aspergillosis in a healthy 22-year-old battle casualty: case report. Surg Infect (Larchmt). 2011;12(5):397–400. 10.1089/sur.2010.065 [DOI] [PubMed] [Google Scholar]
- 19.Leelawattanachai P, Montakantikul P, Nosoongnoen W, et al. Pharmacokinetic/pharmacodynamic study of posaconazole delayed-release tablet in a patient with coexisting invasive aspergillosis and mucormycosis. Ther Clin Risk Manag. 2019;15:589–95. 10.2147/TCRM.S203625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bellanger AP, Navellou JC, Lepiller Q, et al. Mixed mold infection with aspergillus fumigatus and Rhizopus microsporus in a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) patient. Infect Dis Now. 2021;51(7):633–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Molle M, Blaschke-Hellmessen R, Schuler U, et al. Disseminated aspergillosis and mucormycosis. A case report. Mycoses. 1996;39(Suppl 1):59–64. [DOI] [PubMed] [Google Scholar]
- 22.Henn A, Mellon G, Benoit H, et al. Disseminated cryptococcosis, invasive aspergillosis, and mucormycosis in a patient treated with alemtuzumab for chronic lymphocytic leukaemia. Scand J Infect Dis. 2014;46(3):231–4. 10.3109/00365548.2013.866269 [DOI] [PubMed] [Google Scholar]
- 23.Hu ZM, Wang LL, Zou L, et al. Coinfection pulmonary mucormycosis and aspergillosis with disseminated mucormycosis involving gastrointestinalin in an acute B-lymphoblastic leukemia patient. Braz J Microbiol. 2021;52(4):2063–8. 10.1007/s42770-021-00554-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saltini P, Palomba E, Castelli V, et al. Mucormycosis in CAPA, a possible fungal super-infection[J]. J Fungi (Basel). 2021;7(9):708–708. [DOI] [PMC free article] [PubMed]
- 25.Bergantim R, Rios E, Trigo F, et al. Invasive coinfection with Aspergillus and Mucor in a patient with acute myeloid leukemia. Clin Drug Investig. 2013;33(Suppl 1):S51-5. 10.1007/s40261-012-0022-4 [DOI] [PubMed] [Google Scholar]
- 26.Davoudi S, Anderlini P, Fuller GN, et al. A long-term survivor of disseminated aspergillus and mucorales infection: an instructive case. Mycopathologia. 2014;178(5–6):465–70. 10.1007/s11046-014-9785-x [DOI] [PubMed] [Google Scholar]
- 27.Chamilos G, Marom EM, Lewis RE, et al. Predictors of pulmonary zygomycosis versus invasive pulmonary aspergillosis in patients with cancer. Clin Infect Dis. 2005;41(1):60–6. 10.1086/430710 [DOI] [PubMed] [Google Scholar]
- 28.Alexander BD, Lamoth F, Heussel CP, et al. Guidance on imaging for invasive pulmonary aspergillosis and mucormycosis: from the imaging working group for the revision and update of the consensus definitions of fungal disease from the EORTC/MSGERC. Clin Infect Dis. 2021;72(Suppl 2):S79–88. 10.1093/cid/ciaa1855 [DOI] [PubMed] [Google Scholar]
- 29.Enoch DA, Yang H, Aliyu SH, et al. The changing epidemiology of invasive fungal Infections. Methods Mol Biol. 2017;1508:17–65. 10.1007/978-1-4939-6515-1_2 [DOI] [PubMed] [Google Scholar]
- 30.Wingard JR, Carter SL, Walsh TJ, et al. Randomized, double-blind trial of fluconazole versus voriconazole for prevention of invasive fungal infection after allogeneic hematopoietic cell transplantation. Blood. 2010;116(24):5111–8. 10.1182/blood-2010-02-268151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuiper L, Ruijgrok EJ. A review on the clinical use of inhaled amphotericin B. J Aerosol Med Pulm Drug Deliv. 2009;22(3):213–27. 10.1089/jamp.2008.0715 [DOI] [PubMed] [Google Scholar]
- 32.Nau R, Blei C, Eiffert H. Intrathecal antibacterial and antifungal therapies[J]. Clin Microbiol Rev. 2020;33(3). 10.1128/CMR.00190-19. [DOI] [PMC free article] [PubMed]
- 33.Nair AG, Dave TV. Transcutaneous retrobulbar injection of amphotericin B in rhino-orbital-cerebral mucormycosis: a review. Orbit. 2022;41(3):275–86. 10.1080/01676830.2021.1990351 [DOI] [PubMed] [Google Scholar]
- 34.Rumbak M, Kohler G, Eastrige C, et al. Topical treatment of life threatening haemoptysis from aspergillomas. Thorax. 1996;51(3):253–5. 10.1136/thx.51.3.253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krakowka P, Traczyk K, Walczak J, et al. Local treatment of aspergilloma of the lung with a paste containing nystatin or amphotericin B. Tubercle. 1970;51(2):184–91. 10.1016/0041-3879(70)90071-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.